Abstract
Background
Flow diverters are recently developed stent-like endovascular devices developed to treat complex and non-coilable aneurysms. SILK is a type of flow diverter that has been used for nearly 3 years. Only sparse data about it are available. We would like to share our experience with this device.
Methods
Twenty-eight patients were treated with SILK from October 2008 to October 2010. Thirty-one treatment sessions were performed for 32 aneurysms using 31 SILKs. Twenty have been treated with SILK only and eight with SILK and adjuvant stents. Twenty-six (86%) patients performed cross-sectional imaging (MRA/CTA) for follow-up. Eighteen (64%) patients had follow-up brain angiography.
Results
In all patients the SILK could be deployed. No case of early or late aneurysmal rupture was noted. Five patients (17.8%) developed immediate clinical complications, which were permanent in three (10.7%). All the complications occurred in patients harboring aneurysms larger than 15 mm. In two other patients, occlusion of the SILK was noted with no clinical deficit. A complete or near-complete aneurysmal occlusion was found in brain angiography or cross-sectional imaging follow-up in 83.3% of the patients.
Conclusions
SILK is a relatively simple device to use, with a low rate of technical and clinical complications and a high short-term aneurysmal occlusion rate. In aneurysms smaller than 15 mm, the results are excellent. Results are also encouraging in the larger aneurysms, taking into consideration their complexity. The device characteristics and mainly its drawbacks must be well known by the users.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coil embolization has been proven as an effective method of treatment for many intracranial aneurysms. But despite the tremendous evolution in endovascular therapy over the past two decades, some important limitations remain, particularly in the treatment of wide-necked, large and giant, or “non-saccular” fusiform aneurysms. These lesions can frequently be difficult to reconstruct with coils, even when self-expanding intracranial stents are used for the treatment.
Consequently, endovascular treatments of such lesions frequently fail to produce complete aneurysmal occlusion. Even when complete or near-complete occlusion has been achieved after the initial embolization, these aneurysms remain prone to coil compaction and recanalization and thus requiring one more re-treatment session. In some, the option of open surgery with or without bypass must be considered [2, 4, 11–13, 16, 18].
Altering the blood flow into aneurysms, using a stent placed in the parent vessel, was identified and proposed as an effective treatment strategy for aneurysms in the mid-1990s [6]. Such adjuvant stenting was proposed initially for fusiform aneurysms and more recently to support coiling of intracranial wide-neck saccular aneurysms, to reduce incidence of recurrence.
The concept of flow disruption in proximity to the aneurysmal neck and vessel repair has been upgraded with the introduction of the new generation of stent, the flow diverters, which are low-porosity stents with greater metal surface area than the more conventional stents.
They were designed to reconstruct the aneurysmal bearing vessel so that in-sac thrombosis will occur and a neointima will develop along the flow diverter/aneurysmal neck.
Whereas coil embolization aims to fill the aneurysm sac with coils and occlude it by promoting intra-aneurysmal thrombosis, flow diverter stents are deployed in the parent artery across the aneurysm neck in order to redirect the blood flow to the parent artery away from the aneurysm sac and to disrupt both inflow and outflow of blood into the aneurysmal sac. These effects create an intra-aneurysmal environment favorable for thrombosis, which leads to gradual aneurysmal occlusion [3, 8, 10, 14, 15, 17].
In theory, the most effective tool to repair an aneurysm with a very low rate of reopening is the covered stent. The main limitation that causes sparse usage of covered stents for the intra-cranial aneurysms is the high risk of perforator occlusion in the segment distal to the anterior choroidal artery and the posterior inferior cerebellar artery (PICA). The other limitation is the risk of vessel damage, mainly rupture and dissection, due to the stiffness of currently available stents, most of which were designed for cardiology indication.
The available flow diverter stents share the properties of forming a high-coverage mesh along the aneurysmal neck once expanded and gradually induce thrombosis of the aneurysmal sac while preserving patency of adjacent small perforators. These new flow diverters were developed for intracranial usage and are sufficiently flexible for intracranial navigation. They were first used in humans at the end of 2006, but have only recently been available for routine clinical use.
The experience with deployment of flow diverter stents for the treatment of intracranial aneurysms is limited; however, the few reports about this treatment concerning its efficacy and safety have been encouraging. The Pipeline stent (EV3, Irvine, CA) was the first released flow diverter stent and has been evaluated in only a few series showing that the device is safe and the treatment is durable and curative in selected wide-necked, large, and giant aneurysms. Their use had an associated morbidity of less than 10% and aneurysm occlusion rate of 85% [3, 6, 10].
Delayed aneurysm ruptures have been reported, those are rare and the mechanisms are actually not completely elucidated. Stent thrombosis or stenosis was also observed in a low percentage of cases.
The SILK stent (Balt Extrusion, Montmorency, France), a self-expanding flexible flow diverter, was approved for use in humans in Europe in 2007. Sparse data concerning it usage can be found in the literature [1, 9].
The SILK is made of 48 braided Nitinol strands that offer formation of a high-coverage mesh once expanded. This stent has two flared ends and a sinusoidal radio-opaque platinum wire. Many diameters (2–5 mm) and lengths of SILK are available (15–40 mm) for the treatment of aneurysms of different sizes and in different locations.
The SILK flow diverter kit includes a self-expanding stent and a reinforced catheter (Vasco 21) for its delivery. The delivery procedure is similar to other self-expandable intracranial stents, but, in contrast to others, it allows re-sheathing and repositioning, even when up to 90% of it has been deployed.
The aim of this retrospective study is to report our first 2 years of experience with the SILK stent and to add data about the characteristics of the device including pros and cons according to our experience.
Material and methods
Patients’ characteristics
This study was approved by our institutional ethics review board.
Twenty-eight patients were treated in our department with the SILK flow diverter between 1st October 2008 and 30th October 2010. The characteristics of the lesions recorded during the first treatment in our institution, were used for lesion classification in this report. Clinical and imaging follow-up data for this study were collected until the 30th May 2011. All procedures were performed under general anesthesia in a monoplane Philips angio machine with no 3D (three-dimensional) options, using the same treatment protocol, and performed by the same experienced neuro-interventionalist (S.M.). There were 20 (72%) female and eight (28%) male patients. The mean age at presentation was 58 years (range 40-89 years). The presenting symptoms (Table 1) were spontaneous subarachnoid hemorrhage due to an aneurysmal rupture in four (14.3%) patients (one was treated in the acute phase), various neurological deficits (visual disturbances, opthalmoplegia and headaches, ischemic events and ataxia) in 18 (64.7%) patients, and incidental findings in the other six patients (21%).
In eight patients, a flow diverter was inserted due to reopening of previously coiled aneurysms and in all other patients (20) the SILK was the first and primary treatment. Aneurysms larger than 15 mm were found in 19 patients and smaller in nine. Twenty aneurysms were larger than 15 mm and 12 smaller (Tables 2 and 3). Two patients had three aneurysms each at the treated segment. In 20 cases, magnetic resonance imaging (MRI)/MR angiography (MRA) and/or high-quality computed tomographic angiography (CTA) were used as the diagnostic tool and for treatment planning (vessel diameter measurements, neck and aneurysm sizes). A pretreatment diagnostic angiography in a separate session was not performed in most cases. In eight patients with post coiling aneurysmal recanalization, follow-up angiography was also used for treatment planning.
Preparation and procedures
All treatments were performed through a transfemoral approach under general anesthesia. The patients were prepared with Aspirin and Plavix (Clopidogrel) for a minimum of 3 days prior to treatment, except one patient who was treated in the acute phase of subarachnoid hemorrhage (SAH) and therefore was treated with ΙΙA/ΙΙΙB antagonist (Integrilin) at the angiography suit immediately post SILK deployment (bolus and then maintenance dose) and a day later converted to Aspirin and Plavix. The measurements of vessel diameter, neck and aneurysm size that were taken from the CTA or MRA were used for treatment planning. At the angiography suite, we used eyeball measurements to select the best stent/flow diverter diameter, taking into consideration the CTA/MRA data. At that period of time we did not have 3D acquisition in our angiography monoplane machine.
When selecting the size of the SILK flow diverter to insert, we prefer to use stents which are longer and have larger diameter than the treated vessel segment, for example:
-
1.
Concerning the diameter of the SILK flow diverter chosen: if the widest diameter of the treated arterial segment, proximal to the aneurysm is 4 mm, we will choose the 4.5-mm SILK rather than 4.0 mm.
-
2.
Concerning the length of the SILK flow diverter chosen: in an aneurysm with an 8-mm-wide neck, we will deploy a SILK with a minimum length of three-times the neck size (i.e., the 25-mm SILK or longer).
These preferences were used because the SILK has a low resistance force and a tendency to widen and shorten. By selecting the flow diverter using these criteria, we aim to get enough friction with the healthy segment to prevent migration of the flow diverter from the deployment location. In fusiform aneurysms, or in saccular ones with a very wide neck (8 mm and more), we use an adjuvant stent to support the SILK flow diverter. We deploy the adjuvant stent, a self expandable stent, (Leo-Balt Extrusion, Montmorency, France), first as a bridge and then deploy the SILK flow diverter into its lumen. At the end of deployment we usually pass the SILK with the microcatheter to assure its full opening. We do our best to prevent the proximal part of the flow diverter from being deployed in a curvature, as it becomes difficult then to pass through it with a microcatheter.
In saccular aneurysms bigger than 15 mm, we prefer to insert a few coils into the sac, to enhance aneurysmal thrombosis and perhaps to prevent early and late ruptures, which have been reported in the literature [17]. To deploy the SILK, we have to navigate with a Vasco 21 microcatheter, which comes inherent with the SILK package to the segment distal to the aneurysm. This is done with the aid of a 0.014” or 0.016” guide wire. On rare occasions, we navigated distal to the aneurysm with a 14-F microcatheter and then exchange it over a long 0.014” guide wire with the Vasco 21. This was done in complex aneurysms where primary navigation with a large microcatheter was difficult. We prefer to use 7-F long sheaths for supporting the guiding catheter, due to the high back force at the system while navigating with the microcatheter and at the time of SILK deployment.
Results
Thirty-one treatment sessions were performed in 28 patients harboring 32 aneurysms over a 2-year period. Twenty patients have been treated with SILK only and the other eight with SILK and adjuvant stents.
A total of 31 SILK flow diverters and 9 other stents were used; of them, 7 were Leo (Balt Montmary French) stents. We could navigate with the microcatheter to the target area and deploy the stent and flow diverter in all cases. There was no need to perform a balloon dilatation for any of our cases. Due to the size and mild stiffness of the Vasco 21 this microcatheter has the ability to cause vessel spasm; therefore, if the lesion is in a vessel smaller than 3 mm in diameter, we inject an intra-arterial antispasmodic drug before starting the navigation.
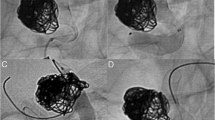
In our first SILK case of a giant fusiform Basilar aneurysm, the SILK shortened and its proximal part fell into the aneurysmal lumen, so a second SILK was deployed parallel to the first, with the aid of a coronary stent that was deployed to support the SILK’s proximal edge. Clinical improvement was noted a few weeks later and the aneurysm was found to be occluded on CTA that was performed 6 months post treatment. From this case, we concluded that the SILK tends to expand and shorten when it is deployed in a wide vascular lumen. This is due to its very low radial resistance. This case had an important impact on the way we used the device later. In six patients, an adjuvant Leo stent was used as a bridge for the SILK. In those cases the neck was longer than 8 mm or the aneurysms were fusiform. To prevent shortening of the SILK, due to its low radial force and tendency to expand and bulge to the aneurysmal sac/lumen, the Leo was deployed first and became the frame for the SILK that was then deployed across its lumen (coaxial system). In two cases an adjuvant stent was deployed to support the SILK, when it was found to be deployed too close to the aneurysmal neck edges.
Flow diverter/stent occlusion was not noted in the SILK only group, but was noted in two of the eight cases treated with the SILK and adjuvant stent. The first was a case of giant wide-neck cavernous carotid aneurysm, recanalized twice after coiling, treated eventually by a Leo-SILK combination. The parent artery was found to be almost completely occluded at 1-year follow-up and the aneurysm was completely occluded. The patient did not have any symptoms due to a complete circle of Willis. This occlusion was most probably due to low patient medical compliance not taking Plavix due to its high cost. A second patient with a giant complex fusiform basilar aneurysm was treated with two long Leo stents for bridging the neck and one SILK flow diverter (Leo-SILK combination). The SILK stent was found to be occluded at 3 months when she arrived to the second part of the treatment. In this case we had to stop Plavix a week after first treatment, due to massive bleeding at a tracheotomy site. We had to discontinue Plavix for more than 10 days. This occlusion was opened by PTA and another Leo-SILK stent combination. This flow diverter occlusion did not have any clinical impact, due to flow in the aneurysmal lumen around the flow diverter. The basilar artery and devices were fully patent 5 months later at the third treatment session.
One of our patients had two treatment sessions with deployment of two SILK stents, due to improper flow diverter length selection in the first session. The flow diverter shortened within 6 months so that its distal part entered the aneurysmal sac, preventing vessel repair. In the second session, a longer SILK was deployed across the first one, getting an immediate change in the aneurysmal flow. After 1 year, a follow-up MRA showed complete aneurysmal occlusion. The patient is due for a follow-up angiography soon.
Complications
Immediate clinical complications, four of them ischemic, were observed in five out of 28 patients (17.8%) in this diverse group. Two patients subsequently improved, one within a week and a second who developed edema around a basilar tip giant aneurysm in less than 3 months. This giant aneurysm was treated with coils and a SILK. Of these three permanent complications, one was in an 89-year-old woman treated with SILK for a giant aneurysm presenting with acute third nerve palsy. This patient developed a carotid dissection during advancement of the guiding catheter with consequent MCA emboli. She subsequently developed a retroperitoneal hematoma due to the anti-aggregate and thrombolytic therapy. She recovered partially but eventually died a month later in a re-habilitation center. The two other patients had hemiparesis due to ischemic infarcts. One of them with giant basilar fusiform aneurysm continued to improve during 7 months’ clinical follow-up, but still needs some daily assistance. In total, we had one case of mortality (3.6%) and two cases of moderate to severe morbidity (7.2%). In-stent thromboses were observed in two cases, both in aneurysms larger than 15 mm treated with SILK and Leo and both patients did not develop new symptoms. The thrombosis was discovered in a 1-year follow-up angiography in one patient and at the time of a second treatment session, performed 3 months from first session in the second patient. All the five clinical complications and the two SILK occlusions developed in patients harboring aneurysm larger than 15 mm.
Follow-up
Clinical follow-up was available for all patients and ranged from 6 months to 2 years. Imaging follow-up was available for 26 patients (86%). Two patients did not have follow-up imaging: one patient died and the second due to severe neurological morbidity. We performed CTA or MRA 3–7 months post treatment. Conventional angiography was usually performed 3–12 months later if the cross-sectional imaging showed more than 60% occlusion of aneurysmal lumen. Follow-up CTA/MRA was performed more than once in three patients. Eighteen patients (18/28, 64%) already underwent a follow-up conventional angiography 6–18 months from treatment. Two patients have had more than one follow-up angiography. In these 18 patients harboring 20 treated aneurysms, control angiography revealed complete aneurysmal lumen occlusion in 12 (14 aneurysms) and near complete in two patients, resulting in overall 16 out of 20 (78.9%) secured aneurysms. In four patients, follow-up angiography revealed partially occluded aneurysms. Three patients had a second treatment session. In one patient the decision was to observe the aneurysm conservatively for further sac thrombosis and not to retreat, due to anatomical considerations and the fact that 60% aneurysmal lumen occlusion was noted. Out of eight patients with MRA/CTA follow-up only, in six patients the lesions were found to be completely occluded and in one near complete occlusion was noted. One patient had only partial occlusion of the aneurysmal lumen. Four of these patients are scheduled for follow-up angiography and four have refused further invasive follow-up studies. CTA/MRA has been performed in 14 patients prior to the follow-up angiography. Thin-slice high-resolution 3D time-of-flight (TOF) sequence was utilized for the MRA with a 1.5-T machine. CTA was performed on a 64 slice CT machine. Good correlation was found between these two non-invasive ways of imaging and conventional angiography in 13 of 14 (93%) cases. Out of all the 26 (30 aneurysms) imaging follow-up cases 25 (83.3%) aneurysms were secured in the early and mid term follow-up. None of our patients developed early or late post treatment aneurysmal rupture, while some of our cases were partially thrombosed giant aneurysms. The patient who had died one month after treatment at the rehabilitation center had died from an infection complication and not from the vascular lesion itself, but we consider the mortality to have been associated with the procedure. One of the patients with severe disability at discharge, due to brain stem infarct, showed impressive clinical improvement at 7-months’ follow-up.
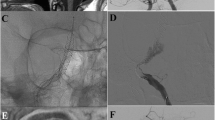
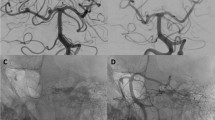
Example case
A 70-year-old female presented with three aneurysms in the clinoid/supraclinoid segment of the right carotid artery (Fig. 1). The largest, a giant partially occluded aneurysm, enlarged dramatically in the year prior to treatment. She was treated by deployment of a Leo stent followed by a SILK flow diverter through it in order to cover the aneurysmal segment. No coils were inserted. Post-treatment evaluation, including CTA at 6 months and cerebral angiography at 10 months after treatment, demonstrated complete occlusion of the aneurysms and dramatic size reduction of the giant aneurysm (Fig. 2).
Discussion
In recent years, flow diverters have become a treatment option for aneurysms that cannot be coiled or are known to have a high rate of re-canalization post coiling.
The purpose of this report is to share our 2-year experience and results of usage of a new flow diverter, SILK, with special emphasis on the feasibility, efficacy and safety as reflected by clinical and radiological outcomes. We started using the device with a classical indication (wide-neck large or fusiform aneurysms) but broadened the indications during this time frame of 2 years according to our experience and the literature. At the beginning we used it mainly to treat complex aneurysms, but fairly quickly we started to use it in less complex cases and also in aneurysms located at bifurcations. The indications broadened also to include smaller aneurysms with wide neck, where we only deploy the flow diverter with no need to add coils. We learned that SILK offers a technically feasible way for treating saccular aneurysms, sometimes less technically demanding than coiling or coiling with balloon assistance.
Because of a long and shallow learning curve, we learned how to use the flow diverter in the best way—understanding the pros and cons of the device and making it feasible to prevent technical complication. We managed to understand the radial force problem of the flow diverter, the risk of shortening, and the need for adjuvant stent, the best deployment way and other unique capabilities. Due to these considerations, we could get to a zero complication rate in small aneurysms below 15 mm with high aneurysmal obliteration rate. In aneurysms larger than 15 mm we did experience complications, but their rate is far lower than the natural history and the lack of efficacy of other treatment options (i.e., coiling) in such aneurysms.
The low rate of complications in aneurysm smaller than 15 mm could be explained by the following. When we started treating this group, it was already on the higher end of the learning curve for usage of the device on complex large/giant aneurysms.
The simplicity of the technique—it’s a one step action—no need for coils or balloon and much less time consuming. The ability to reposition the SILK makes it possible to place it in more exact and desired locations. In non-complex, less than 15 mm aneurysm, the entire procedure could take less than 30 min. It does not require entering the aneurysm lumen, so the risk of rupture and emboli is much reduced.
There is a high rate of aneurysm obliteration, nearly 85% in the follow-up period. At the period after treatment with a flow diverter, there is no slope of recanalization but an increase in the obliteration rate and vessel repair. In most of the cases, at the end of treatment the aneurysm is open and in a few months it obliterates, while with coiling in nearly 20% of the cases aneurysm reopening can be seen after the treatment.
The concept is that we have to focus on the vessel wall repair, like in clipping, and in that having higher occlusion rate with long durability.
There are actually two major disadvantages for the SILK flow diverters:
-
1.
As with all other stents, there is a need for long-term anti-aggregate therapy to prevent thrombosis and vessel occlusion.
This also become a relative contra indication for its use in the acute bleeding phase of the ruptured aneurysm, due to the need to insert drainage devices, or other invasive procedure.
-
2.
Its low radial resistance, which might raise the need of an adjuvant stent as a constructor element, especially in very wide neck saccular and in fusiform aneurysms, where the SILK might bulge or “fall” into the aneurysmal sac.
This need for adjuvant stent usage could increase the risk of in- and post-treatment complications like emboli, flow diverter thrombosis, and stenosis, as we observed in two of our patients. This observation led us to continue treatment with Plavix in those cases of combined devices to a longer period of 6 months and not 3 months as we were giving usually.
If we divide the clinical complications into two groups of patients—those with SILK only and those with SILK and adjuvant stent—we find more complications in the SILK + adjuvant stent group than the SILK only group.
In the SILK only group, we experienced 3/20 (15%) peri-procedural complications, two (10%) permanent. In SILK + adjuvant stent group we experienced 2/8 (25%) peri-procedural clinical complications (12.5% permanent). In that group, we also observed the two cases of flow diverter thrombosis. However, this observation is not statistically significant due to the small number of patients. Performing the treatment with a combination of Leo stent and SILK flow diverter is technically demanding and much more difficult to perform than deployment of SILK alone. The high complications rate in this group can be explained either by those technical difficulties or by the fact that this combination was used in more complex and difficult to treat aneurysms.
The expected course of patients treated with SILK is opposite of that expected with coiling. While with coiling we are looking for compaction of the coils and reopening of the aneurysmal sac, with the SILK we expect the obliteration of the aneurysmal sac that could be visualized by non-invasive cross-sectional techniques like CTA/MRA. When sac obliteration is shown on those studies, conventional angiography is performed to confirm the findings, thus reducing the risk of complications from many follow-up angiography procedures.
In our series there is a high aneurysmal obliteration rate during our follow-up time (4 months to more than a year), with ongoing continuing obliteration of aneurysmal lumen in those cases with partial obliteration in the early post treatment.
These different concepts of treatment and the satisfactory outcome led us to use flow diverter more commonly, specifically for aneurysms known to have a high rate of treatment failure and reopening with coils.
We did not observe any early or late aneurysmal rupture in any of our cases in this group of patients. Most of our complications were due to errors or difficulty in navigation and not due to the SILK itself. This could be due to the need of a larger microcatheter, the Vasco 21, which is more complex to navigate with than the usual small microcatheter for coiling. The need for a long 7-F sheath to support the guiding catheter, due to the significant force applied pushing the device at tortuous vessels, could be a source for one of our complications.
One of the differences between our relative low rate of complication and those published in the literature regarding SILK, is the way we managed it with a “slow learning curve”. We did not perform many cases in a short period of time, rather we gradually performed fewer cases and used the knowledge we obtained for the next case, and this was due to the lack of data (mainly technical) in the literature about that flow diverter. We experienced two cases of SILK occlusions in the group with the SILK/Leo combination.
In the first case, the occlusion could have been due to low compliance of the patient refusing to take Plavix. In the second case, we had to stop Plavix for 10 days due to bleeding from a tracheotomy a few days after treatment. The occlusion in these patients did not produce any clinical sequelae. This is one of the cons of those devices—the need for anti-aggregates for a long period, which can be an obstacle to other invasive treatments and cause bleeding with and without trauma.
One of the complications we encountered was severe brainstem edema post treatment of a large basilar tip aneurysm. This complication is known in the treatment of large aneurysms treated with coils only without a flow diverter [5, 7]. The exact mechanism is not completely clear. Accordingly, the edema observed in our case is probably not directly related to the flow diverter.
One of the main advantages of SILK is the ability to re-sheath and reposition it. Its main disadvantage is its low radial force. A device with a better radial resistance but still having the re-sheath option could be a better one and will eliminate the need for an adjuvant stent placement in wide neck/fusiform aneurysms.
Conclusions
SILK is a simple and relatively easy to use flow diverter with a very low rate of complication in treating aneurysms smaller than 15 mm and shows a high rate of aneurysmal closure in the early and mid term (6–18 months). Acknowledging its capabilities and characteristics helps to utilize it in a better way with lower rates of complications.
SILK is also effective in complex aneurysms that most of the time cannot be treated effectively with other tools. The risk is higher than with the smaller aneurysm, but still the results are better than the expected natural history, with a high rate of aneurysm protection. These results are encouraging.
In coiling, the focus of treatment is the aneurysmal sac, with a consequent vessel repair. Whilst with a flow diverter, the primary focus of treatment is the diseased segment of the vessel and vessel reconstruction, like in clipping.
We continue to use the flow diverter in broad indications, collect cases and data to have a longer follow-up and more cases for evaluation of this promising new tool.
References
Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M (2010) Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLos One 5(9) pii:e12492
Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, Yarnold JA, Rischmiller J, Byrne JV (2007) Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 38:1538–1544
Fiorella D, Woo HH, Albuquerque FC, Nelson PK (2008) Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery 62:1115–1120
Friedman JA, Nichols DA, Meyer FB, Pichelmann MA, McIver JI, Toussaint LG 3rd, Axley PL, Brown RD Jr (2003) Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10-year single-center experience. AJNR Am J Neuroradiol 24:526–533
Hayashi K, Kitagawa N, Morikawa M, Horie N, Kawakubo J, Hiu T, Tsutsumi K, Nagata I (2009) Long-term follow-up of endovascular coil embolization for cerebral aneurysms using three-dimensional time-of-flight magnetic resonance angiography. Neurol Res 31(7):674–680
Higashida RT, Smith W, Gress D, Urwin R, Dowd CF, Balousek PA, Halbach VV (1997) Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case review and of the literature. J Neurosurg 87:944–949
Horie N, Kitagawa N, Morikawa M, Tsutsumi K, Kaminogo M, Nagata I (2007) Progressive perianeurysmal edema induced after endovascular coil embolization. Report of three cases and review of the literature. J Neurosurg 106(5):916–920
Ionita CN, Paciorek AM, Dohatcu A, Hoffmann KR, Bednarek DR, Kolega J, Levy EI, Hopkins LN, Rudin S, Mocco JD (2009) The asymmetric vascular stent: efficacy in a rabbit aneurysm model. Stroke 40:959–965
Lubicz B, Collignon L, Raphaeli G, Pruvo JP, Bruneau M, De Witte O, Leclerc X (2010) Flow-diverter stent for the endovascular treatment ofintracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke 41(10):2247–2253
Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, Berez AL, Tran Q, Nelson PK, Fiorella D (2009) Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery 64:632–642
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R (2002) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. J Stroke Cerebrovasc Dis 11:304–314
Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, Martin N, Viñuela F (2003) Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 98:959–966
Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J (2007) Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol 28:1755–1761
Sadasivan C, Cesar L, Seong J, Rakian A, Hao Q, Tio FO, Wakhloo AK, Lieber BB (2009) An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model. Stroke 40:952–958
Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, Berez A, Nelson PK (2010) Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the Pipeline embolization device. AJNR Am J Neuroradiol 31(6):1139–1147
Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J (2002) Follow up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery 50:239–249
Turowski B, Macht S, Kulcsár Z, Hänggi D, Stummer W (2011) Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): do we need to rethink our concepts? Neuroradiology 53(1):37–41
Vallee JN, Aymard A, Vicaut E, Reis M, Merland JJ (2003) Endovascular treatment of basilar tip aneurysms with Guglielmi detachable coils: predictors of immediate and long-term results with multivariate analysis 6-year experience. Radiology 226:867–879
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Maimon and L. Gonen contributed equally to this paper
Rights and permissions
About this article
Cite this article
Maimon, S., Gonen, L., Nossek, E. et al. Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 years’ experience with 28 patients at a single center. Acta Neurochir 154, 979–987 (2012). https://doi.org/10.1007/s00701-012-1316-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1316-2