Abstract
Background and Purpose
Flow diverter stents (FDSs) are increasingly used for the treatment of intracranial aneurysms. Initially developed for the management of giant and large aneurysms, their indications have progressively expanded. The purpose of our study was to evaluate the safety and effectiveness of FDSs for the treatment of anterior cerebral artery (ACA) aneurysms.
Materials and Methods
Among the 94 consecutive patients treated for 100 intracranial aneurysms by means of FDSs in our institution from October 2010 to January 2015, eight aneurysms (8 %) in seven patients were located on the ACA. Three aneurysms were located on the A1 segment, three aneurysms on the anterior communicating artery (ACom) and two on the A2–A3 junction. In three cases, FDS was used for angiographic recurrence after coiling. Five patients were treated with a Pipeline embolization device, one with a NeuroEndograft and the last one with a Silk FDS.
Results
Treatment was feasible in all cases. No technical difficulty was reported. No acute or delayed clinical complication was recorded. Modified Rankin Scale was 0 for six patients and one for one patient. Mean angiographic follow-up was 9.7 ± 3.9 months (range 6–15).
Total exclusion was observed in five aneurysms (71.4 %) and neck remnant in two (28.6 %) cases. One patient refused the control DSA.
Conclusion
Our series shows the safety and effectiveness of FDSs for the treatment of ACA aneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of flow diverter stents (FDSs) is a paradigm shift in the treatment of intracranial (IC) aneurysms. Indeed, FDSs now help to treat uncoilable IC aneurysms, especially the fusiform and giant/large aneurysms (G/LAs) [1]. The rationale of FDSs is to redirect arterial blood flow in the parent artery thereby leading to progressive thrombosis of the aneurysm’s sac [2] and to reconstruct the parent artery by endothelial regrowth on the inner wall of the device. Despite some specific technical difficulties and a slightly higher complication rate than with regular stent and coiling technique [3, 4], the FDSs have shown their potential for the treatment of G/LAs of the ICA [5], as well as for carotid-ophthalmic aneurysms [6] or fusiform/dissecting aneurysms [7].
The use of FDSs for aneurysms located on the anterior cerebral artery (ACA) has been seldom reported [8]. The purpose of our study was to evaluate the feasibility, safety, and effectiveness of FDSs for the treatment of IC aneurysms located on the ACA.
Materials and Methods
Patients’ Demographics and Aneurysms’ Characteristics
All the data concerning patients’ demographics and aneurysms’ characteristics are summarized in Table 1.
From October 2010 to January 2015, 94 consecutive patients were treated for 100 intracranial aneurysms by means of FDS in our institution. Among them, seven (7.5 %; five females, two males; mean age = 62 ± 11.5 years [range: 39–72]) were treated for eight intracranial aneurysms located on the ACA. None of these aneurysms were acutely ruptured. Revealing symptoms were: transient ischemic attack in one case, homonymous hemianopia in one case and headache in another case. Two aneurysms had been incidentally discovered (patient 4); in three other cases (patients 1, 6, and 7), patients were treated for recanalization of a previously coiled aneurysm (two of them being previously ruptured). Three aneurysms were located on the A1 segment (Fig. 1), three aneurysms on the anterior communicating artery (ACom) and two on the A2–A3 junction (at the pericallosal-callosomarginal arteries’ junction) (Fig. 2). Mean aneurysm maximum diameter was 8.4 ± 7.3 mm (range: 2.6–27 mm); one patient (patient 5) had a giant (27 mm) partially thrombosed A1 segment aneurysm. Average neck width was 4.7 ± 2.2 mm (range: 2.4–9 mm); average dome-to-neck ratio was 1.5 ± 0.75. Aneurysms were saccular in 7/8 cases (87.5 %) and fusiform-shaped in one case (12.5 %).
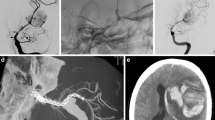
Patient presenting with a left A1 segment aneurysm revealed by left homonymous hemianopia related to aneurysmal compression of the visual pathways (patient # 2). a Left cervical internal carotid artery (ICA) digital subtraction angiography (DSA); lateral projection. Multiple irregularities of the cervical ICA are seen (black arrows), consistent with a fibromuscular dysplasia. Additional mechanical vasospasm is seen in front of the distal aspect of the guiding catheter (white arrow). b Left ICA DSA in anteroposterior (AP) projection. Saccular aneurysm with a large implantation on the left A1 segment is seen (white arrow). c Unsubtracted snapshot during the flow diverter stent (FDS) (3.5/16 mm Pipeline embolization device (PED) [Covidien/eV3, Irvine, CA]) deployment in the A1 segment (single arrow: distal aspect of the FDS; double arrow: proximal aspect). d DSA in working projection after the FDS deployment showing the satisfactory opening of the stent and the patency of the A1 segment. e One-year follow-up DSA showing a complete exclusion of the aneurysm’s sac and patency of the parent artery
Patient presenting with an A2–A3 saccular aneurysm, arising from the pericallosal/callosomarginal arteries’ junction in an azygos anterior cerebral artery (ACA) configuration, revealed by headache (patient # 3). a Right internal carotid artery (ICA) digital subtraction angiography (DSA) in lateral projection showing the A2–A3 saccular aneurysm (arrow). Note that a branch is arising from the inferior aspect of the aneurysm’s neck. b 3D rotational angiography in right oblique anterior view showing more precisely the branch arising from the aneurysm’s neck (arrow). c Snapshot from the road map during the flow diverter stent (FDS) deployment (3/16 mm Pipeline embolization device (PED)). The first two-third of the stent are deployed covering the aneurysm’s neck (black arrows). d Maximum intensity projection (MIP) from computed tomography (CT) plat panel acquisition with diluted contrast media injection (20 % concentration). Satisfactory opening of the stent is demonstrated by this acquisition. e Right ICA DSA in lateral projection just after the stent deployment showing a stagnation of the contrast media in the aneurysm’s sac (arrow). f Right ICA control DSA performed 9 months after the treatment. The aneurysm is totally occluded (grade A) and the FDS is patent. The branch arising from the neck and covered by the stent presents a significant size reduction but remains patent (arrows)
Endovascular Procedures
All the procedures were performed under general anesthesia.
All patients except one were loaded with aspirin (150 mg) and clopidogrel (75 mg) 5 days before the procedure. Then, dual antiplatelet therapy was pursued during 3–6 months after the procedure; aspirin alone was finally pursued for 6–9 months. One patient (patient 4) was resistant to clopidogrel (assessed by platelet aggregation test: Multiplate® [Roche Diagnostics, Switzerland]), despite a double dose; she was thus treated by ticagrelor twice a day for 1year.
In all cases a 6F long sheath was navigated and positioned at the origin of the internal carotid artery (ICA) ipsilateral to the aneurysm. Then, a 5F distal access catheter (DAC) was navigated toward the infra-petrous ICA over a 0.035’’ guide wire (Terumo, Tokyo, Japan). Afterwards, the 5F DAC was navigated over the 0.027’’ delivery microcatheter and positioned in the distal ICA or in the A1 segment. Finally, the delivery microcatheter was navigated over a 0.014’’ microguide wire toward the distal segment of the parent artery, then the FDS was deployed (Figs. 1 and 2). For A1 segment aneurysms, the FDS was deployed from the distal A1 or proximal A2 segment to the proximal A1 segment, paying attention not to open the proximal aspect of the FDS in the carotid terminus. For ACom aneurysms, the FDS was deployed from the A2 segment to the ipsilateral A1 segment and for A2–A3 segment, the FDS was deployed on both sides of the aneurysm’s neck.
Five patients were treated with the Pipeline embolization device (PED, eV3/Covidien, Irvine, CA); one patient (patient # 6) was treated with a Silk device (Balt Extrusion, Montmorency, France) and another (patient # 7) with NeuroEndograft (Stryker Neurovascular,Fremont, CA). No additional coiling was performed in any case of this series. In all patients, only one FDS was used; for patient # 4, the two aneurysms (ACom and A1) were covered by the same FDS.
Clinical Follow-up
Complications were divided into minor and major ones. Minor complications included transient ischemic attack, puncture site hematoma, and headache. Major complications comprised stroke/parenchymal hematoma leading to permanent neurological deficit, puncture site-related retroperitoneal bleeding, and procedure-related death. Clinical outcome was assessed in postprocedure, at discharge and at midterm follow-up (average delay: 19 ± 6.8 months) using the modified Rankin Scale (mRS). Follow-up mRS was compared to the one evaluated in preprocedure.
Angiographic Follow-up
All patients had an angiographic imaging control in postprocedure by means of a DSA in anteroposterior, lateral, and working projections. Flow modification in the aneurysm’s sac was evaluated on postprocedure DSA using the Kamran classification [9]. The Kamran scale is a qualitative grading of the residual filing of the aneurysm’s sac after the FDS deployment from 0: no flow modification to 4: complete aneurysm exclusion. Angiographic follow-up was systematically performed by DSA, except in one patient (patient 7), who refused further angiographic controls. Mean delay for angiographic follow-up was 9.7 ± 3.9 months. On angiographic follow-up, aneurysms’ occlusion rate was graded according to the Roy–Raymond scale [10]: complete occlusion (A), neck remnant (B), and aneurysm remnant (C). The presence of an in-stent stenosis or thrombosis as well as endothelial hyperplasia was also evaluated on DSA angiographic follow-up.
Control magnetic resonance imaging (MRI) was available in 5/7 patients (71.4 %) with a mean delay of 9.6 ± 3 months. In all these five patients, 3D time of flight MR angiography was performed; in 4/7 patients (57.1 %) fluid attenuation inversion recovery (FLAIR)-weighted images were available. On these control MRIs, evolution of the aneurysm’s sac size was evaluated, as well as the presence of a peri-aneurysmal edema and the occurrence of an ischemic stroke.
Ethical Statement
Neither approval of the institutional review board nor patient informed consent are required by the ethics committee of our institution for retrospective analyses of patients’ records and imaging data.
Results
The deployment of the FDS was feasible in all cases. No misdeployment, kinking or twisting of the device was noticed. No additional balloon angioplasty was necessary. No major complication was observed either in postprocedure or at long-term follow-up. Only one patient experienced headache in the postprocedure period, which resolved after administration of paracetamol.
Improvement of her homonymous hemianopia was observed in patient 2 at long-term clinical follow-up. Clinical evaluation showed no modification of the mRS between the pre- and postprocedure period (one patient with mRS 1; six patients with mRS 0).
Immediate angiographic outcome showed Kamran grade 0 in two cases, grade 1 in three cases, grade 2 in two cases, and grade 3 in one case. No grade 4 was observed; median Kamran grade was 1 (IQ: 0.75–2) (Table 2).
On angiographic follow-up (mean delay = 9.7 ± 3.9 months), grade A occlusion was seen in five cases (71.4 %); grade B in two cases (28.6 %); no grade C was seen at follow-up (Table 2). Neither in-stent stenosis nor endothelial hyperplasia was observed.
On available control MRIs, shrinkage of the aneurysm’s sac was observed in 5/5 cases (100 %) (two of them [40 %] having completely regressed). Neither increasing of the aneurysm’s sac size nor peri-aneurysmal edema was observed on follow-up MRI. No sign of ischemic stroke was observed on FLAIR-weighted imaging, especially in the head of the caudate nuclei.
Discussion
Our case series suggests that the treatment of ACA aneurysms by means of FDS may be feasible and safe. Indeed, neither technical failure nor complications were observed in our series. Additionally, no delayed complications were recorded at clinical follow-up (mean delay = 19 months). Angiographic follow-up (mean delay = 9.7 months) showed no residual aneurysm in 71.4 % of the cases and a residual neck in 28.6 % of the cases.
We stress the fact that the treatment of such aneurysms by means of FDS requires a high stability in order to deliver the stent at the accurate site. To overcome the lack of stability in the embolization of such distally located aneurysms, a triaxial access (long sheath + supple guiding catheter or distal access catheter + delivery microcatheter) seems indispensable. With a distal access catheter positioned at the distal aspect of the carotid siphon or in the A1 segment, the stability is dramatically improved and helps avoiding the kick-back phenomenon during the stent deployment. It may also prevent the inopportune release of the proximal aspect of device at the level of the carotid terminus when the FDS is deployed to cover a A1 segment aneurysm.
For A1 segment aneurysms, FDS may be a useful alternative to surgical clipping or parent artery occlusion [11]. Indeed, numerous perforating branches arising from the A1 segment may be sacrificed during the surgical clipping or the parent artery occlusion. Additionally, parent artery occlusion is only feasible when contralateral A1 segment and the ACom are patent.
Another indication for FDS may be the recanalization of ACom aneurysms. Indeed, recanalized ACom aneurysms are prone to recur again after regular coiling. FDS may bring more stable angiographic results in this indication. In our series, two recanalized ACom aneurysms were treated by FDS. Only one patient had an angiographic follow-up (the second one refused the follow-up DSA), with a complete occlusion. It is noteworthy that in these two cases, the FDS was deployed in the ipsilateral A1–A2 segments and that the contralateral A1 segment was patent. In case of agenesis or hypoplasia of the contralateral A1 segment, the use of a FDS may be more hazardous.
In the literature, besides few case reports [8, 12], only three series (two monocentric [13, 14] and one multicentric [15]) have reported the treatment of intracranial aneurysms above the circle of Willis by means of FDS, including ACA aneurysms (Table 3). Indeed, these series, mixing all types of distal aneurysms (ACA, middle, and posterior cerebral arteries’ aneurysms) have reported the treatment with FDS of 21 ACA aneurysms in 17 patients [13], 5 ACA aneurysms in 5 patients [15], and 5 ACom aneurysms in another 5 patients [14], respectively. As reported in our series, no serious neurological events were recorded in the first and last studies [13, 14]. On the contrary, severe neurological event was reported in one out of five cases in the second series [15], consisting in multiple distal emboli. In these three cases series [13–15], complete angiographic exclusion rate at follow up (ranging from 60–83.3 %) was similar to the one observed in our case series.
Interestingly, on control MRI, shrinkage of the aneurysm’s sac was observed for all cases in our study, as reported in most of the published series [16–18]. Neither increasing of the aneurysm size nor peri-aneurysmal edema was seen. This phenomenon may however be observed after FDS treatment, especially in G/LAs of the ICA, and may be responsible for symptomatic cranial nerve compression [18]. Additionally, no silent ischemic strokes were observed on control MRI. In a recent paper [14], head of the caudate nuclei infarcts in the treatment of ACom aneurysms with FDS were reported in 2/5 cases (40 %); only one of these cases (20 %) being symptomatic, but with no permanent deficit on long-term follow-up.
The results of our series, as well as the data from previously published series regarding the interest of FDS for the treatment of unruptured IC aneurysms of the anterior circulation < 10 mm [19], underscore the potential of this new device for the treatment of IC aneurysms above the circle of Willis and provide new arguments for the expansion of their use in other indications than G/LAs.
Our study presents some limitations. First this is a retrospective analysis of a single-center, prospectively collected database. Second, the population of the study is relatively small, which can be explained by the scarcity of indications for flow diverter treatment in this location.
Conclusion
Our series suggests the safety and effectiveness of FDSs for the treatment of ACA aneurysms. FDSs may be useful for the treatment of complex A1 aneurysms or for recanalized ACA aneurysms.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
Abbreviations
- ACA:
-
anterior cerebral artery
- ACom:
-
anterior communicating artery
- DSA:
-
digital subtraction angiography
- FDS:
-
flow diverter stent
- G/LAs:
-
giant/large aneurysms
- IC:
-
intracranial
References
Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PloS one. 2010;5(9).
Ionita CN, Natarajan SK, Wang W, Hopkins LN, Levy EI, Siddiqui AH, Bednarek DR, Rudin S. Evaluation of a second-generation self-expanding variable-porosity flow diverter in a rabbit elastase aneurysm model. AJNR Am J Neuroradiol. 2011;32(8):1399–407.
Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44(2):442–7.
Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ, Moret J. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010;41(1):110–5.
Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol. 2012;33(8):1436–46.
Lanzino G, Crobeddu E, Cloft HJ, Hanel R, Kallmes DF. Efficacy and safety of flow diversion for paraclinoid aneurysms: a matched-pair analysis compared with standard endovascular approaches. AJNR Am J Neuroradiol. 2012;33(11):2158–61.
Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bäzner H, Henkes H. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012;54(4):369–82.
Rouchaud A, Saleme S, Gory B, Ayoub D, Mounayer C. Endovascular exclusion of the anterior communicating artery with flow-diverter stents as an emergency treatment for blister-like intracranial aneurysms. A case report. Interv Neuroradiol. 2013;19(4):471–8.
Kamran M, Yarnold J, Grunwald IQ, Byrne JV. Assessment of angiographic outcomes after flow diversion treatment of intracranial aneurysms: a new grading schema. Neuroradiology. 2011;53(7):501–8.
Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32(9):1998–2004.
Tollard E, Niemtschik L, Darsaut TE, Guilbert F, Roy D, Raymond J, Weill A. Endovascular parent artery occlusion for the treatment of wide-neck A1 segment aneurysms: a single-center experience. AJNR Am J Neuroradiol. 2011;32(1):174–8.
Peschillo S, Cannizzaro D, Missori P, Colonnese C, Santodirocco A, Santoro A, Guidetti G. Reconstructive endovascular treatment of a ruptured blood blister-like aneurysm of anterior communicating artery. J Neurosurg Sci. 2014 Jun 10. [Epub ahead of print]
Pistocchi S, Blanc R, Bartolini B, Piotin M. Flow diverters at and beyond the level of the circle of willis for the treatment of intracranial aneurysms. Stroke. 2012;43(4):1032–8.
Gawlitza M, Januel AC, Tall P, Bonneville F, Cognard C. Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: a single-center series with special emphasis on covered cortical branches and perforating arteries. J Neurointerv Surg. 2015 Apr 15. [Epub ahead of print]
Martínez-Galdámez M, Romance A, Vega P, Vega A, Caniego JL, Paul L, Linfante I, Dabus G. Pipeline endovascular device for the treatment of intracranial aneurysms at the level of the circle of Willis and beyond: multicenter experience. J Neurointerv Surg. 2014 Sep 8. [Epub ahead of print]
Piano M, Valvassori L, Quilici L, Pero G, Boccardi E. Midterm and long-term follow-up of cerebral aneurysms treated with flow diverter devices: a single-center experience. J Neurosurg. 2013;118(2):408–16.
Szikora I, Marosfoi M, Salomváry B, Berentei Z, Gubucz I. Resolution of mass effect and compression symptoms following endoluminal flow diversion for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2013;34(5):935–9.
Slater LA, Soufan C, Holt M, Chong W. Effect of flow diversion with silk on aneurysm size: A single center experience. Interv Neuroradiol. 2015;21(1):12–8.
Chalouhi N, Starke RM, Yang S, Bovenzi CD, Tjoumakaris S, Hasan D, Gonzalez LF, Rosenwasser R, Jabbour P. Extending the indications of flow diversion to small, unruptured, saccular aneurysms of the anterior circulation. Stroke. 2014;45(1):54–8.
Conflict of Interest Statement
Dr N.-A. Sourour is proctor for the Pipeline Embolization Device (eV3/Covidien).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clarençon, F., Di Maria, F., Gabrieli, J. et al. Flow Diverter Stents for the Treatment of Anterior Cerebral Artery Aneurysms: Safety and Effectiveness. Clin Neuroradiol 27, 51–56 (2017). https://doi.org/10.1007/s00062-015-0441-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-015-0441-8