Abstract
Background
It is unclear how soon after a decompressive hemicraniectomy that cranioplasty be safely performed in a patient in whom the ICP has been normalized. Early surgery has been associated with infection, intracerebral hematoma, and complications due to persistent or recurrent brain edema. Delayed cranioplasty of large cranial defects exposes the patient to different conditions known in the literature as the syndrome of the sinking skin flap. The purpose of this study was to investigate the hypothesis that timing of cranioplasty after decompressive hemicraniectomy influences outcome and complications.
Methods
We retrospectively examined outcome after cranioplasty performed at <7 weeks, 7–12 weeks, and >13 weeks after craniectomy in patients with large cranial defects after decompressive hemicraniectomy in our institution between 1997 and 2008.
Results
The time between craniectomy and cranioplasty ranged from 17 days to 4 months depending on several factors such as: the cause of decompression, infection before or after craniectomy, and skin flap concavity. The analysis of the registered postoperative complications revealed that there were no significant differences between the examined groups. The cranioplasty at <7 weeks, in the form of reimplantation of the own skull flap, led to a GOS improvement of 78 %, at 7–12 weeks 46 % and at >13 weeks12 %, respectively. Pairwise comparisons showed that the difference between cranioplasty at <7 weeks versus 7–12 weeks or >13 weeks cranioplasty groups was statistically significant (p = 0.05 and p < 0.001, respectively).
Conclusions
Our study suggests that many patients with large cranial defects after decompressive craniectomy can safely undergo cranioplasty in an early stage; direct answers to these questions of timing of cranioplasty are best addressed by prospective studies. Nevertheless, the present study provides a basis for decision-making in certain patients and for the design of future investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The possible benefits of craniectomy in the therapy of brain edema due to stroke, trauma, SAH, and infection have been reported in many case series [1–5] and unequivocally established by recent, randomized, prospective clinical trials [4–7].
However, after normalization of the intracranial pressure and reversion of the brain tissue shifts, the syndrome known as the “syndrome of the sinking skin flap” [8–10] may cause neurological deterioration [11–14]. Recently, several authors proposed that a negative gradient between atmospheric and intracranial pressure, which is aggravated by changes in the CSF compartment following CSF hypovolemia, to be the mechanism of neurological deterioration after craniectomy [8, 12, 13].
Cranioplasty has been proposed to restore the disorders of CSF circulation, cerebral hemodynamics, and gradients between atmospheric and intracranial pressure [10, 12, 15, 16]. Findings from animal studies and case reports supported by a number of uncontrolled, non-randomized, prospective case series suggest a substantial benefit of cranioplasty for patients with large cranial defects [4–6]. However, many authors reported complications and morbidity after cranioplasty [17–19]. While the likelihood of complications can be stratified according to neurological, medical, and radiological factors, the timing of surgery after cranioplasty can also affect outcome [18, 20]. Early cranioplasty for extended cranial defects after decompressive craniectomy has been associated with infections [17, 21], subdural or epidural fluid collections, seizures, fixed neurologic deficits [17], recurrence of brain edema, hydrocephalus, and hemorrhage [19]. On the other hand, delay in cranioplasty exposes the patient to atmospheric and mechanic pressure of the brain and hemodynamic and metabolic impairment [16]. Moreover, large cranial defects hinder the rehabilitation process [22], are associated with prolonged periods of immobility, and could lead to increased rates of pulmonary infection and thromboembolic events. How soon after a decompressive hemicraniectomy can cranioplasty be safely performed in a patient in whom the ICP has been normalized? This study addresses this topic.
Materials and methods
Study cohort: inclusion and exclusion criteria
We performed a retrospective cohort study among patients with extended cranial bone defects after decompressive hemicraniectomy who underwent cranioplasty between 1997 and 2008. Inclusion criteria were: (1) unilateral hemicraniectomy, (2) diameter of craniectomy defect more than 10 cm, (3) cranial reconstruction with autologous bone flap preserved by freezing, (4) primary pathology as cause for craniectomy included a) traumatic brain injury, b) subarachnoid hemorrhage, c) intracerebral hemorrhage, d) cerebral infarction, and e) infection. Exclusion criteria were: (1) bilateral craniectomy, (2) diameter of craniectomy defect equal or less than 10 cm, (3) cranioplasty materials used other than autologous bone, (4) patients with other primary pathologies than the above mentioned, such as a) pseudotumor cerebri, b) peritumoral edema, and c) venous thrombosis, and 5) patients who were treated in other hospitals.
Medical record review was used to determine the primary cause for hemicraniectomy and consciousness level before craniectomy according to GCS score. Of the 221 patients who were eligible for the study, we excluded 14 patients because of invalid or missing data about time of craniectomy, and seven patients were lost to follow-up.
Timing of cranioplasty
We defined time-to-cranioplasty as the time from decompressive hemicraniectomy at our institutes to the time when surgery to repair the cranial defect was performed. Time-to-cranioplasty is reported in 7-day intervals. We compared patient groups having cranioplasty at < 7 weeks after craniectomy, with those having surgery at 7–12 weeks and at >13 weeks. Delays in treatment due to administrative or scheduling conflict were excluded from the study. The study was based on different policies of treatment represented from two surgeons at the same institute who decided the timing of cranioplasty according to their preference. Allocation to early or late cranioplasty was not randomized.
Data collection
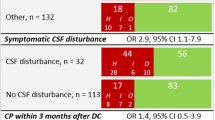
Data were collected by retrospective chart review using standardized, pretested forms. The characteristics of patients with large craniectomy defects admitting cranioplasty are shown in Table 1. Age, sex, and year of admission were noted, preoperative physical examination (GCS score, vital signs, cardiac examination, mental status, motor strength, general condition), electrocardiogram, chest radiograph, blood levels of glucose, coagulation tests), cause of craniectomy, preoperative status as defined by the American Society of Anaesthesiologist’s physical status classification system and postoperative complications. The craniectomy flap characteristics were administered before cranioplasty (side of craniectomy) and intensity of the depth of the craniectomy flap was classified in 3 grades (Figs. 1 and 2).
The difference in GOS in the different timing groups, 14 days after insult, prior to, and 1 year after cranioplasty. Asterisks denote significant difference in outcome between cranioplasty at <7 weeks versus 7–12 weeks and daggers denote significant difference between cranioplasty at <7 weeks versus >13 weeks
The following comorbid conditions were recorded: diabetes mellitus, colonization with multiresistant bacteria, history of cardiovascular disease, thromboembolism, valvular heart disease, arrhythmia, chronic pulmonary disease, malignancy, and hypertension. Cardiovascular disease was defined as history of myocardial infarction, angina or ischemic chest pain, coronary artery disease, heart failure, or peripheral vascular disease. Chronic pulmonary disease was defined as a history of chronic obstructive lung disease, asthma, or other chronic lung disease.
Medical record review was used to determine the original primary outcome at 14 days post-craniectomy and at the time of cranioplasty. These data formed the basis for comparison of outcome after surgery. The median follow-up of the cohort was 43 months (Table 1). We used a self-constructed questionnaire and interviews were conducted by phone in order to assess the final outcome. Outcome analysis was performed using the Glasgow Outcome Scale (GOS).
We evaluated postoperative morbidity and mortality, including serious bacterial infections, new brain infarction and edema, subdural or epidural fluid collections, seizures, intracerebral hematoma, myocardial infarction, and thromboembolism up to the time of discharge or 30 days after surgery. Serious bacterial infections included local infections such as deep wound infections, osteomyelitis, intracerebral abscess, subdural empyema, and systemic infections such as pneumonia and bacteriemia. Deep wound infections and osteomyelitis were identified by neurosurgeons; intracerebral abscess and subdural empyema were identified by a neuroradiologist. Pneumonia required either a chest radiograph consistent with an infiltrate followed by antibiotic treatment, or a physician diagnosis of pneumonia with subsequent antibiotic treatment. Bacteriemia was defined as a positive blood culture; two separate positive cultures were required for organisms that are usually considered contaminants (e.g., staphylococcus epidermidis). A neuroradiologist’s diagnosis identified those with postoperative brain edema and subdural or intracerebral hematoma.
Statistical analysis
Categorical variables were compared using the Chi-squared statistic (or Fisher’s exact test when the expected values were less than five). For continuous variables, we used the Mann–Whitney U test, as appropriate. All analyses were performed using BiAS software.
Results
The median age of the cohort was 53 years (range 6 months to 79 years) and 45 % were women. Stratified by underlying pathological condition, 75 (32 %) underwent decompressive craniectomy due to SAH, 51 (26 %) due to traumatic brain injury, 37 (19 %) due to cerebral infarction, 30 (16 %) due to ICH, and seven (4 %) due to infection. In the overall sample, the median time-to-cranioplasty was 10 weeks, and the median follow-up was 43 months, the minimum was less than 1 year, and the maximum was 5 years.
Factors that delayed time-to-cranioplasty
The time between craniectomy and cranioplasty ranged from 17 days to 14 months, depending on several factors such as: the cause of decompression, infection before or after craniectomy, and skin flap concavity. Out of the 53 (27 %) patients in whom cranioplasty was delayed beyond the 13th week, seven patients (13 %) had an infection as the primary cause of craniectomy and 41 patients (77 %) had a type 3 craniectomy flap with protrusion (Fig. 2). For 147 patients (74 %) in whom cranioplasty was performed earlier than the 13th week, in none of the cases was infection the primary cause for craniectomy, and only 24 patients (16 %) had a type 3 craniectomy flap (p < 0.001).
Perioperative morbidity
Nine patients (5 %) experienced a postoperative complication other than a local infection (one septic complication, one pneumonia, two hemorrhages, two subdural hematomas, one subdural fluid collection, and two brain edema). Timing of cranioplasty (comparing cranioplasty at <7 weeks with cranioplasty at 7–12 weeks and >12 weeks) was not associated with increased risk of postoperative complications (OR: 1.1; 95 % CI: 0.8 to 2.4), and in particular with the development of serious systemic bacterial infections (OR: 1.4; 95 % CI:0.7 to 1.8). There was no statistical significance in the rate of complication based on sex (p = 0.999), patient age (p = 0.889), and initial indication for craniectomy (p = 0.756).
Association between timing of cranioplasty and local infection rate
Twenty-one patients (10 %) experienced a local infection (12 deep-wound infections, six osteomyelitis, and three subdural empyemas). Timing of cranioplasty was not associated with significantly increased deep-wound infections rates, nor was any evidence of an interaction among timing of surgery and mortality. However, in bivariate analyses, cranioplasty at <7 weeks and several measures of comorbid conditions such as diabetes, thromboembolism, and colonization with multiresistant bacteria were independently associated with a trend of increased infection rate (p = 0.073) (Table 2).
Association between timing of cranioplasty and functional outcome
Timing of cranioplasty was associated with significantly better neurologic outcome. The functional outcome (GOS) was better in the cranioplasty at <7 weeks and at 7–12 weeks group than in the cranioplasty at >12 weeks (median score 4 versus 3) (Fig. 1); the odds ratio for a worse functional outcome (GOS ≤ 3) in the >12 weeks group was 7.45; 95 % CI, 3.85 to 14.39.
Among the patient group with cranioplasty at <7 weeks and at 7–12 weeks, GOS was 1.7 times greater than in those at >12 weeks (Fig. 1). The cranioplasty at <7 weeks led to a GOS improvement of 78 %, cranioplasty at 7–12 weeks 46 %, and cranioplasty at >12 weeks 12 %, respectively. Pairwise comparisons showed that the difference between <7 weeks versus 7–12 weeks or >12 weeks cranioplasty groups correlated to the precranioplasty GOS score, was statistically significant (p = 0.05 and p < 0.001, respectively). After adjustment for the baseline parameter of the GOS score at 14 days post-insult, the overall effect of cranioplasty on outcome did not change, however, the between-group differences were no longer significant (p = 0.586).
Discussion
We evaluated the effect of timing of cranioplasty on functional outcome in patients who underwent extended decompressive hemicraniectomy. We found similar results in the analyses of complication rates. There was a significant improvement in neurologic outcome in cranioplasty at <7 weeks and at 7–12 weeks. The shorter the time-to-surgery, the greater the outcome measure was observed.
Time to cranioplasty
No prospective, randomized study has been undertaken to determine the exact timing of cranioplasty in patients with large cranial defects. There is a perception that the standard of care after decompressive craniectomy is to wait at least 6 weeks before cranioplasty can be done, although this is controversial. Some authors tend to recommend that cranioplasty be performed at the latest possible time. Others suggested surgery be postponed even further for 3–6 months [19, 23]. Together, these reports formed the basis for delaying cranioplasty after completion of rehabilitation. These observations were reinforced by a retrospective report examining the results of cranioplasty (patients undergoing cranioplasty earlier than 1 year after penetrating head injury showed increased morbidity) [24]. However, many case series showed contradictory results [25]. Many of these studies were relatively small and may have insufficient power. Experimental studies reported an improvement in cerebral blood flow and metabolism [10, 26–28]. These concerns of late cranioplasty in patients with big cranial defects were reinforced by two additional reports [22, 29]. However, these studies did not determine at what time cranioplasty is most appropriate after intracranial pressure has recovered. In our study, patients were divided into three groups depending on the timing of cranioplasty, at <7 weeks, 7–12 weeks, and >13 weeks. Although time-to-surgery was based on the surgeon’s preference and individual patient’s recovery, we found significant factors for delaying cranioplasty beyond the 13 week after initial surgery such as a) infection as the primary cause of craniectomy (p < 0.001) and b) type 3 craniectomy flap with protrusion (p < 0.001).
Neurologic outcome
We hypothesized that time-to-cranioplasty was associated with better neurologic outcome if it was performed early. Prolonged neurologic improvement and mental disorders can result from decreased cerebral blood flow and disturbed brain metabolism due to craniectomy [2, 14, 30]. Cranioplasty has a markedly positive influence on postural blood flow, cerebrovascular reserve capacity, and cerebral glucose metabolism [10]. It seems logical that longer times-to-cranioplasty would promote the neurologic compromise associated with the syndrome of the sinking skin flap [4, 11, 13, 14]. In our study, patients with big cranial defects after decompressive hemicraniectomy and altered consciousness who underwent cranioplasty at <7 weeks or at 7–12 weeks fared considerably better than those patients undergoing later surgery. Ten of the 15 patients in the <7 weeks and 7–12 weeks cranioplasty groups experienced an improvement in neurological condition; on the other hand, only ten of 75 patients did the same in the >13 weeks cranioplasty group. Furthermore, in <7 weeks and 7–12 weeks cranioplasty groups, GOS was 1.7 times greater than in those with delayed surgery >13 weeks. However, even after post hoc adjustment for the baseline characteristics (GOS at 14 days post-insult); the overall favorable effect of cranioplasty did not change, although the between-group GOS differences were no longer significant. It is unlikely that our findings were due to an increased rate of survival of severely damaged patients in the late >13 weeks cranioplasty group because the rate of vegetative state patients at baseline was similar in the three study groups. However, the decision for the time to-cranioplasty was based on the different preference of two surgeons and no randomization was made. Although we found no difference in preoperative neurologic condition between the groups (Fig. 1), these findings may be influenced from a bias in the selection criteria for the timing of cranioplasty. For patients receiving early cranioplasty the question always arises, whether craniectomy had been indicated in the first place. Recently, a multicenter randomized controlled trial by Cooper and colleagues indicated that decompressive bilateral craniectomy may be associated with a worse functional outcome in patients with diffuse traumatic brain injury, although surgery can immediately and constantly reduce intracranial pressure [31]. Additional studies are needed to provide sound evidence on the role of decompressive craniectomy and timing of cranioplasty.
Complications
We decided to exclude patients with bilateral frontotemporoparietal craniectomies from this retrospective study on the light of reports suggesting that the bilateral approach may have more complications [32]. The overall complication rate was 15 %, which is similar to those recently reported in the literature [17]. Early cranioplasty and several comorbid conditions such as diabetes, colonization with multiresistant bacteria, and thromboembolism appear to increase the risk of deep-wound infections and osteomyelitis. Extrapolation from these observations suggests that these individuals with multiple comorbid conditions would tend to be at risk for increased postoperative infections if cranioplasty is performed early (p = 0.073). Preoperative blood glucose setting, anesthesia protocols, and perioperative medical management could minimize this risk. Further studies should address this topic. Cerebral hemorrhage subsequent to cranioplasty is a well known, although uncommon, complication [32]. In our study, we reported two patients with a postoperative hemorrhage from a series of 200 patients. In one patient, surgery was performed at <7 weeks and in the other one 7–12 weeks. Both hemorrhagic complications took place immediately after surgery. This complication may be attributed to postoperative hyperperfusion in an area of low preoperative perfusion pressure, which resulted in intracerebral hemorrhage.
Limitations
There are several potential limitations of this study. First, this is not a randomized trial and it is possible that we did not adjust for other factors associated with morbidity in these patients. Second, it is possible that our chart review did not detect a possible reason for delayed cranioplasty in some patients. Third, the cause of craniectomy varied among the patients and some patient groups had a smaller number of patients making estimations difficult. Lastly, the decision for craniectomy and cranioplasty depended on the clinical judgement of the individual surgeon and the surgical technique was not standardized, which could be a confounding factor for the observed results. Only prospective randomized trials can adequately address these issues. Nevertheless, this study should provide data for decision-making in the timing of cranioplasty in certain patients with large cranial defects after decompressive hemicraniectomy as well as for the design of future studies to address these unanswered questions.
Conclusions
The results of our study imply that early cranioplasty may contribute to better neurologic outcome as performing cranioplasty as soon as brain edema had normalized did not appear to raise infection rates. While early cranioplasty and several comorbid conditions appear to increase the risk of deep-wound infections and osteomyelitis, this can be averted with aggressive preventive care. We advise optimizing selection and the patient’s medical conditions before surgery.
Abbreviations
- CI:
-
Confidence interval
- CSF:
-
Cerebrospinal fluid
- GCS:
-
Glasgow Coma Scale
- GOS:
-
Glasgow Outcome Scale
- ICH:
-
Intracerebral hemorrhage
- ICP:
-
Intracranial pressure
- OR:
-
Odds ratio
- SAH:
-
Subarachnoidal hemorrhage
References
Adamo MA, Deshaies EM (2008) Emergency decompressive craniectomy for fulminating infectious encephalitis. J Neurosurg 108(1):174–176, Review
Agner C, Dujovny M, Gaviria M (2002) Neurocognitive assessment before and after cranioplasty. Acta Neurochir (Wien) 44(10):1033–1040
Arnaud E (2000) Advances in cranioplasty with osteoinductive biomaterials: summary of experimental studies and clinical prospects. Childs Nerv Syst 16(10–11):659–668
Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB, HAMLET investigators (2009) Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 8(4):326–333
Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, Witte S, Jenetzky E, Hacke W, DESTINY Study Group (2007) Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke 38(9):2518–2525
Hutchinson PJ, Corteen E, Czosnyka M, Mendelow AD, Menon DK, Mitchell P, Murray G, Pickard JD, Rickels E, Sahuquillo J, Servadei F, Teasdale GM, Timofeev I, Unterberg A, Kirkpatrick PJ (2006) Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study (www.RESCUEicp.com). Acta Neurochir Suppl 96:17–20
Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W, DECIMAL, DESTINY, and HAMLET investigators (2007) Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 6(3):215–222
Dujovny M, Agner C, Aviles A (1999) Syndrome of the trephined: theory and facts. Crit Rev Neurosurg 9(5):271–278
Sarov M, Guichard JP, Chibarro S, Guettard E, Godin O, Yelnik A, George B, Bousser MG, Vahedi K, DECIMAL investigators (2010) Sinking skin flap syndrome and paradoxical herniation after hemicraniectomy for malignant hemispheric infarction. Stroke 41(3):560–562
Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K (2000) Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg 93(1):53–61
Bijlenga P, Zumofen D, Yilmaz H, Creisson E, de Tribolet N (2007) Orthostatic mesodiencephalic dysfunction after decompressive craniectomy. J Neurol Neurosurg Psychiatry 78(4):430–433
Fodstad H, Love JA, Ekstedt J, Fridén H, Liliequist B (1984) Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir (Wien) 70(1–2):21–30
Schiffer J, Gur R, Nisim U, Pollak L (1997) Symptomatic patients after craniectomy. Surg Neurol 47(3):231–237, Review
Segal DH, Oppenheim JS, Murovic JA (1994) Neurological recovery after cranioplasty. Neurosurgery 34(4):729–731
Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT (1997) Cranioplasty: cosmetic or therapeutic? SurgNeurol 47(3):238–241, Review
Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M (1997) Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res 19(3):311–316
Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D (2010) Outcomes of cranial repair after craniectomy. J Neurosurg 112(5):1120–1124
Regel JP, Stolke D (2004) Dekompressive Kraniektomie aus neurochirurgischer Sicht. In: Moskopp, Wassmann (Hrsg) Neurochirurgie. Schattauer Verlag, S 231–238
Schimidek H (2000) Operative neurosurgical technique: cranioplasty: indications, technique and prognosis, 4th edn. Elsevier Science, Singapore
Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, Nakaguchi H, Nagashima T (2006) Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien) 148(5):535–540
Erman T, Demirhindi H, Göçer AI, Tuna M, Ildan F, Boyar B (2005) Risk factors for surgical site infections in neurosurgery patients with antibiotic prophylaxis. Surg Neurol 63(2):107–112
Liang W, Xiaofeng Y, Weiguo L, Gang S, Xuesheng Z, Fei C, Gu L (2007) Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J Craniofac Surg 18(3):526–532
Timmons RL (1982) Cranial defects and their repair. In: Youmans JR (ed) Neurological surgery, 2nd edn. WB Saunders, Philadelphia, pp 2228–2250
Rish BL, Dillon JD, Meirowsky AM, Caveness WF, Mohr JP, Kistler JP, Weiss GH (1979) Cranioplasty: a review of 1030 cases of penetrating head injury. Neurosurgery 4(5):381–385
Stiver SI, Wintermark M, Manley GT (2008) Reversible monoparesis following decompressive hemicraniectomy for traumatic brain injury. J Neurosurg 109(2):245–254
Yoshida K, Furuse M, Izawa A, Iizima N, Kuchiwaki H, Inao S (1996) Dynamics of cerebral blood flow and metabolism in patients with cranioplasty as evaluated by 133Xe CT and 31P magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry 61(2):166–171
Richaud J, Boetto S, Guell A, Lazorthes Y (1985) Effects of cranioplasty on neurological function and cerebral blood flow. Neurochirurgie 31(3):183–188
Sakamoto S, Eguchi K, Kiura Y, Arita K, Kurisu K (2006) CT perfusion imaging in the syndrome of the sinking skin flap before and after cranioplasty. Clin Neurol Neurosurg 108(6):583–585
Carvi y Nievas MN, Höllerhage HG (2006) Early combined cranioplasty and programmable shunt in patients with skull bone defects and CSF-circulation disorders. Neurol Res 28(2):139–144
Suzuki N, Suzuki S, Iwabuchi T (1993) Neurological improvement after cranioplasty. Analysis by dynamic CT scan. Acta Neurochir (Wien) 122(1–2):49–53
DECRA Trial Investigators, Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, Wolfe R, Australian and New Zealand Intensive Care Society Clinical Trials Group (2011) Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 364(16):1493–1502
Gooch MR, Gin GE, Kenning TJ, German JW (2009) Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus 26(6):E9
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Archavlis, E., Carvi Y Nievas, M. The impact of timing of cranioplasty in patients with large cranial defects after decompressive hemicraniectomy. Acta Neurochir 154, 1055–1062 (2012). https://doi.org/10.1007/s00701-012-1333-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1333-1