Abstract
Background
Flow diverters are used to treat complex aneurysms that are not amenable to coiling. The aim of the present work was to retrospectively evaluate our experience with the Silk flow diverter. Technical nuances and complications are specifically discussed.
Methods
Retrospectively data was collected on patients treated with Silk between October 2008 and October 2013.
Results
Sixty patients harboring 67 aneurysms were treated using the Silk. Fifteen aneurysms were located in the posterior circulation and 52 in the anterior. A good angiographic result was achieved in 88 % (53/60) of the aneurysms available for imaging follow-up. There were ten treatment-related complications, 80 % were ischemic. Risk of complications increased with aneurysm size and in aneurysms of the posterior circulation.
Conclusions
Silk flow diverters are a good treatment option for aneurysms of the anterior circulation. Additional stents may be required in specific cases due to the Silk’s low radial resistance. Treatment of giant fusiform aneurysms of the posterior circulation with Silk flow diverters is associated with a high rate of severe complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular coil embolization is now the treatment of choice for most intracranial aneurysms [10, 13]. However, coiling has its limitations, mainly in the treatment of wide-neck aneurysms due to the risk of coil protrusion into the parent artery and/or recanalization of the aneurysmal sac [13, 14, 18]. Several techniques were developed to overcome these limitations, including balloon remodeling and stent assisted coil placement. Diversion of blood flow away from the aneurysm lumen was suggested as another alternative in selected cases [4, 13]. Initial experience with flow diversion was done using stents such as the Neuroform (Boston Scientific Neurovascular, Fremont, California, USA) or Leo (Balt, Montmorency, France) that were originally developed for stent-assisted coiling [6]. Recently a new, self-expandable stent was introduced: Silk (Balt, Montmorency, France), which was specifically designed as a flow diverter [1, 2, 8, 9, 11, 16]. The Silk has several potential advantages for aneurysm treatment. It has a tightly woven structure, improving flow diversion from the aneurysm, thus facilitating aneurysm closure while still allowing sufficient blood flow to perforators. It is supplied with a soft microcatheter and is easy to navigate; moreover, it can be re-sheathed and relocated [2, 9]. Currently there are only limited data on the use of Silk in the literature [1, 2, 8, 9, 11, 16].
We have been using the Silk flow diverter in the treatment of complex aneurysms since 2008 and have previously published our short-term experience in 28 patients [9]. With growing experience, we expanded our indications to include also technically challenging small aneurysms (<10 mm diameter), where we estimated that flow diversion would be more effective than balloon remodeling with coils. Reasons for using flow diversion in small aneurysms were: several adjacent small aneurysms where a single Silk was deployed to treat two or three adjacent aneurysms, blister aneurysms, wide neck and dissecting aneurysms.
The present study aimed to evaluate our ongoing experience with Silk in the treatment of brain aneurysms in both the anterior and posterior circulation.
Patients and methods
Patients
We retrospectively reviewed our prospectively collected database for patients who were treated with the Silk flow diverter during a 5-year period, between October 2008 and October 2013. Age, date of diagnosis, the clinical presentation, imaging finding, the neurosurgical and medical treatment modalities, and complications were noted. The study was approved by our institutional review board.
Patients were evaluated clinically before and after each treatment, and periodically during the follow-up period. Clinical outcome was assessed using the modified Rankin score (mRS). It is our practice to perform a cross-axial imaging (computed tomography angiography [CTA] or magnetic resonance angiography [MRA]) as first follow-up at 6 months after treatment, and thereafter digital subtraction brain angiography, depending on the clinical condition of the patient and the radiological characteristics of the lesion.
Procedures
All the endovascular procedures were performed by the same invasive neuroradiologist (S.M.). All treatments were performed under general anesthesia. Catheterization was performed with a transfemoral approach using standard coaxial techniques. When deploying a Silk flow diverter, or a braided Leo stent, a long sheath is used to support the guiding catheter. Patients planned for stent placement were prepared with aspirin (100 mg/day) and clopidogrel (75 mg/day) for a minimum of 3 days prior to treatment.
The measurements of vessel diameter, neck and aneurysm size that were taken from the CTA or MRA were used for treatment planning. We used measurements from CTA/MRA imaging to select the best stent/flow-diverter diameter, taking into consideration the data from three-dimensional angiography when it was performed.
Ideally, for flow diversion the device should appose the entire circumference of the artery with enough radial force to provide a seal and prevent device migration. Therefore, due to the Silk’s relatively low radial force compared with the Leo braided stent [7, 9], we prefer to insert a device that is larger by one size (0.5 mm) than the diameter of the parent vessel and longer than the diseased segment (~6 mm on each side) in order to achieve better apposition to the vessel’s wall and prevent device migration [9]. In addition, in selected cases of fusiform or wide-necked (>8 mm) saccular aneurysms, we use an adjuvant Leo stent as support for the Silk flow diverter. In these cases we deploy the self-expandable braided Leo stent first, as a scaffold, and then deploy the Silk flow diverter into its lumen.
The Silk is deployed by navigating a Vasco microcatheter, which is supplied with the Silk package, to the segment distal to the aneurysm. This is done with the aid of any 0.014 or 0.016 guide wire.
Indications
Silk flow diverters were used for the following indications: Large and giant aneurysms mainly with wide neck (dome-to-neck ratio less than 2 or neck >4 mm); fusiform aneurysms; dissecting aneurysms; two or more adjacent aneurysms. Specifically, small aneurysms were treated in cases where there were several adjacent aneurysms that could be treated with a single device, blister aneurysms, or in cases of small aneurysms with wide neck, where we estimated that flow diversion would be less technically demanding than balloon remodeling. A wide neck was defined as dome-to-neck ratio less than 2 or neck >4 mm; by definition all fusiform aneurysms are wide-necked.
On rare occasions, we used coils with Silk; this was performed according to the treating physician’s judgment, mainly when patients were treated during the acute phase of subarachnoid hemorrhage (SAH) or in patients who were on anticoagulation therapy. Coiling was performed according to standard endovascular technique.
Follow-up policy
Angiographic results were classified into three categories according to the Raymond scale: complete obliteration (Raymond class I), near complete (Raymond class II, i.e. residual neck) or partial obliteration (Raymond class III) [15]. Our policy/routine is to perform non-invasive imaging (CTA/MRA) 3–6 months post treatment, and a formal angiography at a later stage when the cross-sectional angiography shows more than 80 % occlusion of the lumen.
Statistical analysis
Multiple univariate analyses were performed to investigate the association between outcome and categorical demographic and treatment related variables. In order to understand the independent effects of individual covariates on outcome, a multivariate model was generated. The goal was to create the simplest predictive regression model that best fit the study data. To create the predictive model, backward elimination logistic regression was used. Potential predictor variables were included in the modeling if their associated P value on univariate analysis was less than 0.1 or if the variable was thought to be clinically relevant.
Statistical analysis was performed by SAS for windows version 9.2.
Results
Epidemiology
Between October 2008 and October 2013 we treated 60 patients with 67 intracranial aneurysms using the Silk flow diverter. Mean age of patients was 57.3 ± 13.3 years (range, 12–89 years), and 75 % were females (n = 45).
Fifty-two aneurysms (78 %) were located in the anterior circulation (including the PCOM) and 15 in the posterior circulation (22 %); locations of the aneurysms are given in Table 1.
There were 14 giant aneurysm (>25 mm), comprising 20.8 % of the entire cohort, 25 large (10–24 mm), and 28 small (≤10 mm). The small aneurysms comprised 41.8 % of the entire group. All 39 large and giant aneurysms and 22/28 of the small aneurysms were wide-necked.
Eight of the 14 giant aneurysms (57 %) were located in the posterior circulation, comprising 53.3 % (8/15) of posterior circulation aneurysms in our series.
The presenting symptoms were cerebral vascular accident (CVA)/transient ischemic attack (TIA) in eight cases (13.3 %), headaches in 12 cases (20 %), cranial nerve (CN) compression in 13 cases (21.6 %) (CN II, III and VI) and SAH in 12 cases (20 %). In eight cases the bleeding was from the aneurysm that was treated (three were treated during the acute phase) and four aneurysms were discovered after bleeding from another aneurysm. Fifteen cases (25 %) were incidental findings.
Treatments
Sixty-two Silk flow diverters were inserted for the treatment of 67 aneurysms. We were able to successfully navigate and deploy the Silk in all the treated cases. In most cases (54/67 aneurysms), one Silk flow diverter was deployed for treatment of a single aneurysm. In four cases, the same device was used to treat two or more adjacent aneurysms (11/67 aneurysms). In two cases, two Silks needed to be deployed (2/67 aneurysms): in one case because of suboptimal placement of the first Silk in a previous session, and in another case because of shortening of the first Silk deployed at the same session.
Six patients required a second treatment after the insertion of a Silk flow diverter, resulting in 66 treatment sessions. The reasons for the second treatment were for insertion of a second Silk flow diverter in three cases, for reopening of an in-stent thrombosis and insertion of a supporting Leo stent inside the Silk flow diverter in one case, for closure of the vertebral artery on the contra lateral side in one case and for insertion of coils into an ACOM aneurysm due to incomplete occlusion because of Coumadin treatment in one case.
Adjuvant stents were used in 20 cases as a frame for insertion of the Silk flow diverter. This was done in order to bridge across wide-necked (>8 mm) or fusiform aneurysms due to the Silk’s tendency to bulge and shorten in wide lumens [9] (see illustrative case in Fig. 1). Our main adjuvant stent was the Leo stent. Eighteen Leo stents were inserted in 17 cases; three other stents were also used: one Solitaire as a support for the distal part of a Silk in a complex ACOM aneurysm and two coronary stents (one in the first case of Silk shortening, and a second in order to change the curve of the vessel at the aneurysm neck and assist in deflecting the flow from the aneurysm).
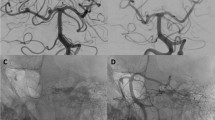
Giant fusiform basilar aneurysm. A 61-year-old man presented with CVA in the brainstem. MR and angiography demonstrated a giant fusiform aneurysm along the entire basilar artery (a, b). During endovascular treatment two telescoping Leo stents were deployed and then a Silk flow diverter was inserted inside (c). The left vertebral artery was occluded at the distal V4 segment (d). Four months postoperatively, MRI showed flow inside the Silk and early thrombosis of the aneurysm lumen outside the stent (e). Follow-up angiography was performed at 1 year postoperatively, demonstrating complete obliteration of the aneurysm (f)
Ten cases (15 %) were treated with flow diversion after reopening of a previously coiled aneurysm.
Immediate complications
There were ten immediate post procedural clinical complications; of them, eight were ischemic (Tables 2 and 3). There were three severe ischemic complications. In two cases of giant fusiform (non-dissecting) vertebrobasilar aneurysms that were treated by flow diversion from one vertebral artery to the basilar artery using Silk, the contralateral vertebral artery was also occluded with coils. Both patients developed brainstem infarction at the level of the vertebro-basilar junction, presumably due to perforant artery occlusion at that level.
Four of the complications resulted in mortalities; three of the four patients who died were severely disabled (mRS 3–4) before treatment.
We had two technical complications that were without any clinical implications. In both cases the Silk device was too short and a second device had to be inserted. This was due to underestimation of the required length.
Follow-up
Imaging follow-up was available for 60 aneurysms in 53 patients; at a mean time of 15 ± 9.5 months after treatment. Digital subtraction angiography (DSA) was performed for 51 aneurysms, MRA for eight and CTA for one.
Imaging follow-up was missing for seven aneurysms: four patients died, one patient was left severely disabled and did not perform imaging follow-up, and two cases were lost to follow-up.
Good angiographic result, defined as complete (Raymond class I; n = 46) or near complete (Raymond class II; n = 7 with small neck remnant), was achieved in 88 % (53/60) of the aneurysms available with imaging follow-up (Table 3). In seven cases we were able to achieve only partial occlusion (Raymond class III). No case of bleeding from the treated aneurysm after discharge was seen during follow-up.
Three cases (5 %) of in-stent flow insufficiency/occlusion were noted during follow-up. In the first case, a Silk placed in the left cavernous carotid was occluded on angiography performed 8 months later, probably due to poor compliance with antiaggregant treatment with Plavix. The patient was neurologically intact due to good collateral circulation through the circle of Willis and it was decided not to intervene. The second case was of a Silk placed for a giant non-dissecting vertebro-basilar aneurysm. When the patient arrived for a second-stage planned occlusion of the contralateral vertebral artery, the Silk device was found to be occluded. It was decided to recanalize using balloon angioplasty (PTA) and a Leo stent placed inside the Silk. On follow-up angiography 8 months later, the artery was still patent. The third patient developed imaging signs of in-stent stenosis after discontinuation of Plavix, presumably due to intimal hyperplasia. This was found on routine follow-up angiography. The stenosis responded favorably to Plavix reinstatement and resolved on follow-up angiography 8 months later.
For further statistical analysis in an attempt to identify factors predictive of outcome of endovascular treatment with flow diverters, aneurysms were subdivided according to maximal diameter as small (≤10 mm), large (11–24 mm) and giant (>24 mm); according to location as anterior circulation versus posterior circulation and by angio-characteristics as fusiform, saccular or dissecting aneurysms. Presenting symptoms were classified as bleeding, ischemic, mass-effect, headaches or incidental (Tables 4 and 5).
There was a trend towards a better angiographic outcome, defined as Raymond classes I and II, in small aneurysms compared to large and giant aneurysms (P = 0.1). Angiographic success rates in small, large, and giant aneurysms were 87, 72, and 64 %, respectively.
Complication rate increased with size: there were no complications in small aneurysms, 16.7 % in large aneurysms and 42.9 % in giant aneurysms (P = 0.003; Table 4). Furthermore, there was a trend towards a higher complication rate in aneurysms of the posterior circulation compared to the anterior circulation (33.3 % vs 11.1 %), but this did not reach statistical significance (P = 0.1; Table 4).
Patients who presented with an ischemic event were more likely to have a poor outcome (P = 0.01; Table 5). There was a trend towards poor clinical outcome in patients harboring larger aneurysms (P = 0.07).
Multivariate models revealed that maximal diameter class (i.e., small, large, or giant) was significantly associated with risk of complications (OR = 3.9, P = 0.04; Table 4) with larger aneurysms associated with a higher rate of complications. Patients who presented with an ischemic event had a significantly higher risk of poor outcome (mRS >3) compared with other clinical presentations (OR = 9, P = 0.007; Table 5).
Discussion
The goal of the present study was to evaluate our results in the use of the Silk flow diverter, and to search for patient or aneurysm characterisitics that are predictive of outcome.
Our main findings are that patients with small aneurysms (<10 mm) had a low complication rate with a higher rate of cure. Endovascular treatment with the Silk flow diverter is therefore an acceptable treatment option in selected patients with small aneurysms and a small ratio of dome to neck (i.e., wide neck).
A relatively high angiographic cure rate (~70 %) was also achieved for large and giant aneurysms, but with higher complication rates of 16.7 and 42.9 % respectively.
For giant fusiform aneurysms of the posterior circulation, the results were particularly unfavorable. These lesions were associated with a high rate of complications (4/7 = 57 %) that were related to brainstem ischemia secondary to perforator occlusion [17]. We now believe that flow diverters are not a good treatment option for these lesions and have changed our policy to try and achieve a more gradual flow diversion using braided stents in a step-wise fashion. We hypothesize that this will achieve slow thrombosis in the aneurysm and allow the brainstem perforators to be preserved.
Flow diverters were originally developed for wide-necked large and giant aneurysms of the carotid cavernous, clinoid and supra-clinoid segments that are known to have a particularly high rate of recurrence [12]. The angiographic cure rates using flow diverters are higher compared with coiling alone; however, usage of these devices is also associated with a long learning curve and a higher complication rate than simple coiling [7]. In our series, we used flow diverters mainly for complex aneurysms (fusiform or wide neck) both in the anterior and posterior circulation, where coiling was not a sustainable treatment option. With time and experience, we have expanded the indications and have found that Silk offers a technically feasible way for treating saccular aneurysms, sometimes less technically demanding than coiling or coiling with balloon assistance.
Technical points
One of the main advantages of flow diverters is that there is no need to enter the aneurysm lumen, reducing the risk of perforation. Also, there is no need for assisting devices like balloons, making the treatment simpler in one aspect, but requiring specific expertise in the use of flow diverters [9].
Our early experience with the Silk flow diverter has taught us that it tends to expand and shorten when it is deployed in a wide vascular lumen [3, 9]. This is due to its low radial resistance. To reduce the risk of the Silk collapsing into the aneurysm sac, there must be a minimal length on both sides of the neck in order to stabilize it (we use 6 mm on each side) [9]. In addition, we tend to use an adjuvant Leo stent as a bridge for the Silk when the neck is wider than 8 mm or for fusiform aneurysms. The Leo is deployed first and serves as a frame for the Silk that is then deployed inside, thereby preventing shortening of the Silk and bulging into the aneurysmal lumen. Additional stents are not required when using other flow diverters (e.g., Pipeline) because of their different physical properties. However, a complete comparison between different flow diverters is beyond the scope of this paper.
On the other hand, one of the advantages of this behavior of the Silk is that familiarity with it allows the surgeon more flexibility in the treatment. Unlike laser-cut stents, the braided Silk has the potential to open in a range of lengths depending on the luminal diameter. Practically this means that the deployment length of the Silk will increase in smaller diameter vessels and will be shorter in larger diameter vessels. In addition Silk can be resheathed and relocated as needed to achieve precise location of the device.
Another lesson learned is that flow diverters will not work in patients on Coumadin, as witnessed in one of our patients. This is most probably due the effect of Coumadin on coagulation, preventing thrombosis in the aneurysmal sac. In these cases we will use stents with coils in the future.
Treatment during the acute post SAH phase
The three cases that were treated during the acute phase post bleeding represent an exception to our usual policy of treating ruptured aneurysms acutely with coils and scheduling them for stent or flow diversion at a later stage if needed. In one of those cases we suspected a high tendency to reopening of the aneurysms based on the morphology and location [5, 14]. The caveat of using flow diverters in the acute phase is the need for antiaggregant treatment that might complicate surgical intervention. Because of the patient’s good clinical condition and relatively mild SAH, we did not think there would be a high likelihood that he would require cerebrospinal fluid (CSF) drainage, and we decided to save the patient another procedure that we thought was inevitable with the use of coils alone. The other two cases were of a blister aneurysm and a vertebral dissection that could not be treated with coils. The options were regular stent or flow diverter and we preferred the latter.
Conclusions
The Silk flow diverter offers a good treatment option for wide-neck aneurysms of the anterior circulation. However, their use is associated with unique technical nuances and requires specific expertise. Additional stents may be required in specific cases due to the Silk’s low radial resistance. Treatment of giant fusiform aneurysms of the posterior circulation with Silk flow diverters is associated with a higher rate of severe complications.
References
Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J, Kadziolka K, Barreau X, Dousset V, Bonafé A (2012) Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol 33(6):1150–1155
Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M (2010) Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One. doi:10.1371/journal.pone.0012492
Cohen JE, Gomori JM, Moscovici S, Leker RR, Itshayek E (2014) Delayed complications after flow-diverter stenting: reactive in-stent stenosis and creeping stents. J Clin Neurosci 21(7):1116–1122
D’Urso PI, Lanzino G, Cloft HJ, Kallmes DF (2011) Flow diversion for intracranial aneurysms: a review. Stroke 42(8):2363–2368
Friedman JA, Nichols DA, Meyer FB, Pichelmann MA, McIver JI, Toussaint LG, Axley PL, Brown RD (2003) Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10-year single-center experience. AJNR Am J Neuroradiol 24(3):526–533
Higashida RT, Smith W, Gress D, Urwin R, Dowd CF, Balousek PA, Halbach VV (1997) Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg 87(6):944–949
Lubicz B, Collignon L, Raphaeli G, Pruvo J-P, Bruneau M, De Witte O, Leclerc X (2010) Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke 41(10):2247–2253
Lubicz B, Van der Elst O, Collignon L, Mine B, Alghamdi F (2015) Silk flow-diverter stent for the treatment of intracranial aneurysms: a series of 58 patients with emphasis on long-term results. AJNR Am J Neuroradiol 36(3):542–546
Maimon S, Gonen L, Nossek E, Strauss I, Levite R, Ram Z (2012) Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 years’ experience with 28 patients at a single center. Acta Neurochir (Wien) 154(6):979–987
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group (2002) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360(9342):1267–1274
Murthy SB, Shah S, Shastri A, Venkatasubba Rao CP, Bershad EM, Suarez JI (2014) The SILK flow diverter in the treatment of intracranial aneurysms. J Clin Neurosci 21(2):203–206
Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D (2011) The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 32(1):34–40
Pierot L, Wakhloo AK (2013) Endovascular treatment of intracranial aneurysms: current status. Stroke 44(7):2046–2054
Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J (2007) Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol 28(9):1755–1761
Roy D, Milot G, Raymond J (2001) Endovascular treatment of unruptured aneurysms. Stroke 32(9):1998–2004
Shankar JJS, Vandorpe R, Pickett G, Maloney W (2013) SILK flow diverter for treatment of intracranial aneurysms: initial experience and cost analysis. J Neurointerv Surg 5(Suppl 3):iii11–iii15
Siddiqui AH, Abla AA, Kan P, Dumont TM, Jahshan S, Britz GW, Hopkins LN, Levy EI (2012) Panacea or problem: flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg 116(6):1258–1266
Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, Organization ES (2013) European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 35(2):93–112
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Funding
No funding was received for this research.
Ethical approval
This retrospective study was approved by our institutional review board. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Strauss, I., Maimon, S. Silk flow diverter in the treatment of complex intracranial aneurysms: a single-center experience with 60 patients. Acta Neurochir 158, 247–254 (2016). https://doi.org/10.1007/s00701-015-2644-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2644-9