Abstract
Hot spring dwelling cyanobacterial strain TA-9 was isolated and characterized using a polyphasic approach. Morphological evaluation of the strain indicated the presence of typical T-type true branching with differently positioned heterocytes. Physiological characterization of the strain was also performed followed by molecular and phylogenetic analyses based on 16S rRNA gene. 16S rRNA gene phylogeny indicated the strain to be strongly supported at an independent node with consistent tree topology being visible. Further, folding of the D1−D1′ and box-B helix of the ITS region differentiated the strain from phylogenetically related species. Thus, the morphological, phylogenetic and folded ITS structures confirm that the strain TA-9 is a new species of the genus Mastigocladus with the name proposed being Mastigocladus ambikapurensis in accordance with the International Code of Nomenclature of algae, fungi and plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are a morphologically diverse group of prokaryotic photosynthetic organisms. It is well known that the environmental conditions that prevailed in the early Precambrian or at the time of the origin of cyanobacteria were quite similar to the environment present in the hot springs (Roy et al. 2014). The transformation of the environmental conditions and metabolic behavior of cyanobacterial communities, i.e., from high temperature, anoxygenic condition, sulfur and reducing gases into low temperature with oxidative characters, ultimately proves the wider adaptability of cyanobacteria to colonize at all possible biotopes such as polar regions, hypersaline lakes, marshy places (Hammer 1986; Whitton and Potts 2007) and hot springs with minor or major multidirectional modifications (morphological, physiological, biochemical and molecular) that are the real causes of the diversity (Finsinger et al. 2008; Padisák et al. 2016; Singh et al. 2014; Singh et al. 2016, 2017, 2018; Mishra et al. 2020).
Hot springs are very specific, geographically isolated habitats and their constant physicochemical properties allow the growth of unique and endemic microbial communities (Castenholz 1996; Papke et al. 2003; Klatt et al. 2011). Although these hot springs have been defined by extremely and moderately high temperature and sulfur content along with variable pH (Miller et al. 2006; Wang et al. 2013), they are rich in microbial communities including thermophilic cyanobacteria. These cyanobacterial communities are of great interest due to their high primary productivity, thermostable bioactive molecules, metabolites, enzymes, biofuel production, energy metabolism and organic matter cycling (Miller et al. 2009; Klatt et al. 2011; Nozzi et al. 2013). Cyanobacterial components that are commonly found in hot springs include unicellular (Synechococcus sp.), filamentous (Lyngbya, Phormidium, Calothrix and Leptolyngbya spp.) and branched filamentous (Fischerella/Mastigocladus) (Ward et al. 2012; Papke et al. 2003; Steunou et al. 2008). The hot springs of North American part (United State, Costa Rica, Greenland), Japan, Jordan, Bulgaria, Greece, Chile, Thailand, Russia and China have been studied for cyanobacterial community evaluation but extensive research has only been confined to Yellowstone National Park, the USA (Copeland 1936; Miller et al. 2006, 2009; Stewart 1970; Loiacono et al. 2012; Hamilton et al. 2011). The distribution pattern of blue-green algae was extensively studied in thermal gradient of hot spring Yellowstone National Park by Copeland (1936). More than 300 hot springs are reported from India but very few have been explored in context to cyanobacterial diversity (Singh et al. 2018; Mongra 2012; Thomas and Gonzalves 1965; Jha and Kumar 1990; Bhardwaj and Tiwari 2011; Debnath et al. 2009; Roy et al. 2014, 2015; Bhattacharya et al. 2016). Chhattisgarh is the ninth largest state located in east central India where the first geo-thermal power project was established to use hot water of the Tatapani hot spring for generating electricity (Sarolkar and Das 2015). Such water is also known for its medicinal properties to cure skin diseases. Tatapani hot spring is located in Balarampur district of Chhattisgarh, India (23.6986°N and 83.68404°E) and covers an area of about 0.1km2. The temperature ranges from 50 to 90 °C with marshy ground, high mineral deposits and alkaline pH (>7.0) (Shanker et al. 1987). The geological origin of water quality of hot springs is crucial for the diversity of microbial community, and the water quality is decided by the rock channels (Singh et al. 2018). In this context, the area of Tatapani hot spring is surrounded by Archaean rocks with biotite-chloriteschist, biotite- gneiss and calc-granulite bands (Sarolkar 2018). The luxuriant growth of blue-green mats has created an interest to isolate and characterize thermophilic cyanobacteria and among them, a branched, filamentous and heterocytous cyanobacterium was evaluated based on morphological parameters and it showed comparative resemblance with a member of the genus Mastigocladus. True branching heterocytous cyanobacteria are comprised of morphologically complex species along with uniseriate to multiseriate trichomes, homothallic to heterothallic forms and different branching patterns (T-type, V-type and Y-type) (Golubic et al. 1996; Gugger and Hoffmann 2004). They have four distinct types of cells: vegetative cells for oxygenic photosynthesis; heterocytes for nitrogen fixation; hormocytes for the development of hormogonia and the resting cells akinetes.
True branching cyanobacteria are classified under the families Hapalosiphonaceae, Stigonemataceae, Symphonemataceae and Capsosiraceae according to the recent classificatory system (Komárek et al. 2014). Family Hapalosiphonaceae forming a monophyletic clade includes widely studied genera Hapalosiphon, Westiellopsis, Fischerella, Mastigocladus, and Nostochopsis; however, these genera do not form monophyletic clades (Gugger and Hoffmann 2004). Genus Pelatocladus, Neowestiellopsis, Aetokthonos,and Reptodigitus are other recently described genera of family Hapalosiphonaceae (Miscoe et al. 2016; Wilde et al. 2014; Kabirnataj et al. 2018; Casamatta et al. 2020).
The type species of genus Mastigocladus, i.e., Mastigocladus laminosus is characterized usually by T-type or V-type true branching with the cells in the branches having narrowed ends, intercalary and solitary heterocytes (rarely in pairs) with rare akinete formation (Kirchner 1898). This genus has only one species as of now which is also the type, Mastigocladus laminosus Cohn ex Kirchner (Kirchner 1898). This species is a good example of an extremophile, and it was originally described from thermal waters of Karlovy Vary, Czech Republic. M. laminosus is a common organism from hot springs throughout the world though it depends on special set of environmental conditions with pH > 7.5, temperature less than 60 °C and relatively low salinity (Kaštovský and Johansen 2008).
The taxonomy of genus Mastigocladus is confusing as many thermal isolates have been assigned to the genus Fischerella (Kaštovský and Johansen 2008). In spite of this, one of the unbranched thermal strain Kaštovský 1996/2 which was earlier identified as M. laminosus f. nostocoides but later on it was described as a novel genus Cyanocohniella calida based on polyphasic approach (Kaštovský et al. 2014). The phylogenetic reconstructions revealed that thermal stains form a monophyletic clade among true branching cyanobacteria. However, Alcorta et al. (2019) state that Fischerella thermalis is a monophyletic, thermophilic species of genus Fischerella, but Fischerella thermalis is distantly related to other species of Fischerella, which are non-thermal isolates and it is considered that the genus Fischerella was associated mainly with soil form and all true branching thermal isolates should also be placed in the genus Mastigocladus (Kaštovský and Johansen 2008). Recent studies based on intense phylogenetic evaluation of the true branched cyanobacteria have also recommended revision in taxa identified as Fischerella to the genus Mastigocladus along with also anticipating more new species of the genus Mastigocladus (Mishra et al. 2020).
The present study aims to characterize and identify the true branching thermal strain TA-9 which has been isolated from the unexplored Tatapani hot spring from Chhattisgarh, India. Polyphasic evaluation of the cyanobacterial strain TA-9 has been done on the basis of different morphological, physiological (Chlorophyll a, carotenoids, accessory pigment phycocyanin, phycoerythrin and allophycocyanin, protein and carbohydrate content), molecular and phylogenetic attributes.
Materials and methods
Study area and sample collection
Sample was collected from Tatapani hot spring located in Balarampur district of Chhattisgarh, India (23.6986°N and 83.68404°E). Tatapani is an unexplored hot spring of Chhattisgarh, covering an area of about 0.1 km2. The cyanobacterial strain TA-9 was collected on May 2019 as a dark green mat, submerged and attached to the wall of the hot spring pool having the temperature of 52 °C and pH of 8.2, in pre-sterilized falcon tubes with hot spring water.
Analysis of hot spring water
The physicochemical properties of water such as pH, temperature, conductivity, total dissolved solids (TDS) and salinity were measured on-site by digital Multi-Parameter PCST35. Furthermore, water samples were analyzed in the laboratory for other chemical attributes such as alkalinity, sulfate, total nitrogen and metal ions. The alkalinity was measured by titrimetric method, sulfate was analyzed spectrometrically, whereas nitrogen was analyzed titrimetrically by using kjeldahl method. The metal ions were analyzed by using atomic absorption spectroscopy (American Public Health Association 2005).
Isolation, purification and culturing
The collected cyanobacterial mat was repeatedly washed in double-distilled water, and washed sample was serially diluted in sterilized BG-110 medium (Rippka et al. 1979). Isolation was done by spreading and streaking technique on solid media, on 1.2% agar. Purification was done by alternative sub-culturing between liquid and solid BG-110 medium. The culture was established and maintained under illumination of approximately 50–55 µE m−2 s−1 with a photoperiod of 14/10 h light/dark cycle at 50 °C. pH was adjusted to 7.6.
Phenotypic analysis
Phenotypic observation, micrometry and microphotography of cyanobacterial strain TA-9 were done through Nikon Eclipse 50i microscope fitted with camera (Nikon DS-Fi1). The shape, size and position of vegetative cells and heterocytes along with other morphological features such as branching pattern, the presence/absence and color of sheath were also observed and documented. Keys of Desikachary (1959) and Komárek (2013) were consulted for the morphological description and primary identification.
Physiological characterization
Growth evaluation of the strain TA-9 was carried out at various temperatures (30, 40, 50 and 60 °C) in terms of chlorophyll a content, and rest of the experiments were done after selection of the optimum temperature, i.e., 50 °C. The photosynthetic pigments were estimated in terms of chlorophyll a, carotenoids and the accessory pigments (phycocyanin, phycoerythrin and allophycocyanin). Chlorophyll a content of the cyanobacterium was estimated as per the protocol of MacKinney (1941). After centrifugation of 5 ml of culture at 5000 rpm for 5 min, the pellet obtained was treated with 90% methanol and left for 1 h. The absorbance of chlorophyll-containing solution was taken at 665 nm. The chlorophyll a content was expressed in terms of µg mg−1 dry weight. The absorbance of the above methanol extract was taken at 420 nm for estimation of carotenoid content in terms of µg mg−1 dry weight (MacKinney 1941).
The cyanobacterial cultures were centrifuged at 8,000 rpm for 5 min. The obtained pellets were suspended in potassium phosphate buffer (pH 6.8, 50 mM) and kept at 4 ºC. The pellets were then crushed with acid-washed sand using mortar and pestle. The crushed samples were centrifuged at 8,000 rpm for 5 min. The supernatant was collected in test tubes with potassium phosphate buffer (pH 6.8, 50 mM). The absorbance was taken at 562, 615 and 652 nm for estimation of phycocyanin, allophycocyanin and phycoerythrin, respectively, in terms of µg mg−1 dry weight (Bennett and Bogorad 1973). Total cellular protein content of the cyanobacterium was determined by using the protocol of Bradford (Bradford 1976), whereas the carbohydrate content was estimated by the phenol sulfuric acid method as per the protocol given by Dubois et al. (1956). All the physiological measurements were done in six replicates for twelve days.
Genomic DNA extraction, PCR and sequence analysis
The genomic DNA was extracted from exponentially grown culture using HiPurA™ Bacterial Genomic DNA Purification Kit (MB505–250PR) as per protocol given by manufacturer. 16S rRNA gene was amplified using cyanobacterial specific primer pA (5′-AGAGTTTGATCCTGGCTCAG-3′) and B23S (5′-CTTCGCCTCTGTGTGCCTAGGT-3′) (Edwards et al. 1989; Gkelis et al. 2005). Sequencing of the amplified products was carried out by Sanger’s method on a 3730xl DNA analyzer (Applied Biosystems, Foster City, CA, the USA). A sequence of 1433 bps of 16S rRNA and 816 bps of 16S-23S ITS region was obtained and submitted to the NCBI database with the accession number MN966421 and MT484274, respectively. The sequences were compared with the EzBioCloud database and NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Phylogenetic analysis
Phylogeny of strain TA-9 was reconstructed from 311 nucleotide sequences of the16S-rRNA gene imported from data base including outgroup Synechococcus sp. PCC7335 (AB015062). Sequences were aligned using MEGA5.2 software with the Clustal W algorithm, and the length of the alignment was 1131 nucleotides (Online Resource 4). Phylogenetic trees were reconstructed using maximum likelihood (ML), neighbor joining (NJ) and maximum parsimony (MP) algorithms in MEGA 5.2 selecting the model with the lowest BIC (Bayesian information criterion) score (Tamura et al. 2011). All trees were mapping into a single tree.
16S-23S ITS secondary structure analysis
Secondary structure analysis of folded D1-D1′ and box-B regions of 16S-23S ITS (Internal Transcribed Spacer) region was performed using Mfold web server (Zuker 2003). The secondary structure of ITS region of strain TA-9 was compared with phylogenetically related taxa.
Results
Habitat and morphological analysis
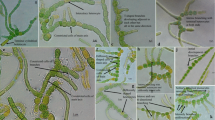
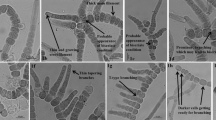
The cyanobacterial strain TA-9 was isolated from the hot spring having temperature around 52 °C and pH 8.2. The salinity, total dissolved solids, conductivity and alkalinity were 324 ppm, 475 ppm, 668 µS cm−1 and 110 mg l−1, respectively. The sulfate and nitrogen concentration was found to be 87 and 1.26 mg l−1, respectively. The ionic dominance of major cations and anions was found to be Na+ > K+ > Ca++ > Ni++ > Mg++ (Table 1). In the natural condition, strain TA-9 appeared as macroscopic, dark green, soft spongy mats adhering to the wall of the pool in the submerged condition. The initial examination of the sample showed clearly the presence of true branched cyanobacterial components. The sample on subsequent isolation, purification and sub-culturing in the laboratory showed bluish green creeping growth with dry surface on solidified medium. The culture in the liquid medium formed small clumps and grew by sticking to the wall of flask. The strain showed uniseriate filaments with typical T-type true branching and rare false branching. Interestingly, the position of heterocytes was intercalary, terminal and lateral sessile (Fig. 1a−j; Table 2). Main filaments had a distinct colorless sheath and the branches exhibited narrowing and tapering at the ends. Akinetes and hormogonia were not visible in the cyanobacterial strain TA-9 even after prolonged culturing. Monocytes were also observed.
Morphological attributes of strain TA-9. a Intercalary heterocytes in main filament; consecutive lateral T-type branches adjacent to heterocyte. b Terminal heterocyte in main filament. c Initiation of branching. d Transverse division in vegetative cell; false branching; initiation of lateral branches from both side. e Initiation of heteropolarity; development of lateral sessile heterocyte. f Circular- and oblong-shaped heterocyte in main filament. g Emergence of lateral branch from heterocyte; lateral sessile heterocyte; enlarged vegetative cell. h Intercalary heterocyte in secondary filament. i Development of lateral heterocyte on terminal cell; intercalary heterocyte in older secondary filament. j Two adjacent T-type branches with tapered end; monocyte liberated from main filament. Scale bar 10 µm (a−j)
Physiological analysis
When the growth was compared at the different temperatures in terms of chlorophyll a, it was very clear that 50 °C supported maximum growth followed by 40 °C. However, after 4th day of incubation, a drastic reduction was observed in the chlorophyll a content at 60 °C, whereas no significant change was investigated till 12th day at 30 °C (Online resource 1). So, in nutshell, it has been proved that 50 °C was the optimum, while 40 °C was the minimum. That’ why, only 50 °C (optimum temperature for growth) was taken for further investigations. In Fig. 2a, it was evident from the data that the chlorophyll a content rapidly increased as the culture aged in comparison to carotenoid content. Generation time was calculated on the basis of chlorophyll a, and it was found to be 34.45 h. On the basis of chlorophyll a, it was found that the exponential growth was observed till 6th day of incubation and after that linearity was established which indicated the growth toward the stationary phase. When all the accessory pigments content was compared, it was evident that phycocyanin content was increased majorly up to the 8th day as compared to initial day of incubation (Fig. 2b). No significant change was observed in case of allophycocyanin and phycoerythrin content throughout the experimentation period. The phycoerythrin content was lowest among all the accessory pigment content from initial to last day of incubation. Interestingly, allophycocyanin content was always higher than phycocyanin as the culture aged but only up to the 6th day of incubation thereafter, phycocyanin content was increased as compared to allophycocyanin (Fig. 2b). The incremental pattern of carbohydrate and protein content was almost same and rapid up to the 8th day of incubation (Fig. 2c, d) but the ratio of carbohydrate content was always higher than protein throughout the experimental period.
Molecular and Phylogenetic analysis
The 16S rRNA gene sequence homology was searched from the NCBI database and cyanobacterial strain TA-9 showed 97.91% sequence similarity with Mastigocladus sp. CPH1 (KX035101), 97.33% similarity with Fischerella sp. MV11 (DQ786170), 97.26% similarity with Fischerella sp. MV9 (DQ786169), 97.12% similarity with Fischerella sp. RV14 (DQ786172) and 96.56% similarity with Fischerella sp. NIES-3754 (AP017305).
Phylogenetic evaluation was performed based on 16S rRNA gene using 310 nucleotide sequences of heterocytous cyanobacteria along with Synechococcus sp. PCC7335 (AB015062) as outgroup. The 16S rRNA-based NJ dendrogram (Fig. 3) showed that the cyanobacterial strain TA-9 was clustered separately from its closely related taxa on a different node with strong bootstrap support (99/99/93) and consistent tree topology closer to the cluster of other true branching thermal isolates. The ML and MP trees also showed an overall similar pattern of clustering, thus strengthening the phylogenetic findings based on the 16S rRNA gene. The percentage pairwise similarity within the clade was found to be 96.3%-98.1% (online Resource 3).
16S-23S ITS secondary structure analysis
Secondary structure of D1-D1′ helix region of strain TA-9 comprised of 107 nucleotides and showed different and distinct folding patterns from the other phylogenetically related strains (Fischerella sp. MV11, MV14, CY2 and CY9) (Fig. 4). Broadly, strains CY9 and CY2 had different folding patterns itself and hence, the differences between TA-9 and these two strains were very evident. Interestingly, strains RV14 and MV11 had very similar structures of the D1-D1′ region as compared to strain TA-9. But the continuous formation of two topmost loops in strain TA-9 differentiated it from the strains RV14 and MV11. Folded secondary structures of box-B region of strain TA-9 again showed difference from the rest of the phylogenetically related strains having prominent in the sequence of nucleotides though the folding patterns were almost similar in all the five strains (Fig. 5). The V3 region could not be folded as direct sequencing was performed due to which V3 region could not be sequenced.
Discussion
In this study, a hot spring dwelling cyanobacterial strain TA-9 was isolated from the Tatapani hot spring. It is an important hot spring along the Narmada-Son-tectonic lineament. The rocks around Tatapani hot springs belong to Chhotanagpur Gneissic Complex (north, south, south-western part) and Gondwana subgroup (north-western part) that determine the physicochemical nature of thermal water. It was evident from the data (Table 1) that the thermal water has low salinity, alkaline pH (> 7.5) and temperature range of 40–60 °C. These features of thermal water were also supported by other findings where the growth of Mastigocladus has already been reported throughout the world (Kaštovský and Johansen 2008). However, the ecology of Tatapani hot spring was slightly different from the hot spring of Karlovy Vary (pH 6.91, high mineral composition and temperature of 36–53 °C) where the Mastigocladus laminosus was the dominating species (Kaštovský and Komárek 2001).
Phenotypically the strain TA-9 was characterized by uniseriate heteropolar filaments with typical T-type true branching and rare false branching. However, V or reverse Y-type of branching is also an important character of genus Mastigocladus (Komárek 1992), but according to Kaštovský and Johansen (2008), the T-type of branching is very common, while V-type branching is limited to the genus. In strain TA-9, V or reverse Y-type of branching was not visible but the position of heterocytes was found to be unique (intercalary, terminal and lateral sessile). The lateral sessile heterocytes were distinctive feature of the strain TA-9 that is non-typical for genus Mastigocladus, thus providing significant difference from the only well-established species of the genus, Mastigocladus laminosus which exhibits only intercalary heterocytes (Komárek 2013) (Online Resource 2).
Due to lack of data, the physiological evaluation could not be compared with the closely related species but these observations are still important as they could be good markers when more species will be eventually added to the genus Mastigocladus. At the present moment, the physiological data thus could not be compared with the other closely related taxa.
Phylogenetically, the strain TA-9 clustered separately on a different node with strong bootstrap support and consistent tree topology close to the cluster of other true branching thermal isolates. All true branching cyanobacteria represented two broad groups: The first one consists of only thermal strains that should belong to the genera Mastigocladus. While many of the strains in this cluster have been identified as Fischerella, but possibly they all should be revised and must be designated as members of the genus Mastigocladus (Kaštovský and Johansen 2008). This assumption is in sync also with many other studies where either this issue or similar kind of phylogeny has been observed (Mishra et al. 2020; Kaštovský and Johansen 2008). The second major group consisted of non-thermal strains and comprised mainly of genera Hapalosiphon, Fischerella, Westiellopsis, Neowestiellopsis, Pelatocladus, Reptodigitus and Nostochopsis. While the generic clusters were usually monophyletic and consistently supported, the presence of some misidentified strains deep inside these clusters is indeed an alarming issue which must be solved by cyanobacterial taxonomists. The clusters needing particular attention include Neowestiellopsis, Westiellopsis and Hapalosiphon. In agreement with some past studies, we believe that the soil-dwelling strain Hapalosiphon sp. 804–1 (AJ544078) must be assessed again using the polyphasic as it could eventually be a new generic entity although at the present moment we do not have any concrete evidence (Mishra et al. 2020). Most probably, a larger taxon sampling could provide some better insights into the taxonomic identity of this strain. Also in congruence with past studies, the position of Fischerella IAMM-263 (AB093491) continues to be vague and uncertain (Mishra et al. 2020; Kabirnataj et al. 2018). The position of the strain TA-9 observed in all the phylogenies was strongly supported and distinct which indicated that it could be a new taxa of the genus Mastigocladus.
In spite of having strong morphological and phylogenetic evidence of the strain TA-9 being a new member of the genus Mastigocladus, we performed the folding of the secondary structures of the ITS region in order to differentiate between the phylogenetically related taxa (Mishra et al. 2020; Boyer et al. 2002; Kabirnataj et al. 2018; Bohunická et al. 2015; Berrendero et al. 2016; Saraf et al. 2018; Shalygin et al. 2017) and the folded structures of the D1-D1′ and box-B regions gave enough evidence of the strain TA-9 being different from all the phylogenetically related members of the Mastigocladus cluster. Thus, the employment of the polyphasic approach and comparative evaluation using morphological, phylogenetic and folded ITS structures indicated clearly that the strain TA-9 was indeed a new member of the genus Mastigocladus.
Taxonomic treatment
Mastigocladus ambikapurensis Jaiswal & Singh, —HOLOTYPE: Tatapani hot spring, Ambikapur, Chhattisgarh, India (23.6986°N; 83.68404°E). Portion of a culture of Mastigocladus ambikapurensis is preserved in metabolically inactive form in Global Collection of Cyanobacteria (GCC; Registered Number 1165), Varanasi, India and is available under the accession number GCC20207.
Etymology: The epithet ambikapurensis (am.bi.ka.pu.ren'sis. N.L. masc. adj.ambikapurensis) refers to district Ambikapur from where the strain was isolated.
Description: Dark greenish, soft spongy, macroscopic mats were found to grow in submerged condition by adhering to the wall of the hot spring in natural condition. In pure culture condition, colonies grow as bluish green creeping mat on solid media, while a clumped appearance was visible in the liquid medium. Usually, filaments are densely entangled to each other. Filaments are uniseriate, and branches appear from both the sides of the filament. Initiation of branching can be seen even in young filaments. Typical T-type true branching is prominent though at some places, false branching is also seen. However, V or reverse Y-type branching was never been observed. Branches are usually narrower than the main filaments and also exhibit tapering ends with elongated cells. The vegetative cells of the main axis exhibit granular cytoplasm and their shape varies from globose to spherical having deep constrictions at the cross walls but sometimes there was a little bit distortion in the branch primordial cells. The vegetative cells of the main filament are 5.2–8.0 μm in length and 6.5–8.5 μm in width, whereas the size of the vegetative cells of the branches is varied from initial to terminal position. The size of the initial cell of the branch is 5.2–6.8 μm in length and 7.5–8.2 μm in width, whereas the size of the terminal cells ranges from 5.5–6.6 μm in length and 3.5–4.5 μm in width. Heterocytes are prominent and can be intercalary, terminal and lateral sessile and the shape varies from spherical, cylindrical to barrel shaped. Intercalary heterocytes 5.5–7.8 μm in length to 5.2–8.0 μm in width. Lateral heterocytes are slightly smaller than the others, i.e., 4.8–6.0 μm in length to 5.0–6.5 μm in width and are circular to oval in shape with slightly broader base. Terminal heterocytes 5.2–5.5 μm in length to 5.0–5.5 μm in width. Circular monocytes are 8.2–10.0 μm in length and width and emerging from the main filaments and comparatively larger in size. Akinetes and hormogonia were not visible.
Diagnosis: The strain TA-9 exhibits prominent T-type branching, rare false branching and distinctive positioned heterocytes (intercalary, terminal and lateral sessile) and the above said features show significant differences from Mastigocladus laminosus having T-type and reverse Y-type of branching pattern with only intercalary heterocytes (Fig. 1a, j; Online Resource 2). The presence of lateral sessile heterocytes, which is smaller than intercalary and terminal heterocytes, is typical distinctive character of this novel species (Fig. 1e, g; Table 2). The branches of strain TA-9 have less tapered ends as compared to M. laminosus. Monocytes are present, whereas hormogonia and akinetes are not detected. Monocytes are larger than the heterocytes and the vegetative cells (Fig. 1j). Phylogenetically, the strain TA-9 establishes single clade with other thermal isolates of true branching but on separate node (Fig. 3). Secondary structures of the ITS region of strain TA-9 prove significant differences from the other phylogenetically closely related taxa (Fig. 4 and Fig. 5).
Habitat: TA-9 was isolated from a hot spring having temperature around 52 °C and pH 8.2. The salinity, total dissolved solids, conductivity and alkalinity were measured on-site with the values being 324 ppm, 475 ppm, 668 µS cm−1and 110 mg l−1, respectively. The sulfate and nitrogen concentration was found to be 87 and 1.26 mg l−1, respectively. The ionic dominance of major cations and anions was found to be Na+ > K+ > Ca++ > Ni++ > Mg++ (Table 1).
Distribution area: Strain TA-9 is reported here for the first time in India and is expected to occur in hot springs with temperature ranging from 40 °C to 60 °C worldwide.
References
American Public Health Association (2005) Standard methods for the examination of water and wastewater. APHA, Washington
Alcorta J, Vergara-Barros P, Antonaru LA, Alcamán-Arias ME, Nürnberg DJ, Díez B (2019) Fischerella thermalis: a model organism to study thermophilic diazotrophy, photosynthesis and multicellularity in cyanobacteria. Extremophiles 23:635–647. https://doi.org/10.1007/s00792-019-01125-4
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419. https://doi.org/10.1083/jcb.58.2.419
Berrendero Gómez E, Johansen JR, Kaštovský J, Bohunická M, Čapková K (2016) Macrochaete gen. nov. (Nostocales, Cyanobacteria), a taxon morphologically and molecularly distinct from Calothrix. J Phycol 52:638–655. https://doi.org/10.1111/jpy.12425
Bhardwaj KN, Tiwari SC (2011) Cyanobacterial diversity of two hyper-thermal springs, Ringigad and Soldhar in Tapoban geothermal field, Uttarakhand Himalaya. Curr Sci 99:1513–1515
Bhattacharya S, Roy S, Ray S (2016) Species composition of cyanobacterial component of mats collected from two hot springs of West Bengal, India—first report. Phykos 46:32–39
Bohunická M, Pietrasiak N, Johansen JR, Gómez EB, Hauer T, Gaysina LA, Lukešová A (2015) Roholtiella, gen. nov. (Nostocales, Cyanobacteria)—a tapering and branching cyanobacteria of the family Nostocaceae. Phytotaxa 197:84–103. https://doi.org/10.11646/phytotaxa.197.2.2
Boyer SL, Johansen JR, Flechtner VR, Howard GL (2002) Phylogeny and genetic variance in terrestrial Microcoleus (Cyanophyceae) species based on sequence analysis of the 16S rRNA gene and associated 16S–23S ITS region 1. J Phycol 38:1222–1235. https://doi.org/10.1046/j.1529-8817.2002.01168.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Casamatta DA, Villanueva CD, Garvey AD, Stocks HS, Vaccarino M, Dvořák P, Hašler P, Johansen JR (2020) Reptodigitus chapmanii (Nostocales, Hapalosiphonaceae) gen. nov.: a unique Nostocalean (Cyanobacteria) genus based on a polyphasic approach1. J Phycol 56:425–436. https://doi.org/10.1111/jpy.12954
Castenholz RW (1996) Endemism and biodiversity of thermophilic cyanobacteria. Nova Hedwigia Beih 112:33–48
Copeland JJ (1936) Yellowstone thermal Myxophyceae. Ann New York Acad Sci 36:4–223. https://doi.org/10.1111/j.1749-6632.1936.tb56976.x
Debnath M, Mandal NC, Ray S (2009) The study of cyanobacterial flora from geothermal springs of Bakreswar, West Bengal, India. Algae 24:185–193. https://doi.org/10.4490/ALGAE.2009.24.4.185
Desikachary TV (1959) Cyanophyta. ICAR monograph on algae. Indian council of Agricultural Research, New Delhi
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Analytical Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucl Acids Res 17:7843–7853. https://doi.org/10.1093/nar/17.19.7843
Finsinger K, Scholz I, Serrano A, Morales S, Uribe-Lorio L, Mora M, Sittenfeld A, Weckesser J, Hess WR (2008) Characterization of true-branching cyanobacteria from geothermal sites and hot springs of Costa Rica. Environm Microbiol 10:460–463. https://doi.org/10.1111/j.1462-2920.2007.01467.x
Gkelis S, Rajaniemi P, Vardaka E, Moustaka-Gouni M, Lanaras T, Sivonen K (2005) Limnothrix redekei (Van Goor) Meffert (Cyanobacteria) strains from Lake Kastoria, Greece form a separate phylogenetic group. Microb Ecol 49:176–182. https://doi.org/10.1007/s00248-0032030-7
Golubic S, Hernandez- Marine M, Hoffmann L (1996) Developmental aspects of branching in filamentous Cyanophyta/Cyanobacteria. Algol Stud 83:303–329. https://doi.org/10.1127/algol_stud/83/1996/303
Gugger MF, Hoffmann L (2004) Polyphyly of true branching cyanobacteria (Stigonematales). Int J Syst Evol Microbiol 54:349–357. https://doi.org/10.1099/ijs.0.02744-0
Hammer UT (1986) Saline lake ecosystems of the world. Springer Science and Business Media, Berlin
Hamilton TL, Lange RK, Boyd ES, Peters JW (2011) Biological nitrogen fixation in acidic high-temperature geothermal springs in Yellowstone National Park, Wyoming. Environm Microbiol 13:2204–2215. https://doi.org/10.1111/j.1462-2920.2011.02475.x
Jha M, Kumar HD (1990) Cyanobacterial flora and physicochemical properties of Saptadhara and Brahma Kund hot springs of Rajgir, Bihar, India. Nova Hedwigia 50:529–534
Kabirnataj S, Nematzadeh GA, Talebi AF, Tabatabaei M, Singh P (2018) Neowestiellopsis gen. nov., a new genus of true branched cyanobacteria with the description of Neowestiellopsis persica sp. nov. and Neowestiellopsis bilateralis sp. nov., isolated from Iran. Pl Syst Evol 304:501–510. https://doi.org/10.1007/s00606-017-1488-6
Kaštovský J, Komárek J (2001) Phototrophic microvegetation of thermal springs in Karlovy Vary, Czech Republic. In: Elster J, Seckbach J, Vincent WF, O. Lhotský, (eds) Algae and extreme environments. Nova Hedwigia Beih 123:107–119
Kaštovský J, Gomez EB, Hladil J, Johansen JR (2014) Cyanocohniella calida gen. et sp. nov. (Cyanobacteria: Aphanizomenonaceae) a new cyanobacterium from the thermal springs from Karlovy Vary, Czech Republic. Phytotaxa 181:279–292. https://doi.org/10.11646/phytotaxa.181.5.3
Kaštovský J, Johansen JR (2008) Mastigocladus laminosus (Stigonematales, Cyanobacteria): Phylogenetic relationship of strains from thermal springs to soil- inhabiting genera of order and taxonomic implication for the genus. Phycologia 47:307–320. https://doi.org/10.2216/PH07-69.1
Klatt CG, Wood JM, Rusch DB, Bateson MM, Hamamura N, Heidelberg JF, Grossman AR, Bhaya D, Cohan FM, Kühl M, Bryant DA (2011) Community ecology of hot spring cyanobacterial mats: predominant populations and their functional potential. ISME J 5:1262–1278. https://doi.org/10.1038/ismej.2011.73
Kirchner O (1898) Schizophyceae. – In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, Teil I, Abt. 1a. Wilhelm Engelmann, Leipzig, pp. 4−92
Komárek J (1992) Diversita a moderní klasifikace sinic (Cyanoprocaryota). Habilitation Thesis, Institute of Botany, Třeboň
Komárek J (2013) Cyanoprokaryota. 3. Heterocytous genera. In: Budel B, Gartner G, Krienitz L, Schagerl M (eds) Suswasserflora von Mitteleuropa/freshwater flora of Central Europe 19/3. Springer Spectrum, Berlin, pp 1–1130
Komárek J, Kaštovský J, Mareš J, Johansen JR (2014) Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) using a polyphasic approach. Preslia 86:295–335
Loiacono ST, Meyer-Dombard DA, Havig JR, Poret-Peterson AT, Hartnett HE, Shock EL (2012) Evidence for high-temperature in situ nifH transcription in an alkaline hot spring of Lower Geyser Basin, Yellowstone National Park. Environm Microbiol 14:1272–1283. https://doi.org/10.1111/j.1462-2920.2012.02710.x
MacKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322. https://doi.org/10.1016/s0021-9258(18)51320-x
Miller SR, Purugganan M, Curtis SE (2006) Molecular population genetics and phenotypic diversification of two populations of the thermophilic cyanobacterium Mastigocladus laminosus. Appl Environm Microbiol 72:2793–2800. https://doi.org/10.1128/AEM.72.4.2793-2800.2006
Miller SR, Williams R, Strong AL, Carvey D (2009) Ecological specialization in a spatially structured population of the thermophilic cyanobacterium Mastigocladus laminosus. Appl Environm Microbiol 75:729–734. https://doi.org/10.1128/AEM.72.4.2793-2800.2006
Miscoe LH, Johansen JR, Kociolek JP, Lowe RL, Vaccarino MA, Pietrasiak N, Sherwood AR (2016) The diatom flora and cyanobacteria from caves on Kauai, Hawaii. Acta Bot Hungar 58:3–4
Mishra D, Suradkar A, Saraf A, Singh P (2020) Phylogenetic evaluation of the true-branched heterocytous cyanobacteria and description of soil dwelling Westiellopsis akinetica sp. nov. FEMS Microbiol Lett 367:046. https://doi.org/10.1093/femsle/fnaa046
Mongra AC (2012) Distribution pattern of cyanobacteria in hot water springs of Tattapani, Himachal Pradesh, India. JAIR 1:2278–5213
Nozzi NE, Oliver JWK, Atsumi S (2013) Cyanobacteria as a Platform for Biofuel Production. Frontiers Bioengin Biotechnol 1:7. https://doi.org/10.3389/fbioe.2013.00007
Padisák J, Vasas G, Borics G (2016) Phycogeography of freshwater phytoplankton: traditional knowledge and new molecular tools. Hydrobiologia 764:3–27. https://doi.org/10.1007/s10750-015-2259-4
Papke RT, Ramsing NB, Bateson M, Ward DM (2003) Geographical isolation in hot spring cyanobacteria. Environm Microbiol 5:650–659. https://doi.org/10.1046/j.1462-2920.2003.00460.x
Rippka R, Deruelles J, Waterbury JB, Herdman MR, Stanier RY (1979) Generic assignments, strain histories and properties of pure culture of cyanobacteria. J Gen Microbiol 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Roy S, Bhattacharya S, Debnath M, Ray S (2015) Diversity of cyanobacterial flora of Bakreswar geothermal spring, West Bengal, India-II. Algol Stud 147:29–44. https://doi.org/10.1127/1864-1318/2014/0178
Roy S, Debnath M, Ray S (2014) Cyanobacterial flora of the geothermal spring at Panifala, West Bengal, India. Phykos 44:1–8
Saraf A, Dawda HG, Suradkar A, Behere I, Kotulkar M, Shaikh ZM, Kumat A, Batule P, Mishra D, Singh P (2018) Description of two new species of Aliinostoc and one new species of Desmonostoc from India based on the polyphasic approach and reclassification of Nostoc punensis to Desmonostoc punense comb. nov. FEMS Microbiol Lett 365:272. https://doi.org/10.1093/femsle/fny272
Sarolkar PB, Das AK (2015) Assessment of Tatapani Geothermal Field, Balarampur District, Chhattisgarh State, India. Assessment, vol. 19, p. 25
Sarolkar PB (2018) Geothermal energy in India: poised for development. In Proceedings of 43rd Workshop on Geothermal Reservoir Engineering.
Shalygin S, Shalygina R, Johansen JR, Pietrasiak N, Berrendero Gómez E, Bohunická M, Mareš J, Sheil CA (2017) Cyanomargarita gen. nov. (Nostocales, Cyanobacteria): convergent evolution resulting in a cryptic genus. J Phycol 53(4):762–777. https://doi.org/10.1111/jpy.12542
Shanker R, Thussu JL, Prasad JM (1987) Geothermal studies at Tattapani hot spring area, Sarguja district, central India. Geothermics 16:61–76. https://doi.org/10.1016/0375-6505(87)90079-4
Singh SS, Kunui K, Minj RA, Singh P (2014) Diversity and distribution pattern analysis of cyanobacteria isolated from paddy fields of Chhattisgarh. India. J Asia-Pacific Biodivers 7:462–470. https://doi.org/10.1016/j.japb.2014.10.009
Singh P, Minj RA, Kunui K, Shaikh ZM, Suradkar A, Yogesh SS, Mishra AK, Singh SS (2016) A new species of Scytonema isolated from Bilaspur, Chhattisgarh, India. J Syst Evol 5:519–527. https://doi.org/10.1111/jse.12202
Singh P, Minz RA, Kunui K, Shaikh ZM, Suradkar A, Shouche YS, Mishra AK, Singh SS (2017) A new species of Scytonema isolated from Bilaspur, Chhattisgarh, India using the polyphasic approach. Pl Syst Evol 303:249–258. https://doi.org/10.1007/s00606-016-1370-y
Singh Y, Gulati A, Singh DP, Khattar JIS (2018) Cyanobacterial community structure in hot springs of Indian North-Western Himalaya: A morphological, molecular and ecological approach. Algal Res 29:179–192. https://doi.org/10.1016/j.algal.2017.11.023
Steunou AS, Jensen SI, Brecht E, Becraft ED, Bateson MM, Kilion O, Bhaya D, Ward DM, Peters JW, Grossman AR, Kühl M (2008) Regulation of nif gene expression and the energetics of N2 fixation over the diel cycle in a hot spring microbial mat. ISME J 2:364–378. https://doi.org/10.1038/ismej.2007.117
Stewart W (1970) Nitrogen fixation by blue-green algae in Yellowstone thermal areas. Phycologia 9:261–268. https://doi.org/10.2216/i0031-8884-9-3-261.1
Tamura K, Peterson D, Peterson N, Stecher DA, Lane DJ (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molec Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Thomas J, Gonzalves EA (1965) Thermal algae of Western India. Hydrobiologia 26:41–54. https://doi.org/10.1007/BF00142252
Wang S, Hou W, Dong H, Jiang H, Huang L, Wu G, Zhang C, Song Z, Zhang Y (2013) Control of temperature on microbial community structure in hot springs of the Tibetan plateau. PLoS ONE 8:e62901. https://doi.org/10.1371/journal.pone.0062901
Ward, David M, Richard W, Castenholz, Scott R, Miller (2012) Cyanobacteria in geothermal habitats. In: Whitton B (ed) Ecology of cyanobacteria II. Springer, Dordrecht, pp. 39–63. https://doi.org/10.1007/978-94-007-3855-3_3
Whitton BA, Potts M (2007) The ecology of cyanobacteria: their diversity in time and space. Springer Science and Business Media, Berlin
Wilde SB, Johansen JR, Wilde HD, Jiang P, Bartelme B, Haynie RS (2014) Aetokthonos hydrillicola gen. et sp. nov.: Epiphytic cyanobacteria on invasive aquatic plants implicated in Avian Vacuolar Myelinopathy. Phytotaxa 181(5):243–260. https://doi.org/10.11646/phytotaxa.181.5.1
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucl Acids Res 31:3406–3415. https://doi.org/10.1093/nar/gkg595
Acknowledgements
We thank the Head, Department of Botany, Banaras Hindu University, Varanasi for providing the necessary facilities and encouragement. One of us (TPJ) was financially supported by University Grants Commission, New Delhi. We thank Prof. Aharon Oren helping with the scientific names and etymology. The authors are also grateful to Prof. B. K. Sharma, Institute of Agriculture Science, Banaras Hindu University, Varanasi for providing microscopic facilities. Further, authors are also thankful to the ICAR-AMAAS Network project for financial assistance. Accession numbers generated in this study (all available on the public database): MN966421, MT484274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Yvonne Nemcova.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
Online Resource 1. Growth behavior of strain TA-9 at different temperatures in terms of chlorophyll a.
Online Resource 2. Comparison of morphological features of Mastigocladus ambikapurensis TA-9 with Mastigocladus laminosus.
Online Resource 3. Pairwise percentage similarity of Mastigocladus ambikapurensis TA-9 within the clade of Mastigocladus.
Online Resource 4. 16S rRNA alignment file with the strain TA-9 and all the phylogenetically related taxa.
Rights and permissions
About this article
Cite this article
Jaiswal, T.P., Chakraborty, S., Singh, P. et al. Description of hot spring dwelling Mastigocladus ambikapurensis sp. nov., using a polyphasic approach. Plant Syst Evol 307, 33 (2021). https://doi.org/10.1007/s00606-021-01755-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00606-021-01755-2