Abstract
St. John’s wort (Hypericum perforatum L.), as the most important medicinal species of the genus Hypericum, is medicinally used for many purposes such as treatment of mild and moderate depression. Considering importance of knowledge on genetic structure of wild relatives of crop plants, the present study was aimed to assess genetic variability among ten wild populations of H. perforatum growing in different climatic regions of Iran via ISSR markers. In general 15 selected primers generated 191 polymorphic fragments with an average of 12 in each primer. According to the UPGMA constructed dendrogram and PCoA, studied populations were classified into four main groups which was, to the some extent, in accordance with their geographical origins. Genetic distances of these populations ranged from 0.109 (Nor and Nowshahr) to 0.345 (Ardebil and Galogah). Also, Nei gene diversity (H e) values were calculated to be from 0.066 to 0.216 and Shannon’s information indices (I) varied from 0.321 to 0.097. Nei’s genetic differentiation index (G st) was high (0.573) indicating the high levels of genetic variation among the studied populations. Finally, the gene flow (N m) value was found to be 0.372. Obtained information is useful for use of Iranian H. perforatum germplasm for breeding programs and for development of effective conservation strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Hypericum belongs to the Hypericaceae (Guttiferae) family and comprises at least 469 species worldwide, out of which 19 are found in Iran (Crockett 2010; Lebaschi et al. 2003). St. John’s wort (Hypericum perforatum L.) is the most important medicinal species of the genus and its main uses in medicine includes treatment of mild and moderate depression, skin wounds and burns (Barnes et al. 2001). The plant contains a vast array of secondary metabolites, among which naphthodianthrones (hypericin and pseudohypericin), acylphloroglucinols (hyperforin and adhyperforin) and essential oil can be mentioned (Morshedloo et al. 2012; Radusiene et al. 2005; Verotta 2002; Greeson et al. 2001). Antiviral, antiretroviral and antitumor activities (Orčić et al. 2011; Percifield et al. 2007; Glisic et al. 2006; Agostinis et al. 2002; Meruelo et al. 1988) of extracts from this plant make it promising for treatment of HIV and cancer.
According to Brutovská et al. (2000), H. perforatum is probably originated from autopolyploidization of an ancestor closely related to diploid H. maculatum. However, it was recently implied that the plant has a single evolutionary origin and arose from independent and recurrent polyploidization of two different ancestral gene pools along with occurrence of substantial gene flow within and between H. perforatum and H. maculatum (Koch et al. 2013). As both sexual and aposporic processes can simultaneously occur in a given plant, St. John’s wort is considered as a facultative apomict species (Percifield et al. 2007; Mártonfi et al. 1996).
The prosperity of breeding programs depends largely on the genetic variability of plants, or in fact, the presence of a rich gene pool. Wild relatives of crops contain genes with the great potential for use in breeding programs and constitute a part of their gene pool (Pandey et al. 2008). In addition, the study of intra-specific levels of genetic variation and investigation of genetic structure of wild populations is crucial for development of effective conservation strategies.
Different techniques including morphological, biochemical and especially molecular markers let scientists to study genetic variability of plants. As molecular markers present reproducible results regardless of environmental conditions, they have gained nowadays considerable attention for studies relating to the genetic diversity (Farooq and Azam 2002). Molecular markers have been extensively served in a wide range of species including horticultural, medicinal and food crops for different purposes. In medicinal plants, applications included the study of genetic variations, identification of cultivars, genotyping, cross-breeding studies, phylogenetic analyses, pharmacognostic and species characterization and also genetic mapping (Percifield et al. 2007; Joshi et al. 2004). In this respect, St. John’s Wort has been the subject of many studies (Tonk et al. 2011; Percifield et al. 2007; Barcaccia et al. 2006).
As a kind of PCR-based molecular marker, ISSR (inter simple sequence repeat) technique amplifies DNA fragments located between two identical microsatellite repeats oriented in opposite direction. Microsatellite motifs, which are used as primers for ISSRs in unanchored or more usually anchored at 3′ or 5′ end, can be di- to penta-nucleotides (Semagn et al. 2006). These primers can be undertaken for any plant species which contains a sufficient number and distribution of SSR motifs in its genome (Domyati et al. 2011). ISSR markers have the potential to reveal similarities at the intra- and interspecific levels (Chennaoui-Kourda et al. 2007; Davila et al. 1998; Ghariani et al. 2003). Simplicity, reproducibility, requirement of only a small amount of DNA and relatively low cost of performing are advantages of this system of genotyping. In addition, ISSR markers don’t require information on DNA sequence and produce a large number of fragments per primer (Wang et al. 2008; Semagn et al. 2006; Reddy et al. 2002; Pasqualone et al. 2001).
In the present study, we decided to assess genetic diversity in ten natural populations of H. perforatum growing in Iran using ISSR markers. Our results can clarify relationships in order to establish basic information for conservation and use of Iranian H. perforatum germplasm in breeding programs of this important medicinal plant.
Materials and methods
Plant materials and DNA extraction
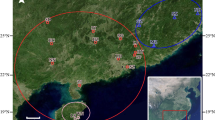
Ten wild populations of St. John’s Wort were collected at the flowering stage in August 2011 from different parts of Iran. The origin and geographical characteristics of studied populations are presented in Table 1 and Fig. 1. Voucher specimens were deposited at the Herbarium of Department of Horticulture, Faculty of Agricultural Sciences and Engineering, University of Tehran, Iran. For performing ISSR analyses, five single plants were randomly chosen from each population and their young leaves were frozen in liquid nitrogen. Genomic DNA was isolated according to the protocol described by Doyle and Doyle (1990) with little modifications. Quality and quantity of extracted DNAs were evaluated by electrophoresis on 1.5 % agarose gel and spectrophotometrically using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). The isolated DNAs were diluted to 10 ng/µl and stored at −20 °C until use.
ISSR analysis
Out of thirty-two primers tested for amplification of the genomic DNAs, fifteen were finally selected (Table 2). Primers used were synthesized by Bioneer (South Korea) and Metabion-Deutschland companies. Amplification mixtures (15 µl) contained 3 µl of template DNA (10 ng/µl), 7.5 µl PCR mastermix (Sinagene, Iran), 1/5 µl ISSR primer (10 pmol/µl) and 3 µl double distillated water. Amplification was carried out in a Bio Rad thermocycler (iCycler, USA) programmed as follows: initial denaturation at 94 °C for 5 min; 32 cycles each including 94 °C for 1 min, 40–58 °C for (depended on the primer) 1 min and 72 °C for 2.5 min; and final extension at 72 °C for 7 min.
The amplification products were separated on 1.5 % (w/v) agarose gels and 1× TBE buffer (pH 8.5) for 120 min at 70 v, stained with ethidium bromide and photographed under UV light.
Data analyses
Amplified fragments were scored as 1 for the presence and 0 for the absence. Fragments with the same molecular weight were considered as monomorphic. Only reproducible bands were considered for data analysis. The dendrogram was constructed using the UPGMA (unweighted pair group method with the arithmetic averaging) cluster analysis (Sheath and Sokal 1973) by NTSYSpc software ver.2.02. Resolving power (Rp) accounted according to the Prevost and Wilkinson (1999) formula:
where, p is the proportion of species presenting the I band. The polymorphic information content (PIC) value for each locus was calculated using the following formula:
in which, PICi is the polymorphic information content of the locus i, fi is the frequency of the amplified fragments and 1–fi is the frequency of non-amplified fragments (Roldàn-Ruiz et al. 2000). The frequency was calculated as the ratio between the number of amplified fragments at each locus and the total number of accessions. The PIC of each primer was calculated using the average PIC value from all loci of each primer.
A genetic distance matrix was created based on obtained ISSR data using Genalex 6.1 (Peakall and Smouse 2006). Nei’s genetic distances were calculated according to Nei (1972). Also, principal coordinates analysis (PCoA), analysis of molecular variance (AMOVA), effective number of alleles per locus (N e) and observed number of alleles per locus (N a) were calculated. Genetic diversity measures were tested using gene diversity statistics (Nei 1973). Shannon’s information index (I) was also used to examine partitioning of genetic diversity within and among populations. In addition, genetic construction was computed using Nei’s (1978) gene diversity statistics, including the total genetic diversity (H t), genetic diversity within populations (H s), and the relative magnitude of gene differentiation coefficients (G st, G st = (H t – H s)/H t) among populations. Finally, the value of gene flow (N m = 0.5 (1 – G st)/G st)) was calculated according to the McDermott and McDonald (1993). These analyses were performed using POPGENE32 program.
Results and discussion
Polymorphism of ISSR markers
Primers capable of polymorphic bands generation were served for analysis of populations. These primers generated fragments differing in number, size and pattern (Fig. 2). Sequences of used primers along with their percentages of polymorphic bands and resolving powers are summarized in Table 2. Length of amplified fragments was in the range of about 500–3,000 bp. In total, 15 ISSR primers amplified 191 polymorphic bands (97.4 %) with an average of 12 in each primer indicating the potentially polymorphic nature of microsatellite regions that amplify by ISSR technique (Morgante and Olivieri 1993). The primer IS8 was produced the highest (24), while the primers IS25 and UBC-810 gave the lowest number of polymorphic bands (8) in tested genotypes. Considering sequences of IS25 and UBC-810 primers [(GACA)5 and (GA)8T, Table 2], it was also previously reported that primers containing GACA and GA repeats often show least frequency in the genome (Kumar et al. 2013; Singh et al. 2012; Chennaoui-Kourda et al. 2007). In this study, PIC values ranged from 0.37 (the primer UBC-810) to 0.24 (the primer IS06) with a mean of 0.3. However, Rp values for used ISSR markers differed from 3.18 (IS24) to 10.12 (IS8) with an average of 5.62. According to the results, ACG repeats should be abundant in the genome of H. perforatum and has the potential for population’s discrimination. In agreement to our results, similar PIC and Rp mean values were also reported in a previous study by Tonk et al. (2011) on H. perforatum using RAPD markers. However, the higher mean PIC values were obtained using ISSR markers on Dasypyrum villosum (L.), Triticum L. and Secale L. (Grądzielewska 2011) and rice (Sarla et al. 2005) (0.5, 0.62, 0.62 and 0.82, respectively).
Genetic distances and population relationships
According to the results, genetic distances among studied populations ranged from 0.109 to 0.345 with an average of 0.226 (Table 3) indicating relatively the high levels of genetic distances among Iranian H. perforatum populations. The lowest distance value (0.109), and therefore the highest similarity, was observed between populations ‘Nor’ and ‘Nowshahr’. These populations are grown in a similar altitude (3–4 m above sea level) in the Mazandaran province. On the contrary, populations Ardebil and Galogah, belonging to different provinces and growing in 2,100 and 25 m above sea level, respectively, exhibited the maximum distance value (0.345). The low genetic similarity of these populations may be related to their high geographical distance. Similar to our results, RAPD markers also revealed relatively the high genetic distances values among H. perforatum populations (0.121–0.301) (Hazler Pilepic et al. 2008).
Although studied H. perforatum populations nearly covered all types of its habitats, Pearson’s correlation analyses showed no significant correlation between genetic and geographical factors. In this respect, similar results have been previously reported on H. perforatum (Barcaccia et al. 2006) and other species (DeWalt et al. 2011; Li et al. 2003; Fu et al. 2001).
In order to reveal populations’ relationships, the genetic distances matrix was made using NTSYSpc software ver.2.02. The UPGMA-based constructed dendrogram grouped studied populations into three main groups at the distance of 0.24 (Fig. 3). Group A consisted of only one member, the Kharw population. Plants of this population grow in a closed valley environment; interruption of its habitat from other habitats of wild growing Iranian H. perforatum may be resulted in an isolated population (Hamrick and Godt 1989). The second group (B) included populations Saghez and Ardebil and seven other populations were placed in the third group (C). The latest was subsequently divided into two sub-groups including plants of Torghabe (Mashhad) population and other six remaining ones (Galogah, Shirgah, Javaherdeh, Nowshahr, Nor and Toskestan) (Fig. 3). Biplot analysis also grouped these populations in accordance with UPGMA-based constructed dendrogram. Considering breeding system of this species (sexual and apomixes) and geographical origins of collected materials and therefore occurrence of different levels of gene flow among them, grouping of studied populations nearly according to their origins seems to be reasonable.
UPGMA dendrogram of the analyzed populations generated from ISSR data and estimated according to the Nei (1972) formula
Principal coordinate analysis (PCoA) via covariance matrix with data standardization produced similar results. According to the results, the variance explained by the first, second and third component was 27.9, 21.1 and 18.5 %, respectively. Therefore, the first three principle components explained 67.5 % of the total variation (Table 4). Results of biplot analysis are represented in Fig. 4. As mentioned previously, grouping of our populations was similar to that of constructed dendrogram, representing close relationships among geographical origins and their dispersal patterns. Similar results using ISSR markers have been also reported in many other plants including species of the genera Sulla and Lathyrus and Rheum tanguticum (Hu et al. 2010; Chennaoui-Kourda et al. 2007; Belaid et al. 2006).
Indices of genetic diversity and population genetic differentiation
According to Hardy–Weinberg equilibrium, Nei gene diversity (H e) values in H. perforatum populations studies ranged from 0.066 (Ardebil population) to 0.216 (Toskestan population) with an average of 0.119, indicating considerable levels of genetic diversity in H. perforatum plant. A high level of variation among H. perforatum populations was also reported by Percifield et al. (2007) which confirms results of the present study. Similar results have been reported on this species using the RAPD markers by Hazler Pilepic et al. (2008). The high genetic diversity of H. perforatum populations is as a result of its mating systems. In fact, propagation method(s) of plant species is considered as one of the most important factors determining their levels of genetic diversity (Hamrick 1982; Hamrick and Godt 1989). Self-incompatibility is a wide spread phenomenon in the genus Hypericum (Robson 1981), resulting in the high levels of genetic variability (Borba et al. 2001). Furthermore, this perennial plant produces a great number of seeds every year in favor of the high amounts of diversity in this species (Zhao et al. 2007). Since widespread species may possess the higher levels of genetic diversity than narrowly distributed plants (Hamrick and Godt 1996; Singh et al. 1998), the wide range of H. perforatum distribution is an important factor in this respect.
Moreover, Shannon’s information indices (I) varied between 0.321 and 0.097 with a mean of 0.177. Among 10 studied populations, plants of the Toskestan population exhibited the highest levels of genetic variability (H e = 0.216, I = 0.321, PPL = 58.53 %), whereas those of Ardebil population showed the lowest ones (H e = 0.066, I = 0.097, PPL = 17.99 %) (Table 5). The high levels of intraspecific genetic variability have also been reported in the previous studies on Rheum tanguticum (Hu et al. 2010), the genus Lathyrus (Belaid et al. 2006) and castor (Gajera et al. 2010). The fact that ISSR markers are efficient for assessing genetic diversity at the intraspecific level, in addition to this study, is also frequently demonstrated in a line of previous studies (Hu et al. 2010; Belaid et al. 2006; Ghariani et al. 2003; Cavan et al. 2000; Charters et al. 1996).
According to Nei (1978), our populations have been estimated to have high value of genetic differentiation coefficient (G st) (0.573). In this respect, populations with G st values <0.05, between 0.05 and 0.15 and >0.15 have the low, medium and high levels of genetic differentiation, respectively. Estimated value for studied populations of H. perforatum growing in Iran is also greater than the average G st value of 0.187 for insect-pollinated outcrossing plants (Hamrick 1989). ISSR markers also revealed a high value of G st in Dysosma pleiantha populations in a previous study by Zong et al. (2008).
According to Hamrick (1993), as more than 80 % of the total genetic diversity resides within populations, five strategically placed populations should maintain 99 % of their total genetic diversity. However, an average genetic diversity of 42.67 % among our populations implies the need to conserve more populations to maintain genetic diversity within this species. Furthermore, the low level of gene flow (0.372) indicates low movement of genes from one population to another and the low level of migrants exchange among populations. It has been noted that the gene flow rate of more than four migrants per generation is theoretically sufficient to prevent genetic differentiation due to genetic drift (Slatkin 1987). Considering the low level of gene flow rate among studied wild populations of H. perforatum, therefore, genetic drift might be inevitable. In H. perforatum, the low rate of gene flow may be due to factors such as prevailing apomixes and short distance of seed dispersal as stated by Hazler Pilepic et al. (2008).
Conclusion
Because of its valuable medicinal properties and high demand, H. perforatum needs more attention toward the conservation. As this plant is widely used in traditional systems of medicine in the world, an ex situ long-term conservation program seems to be recommended for its suitable and efficient uses. In the present study, ISSR markers revealed relatively a high level of genetic variability among Iranian St. John’s wort populations suggesting that the ISSR technique is efficient and powerful for assessment of genetic diversity at the intraspecific level. Data on the genetic structure of these populations may be helpful for developing sustainable conservation strategies and also for using this gene pool in breeding programs of this valuable medicinal plant. In other words, populations with the high levels of intraspecific genetic diversity (such as Toskestan) may be considered for exploring desired traits among its individuals for use in breeding programs of this plant. On the other hand, considering the direct relationship between the levels of genetic diversity and populations threatening, the low levels of genetic diversity in populations such as Ardebil should be increased via different strategies such as introducing new individuals into them for increasing the level of genetic diversity and, as a result, increasing their constancy.
References
Agostinis P, Vantieghem A, Merlevede W, de Witte PA (2002) Hypericin in cancer treatment: more light on the way. Int J Biochem Cell Biol 34(3):221–241
Barcaccia G, Arzenton F, Sharbel T, Varotto S, Parrini P, Lucchin M (2006) Genetic diversity and reproductive biology in ecotypes of the facultative apomict Hypericum perforatum L. Heredity 96(4):322–334
Barnes J, Anderson LA, Phillipson JD (2001) St John’s wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol 53(5):583–600
Belaid Y, Chtourou-Ghorbel N, Marrakchi M, Trifi-Farah N (2006) Genetic diversity within and between populations of Lathyrus genus (Fabaceae) revealed by ISSR markers. Genet Resour Crop Evol 53(7):1413–1418
Borba EL, Semir J, Shepherd GJ (2001) Self-incompatibility, inbreeding depression and crossing potential in five Brazilian Pleurothallis (Orchidaceae) species. Ann Bot 88:89–99
Brutovská R, Čellárová E, Schubert I (2000) Cytogenetic characterization of three Hypericum species by in situ hybridization. Theor Appl Genet 101(1–2):46–50
Campbell MH, May CE, Southwell IA, Tomlinson JD, Michael PW (1997) Variation in Hypericum perforatum L. (St. John’s wort) in New South Wales. Plant Prot Q 12:64–66
Cavan G, Potier V, Moss S (2000) Genetic diversity of weeds growing in continuous wheat. Weed Res 40(3):301–310
Charters Y, Robertson A, Wilkinson M, Ramsay G (1996) PCR analysis of oilseed rape cultivars (Brassica napus L. ssp. oleifera) using 5′-anchored simple sequence repeat (SSR) primers. Theor Appl Genet 92(3–4):442–447
Chennaoui-Kourda H, Marghali S, Marrakchi M, Trifi-Farah N (2007) Genetic diversity of Sulla genus (Hedysarea) and related species using inter-simple sequence repeat (ISSR) markers. Biochem Syst Ecol 35(10):682–688
Crockett SL (2010) Essential oil and volatile components of the genus Hypericum (Hypericaceae). Nat Prod Commun 5(9):1493–1506
Davila J, Sánchez de la Hoz M, Loarce Y, Ferrer E (1998) The use of random amplified microsatellite polymorphic DNA and coefficients of parentage to determine genetic relationships in barley. Genome 41(4):477–486
DeWalt SJ, Siemann E, Rogers WE (2011) Geographic distribution of genetic variation among native and introduced populations of Chinese tallow tree, Triadica sebifera (Euphorbiaceae). Am J Bot 98(7):1128–1138
Domyati F, Rania A, Younis A, Edris S, Mansour A, Sabir G, Bahieldin A (2011) Molecular markers associated with genetic diversity of some medicinal plants in Sinai. J Med Plants Res 5(2):200–210
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Farooq S, Azam F (2002) Molecular markers in plant breeding-I: concepts and characterization. Pak J Biol Sci 5(10):1135–1140
Fu Y, Qian J, Ma Y, Li J, Zheng S (2001) Genetic differentiation research on populations of wild soybeans in different scales. Acta Ecol Sin 22(2):176–184
Gajera BB, Kumar N, Singh AS, Punvar BS, Ravikiran R, Subhash N, Jadeja G (2010) Assessment of genetic diversity in castor (Ricinus communis L.) using RAPD and ISSR markers. Ind Crop Prod 32(3):491–498
Ghariani S, Trifi-Farah N, Chakroun M, Marghali S, Marrakchi M (2003) Genetic diversity in Tunisian perennial ryegrass revealed by ISSR markers. Genet Resour Crop Evol 50(8):809–815
Glišić SB, Popadić SV, Skala DU (2006) St. John’s Wort Hypericum perforatum L.: supercritical extraction, antimicrobial and antidepressant activity of extract and some component. Hem Ind 60(3–4):61–71
Grądzielewska A (2011) Application of the ISSR method to estimate the genetic similarity of Dasypyrum villosum (L.) P. Candargy Greek populations to Triticum and Secale species. Biodiv Res Conserv 21(1):7–12
Greeson JM, Sanford B, Monti DA (2001) St. John’s wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 153(4):402–414
Hamrick JL (1982) Plant population genetics and evolution. Am J Bot 69:1685–1693
Hamrick JL (1989) Isozymes and the analysis of genetic structure in plant populations. In: Soltis DE, Soltis PE (eds) Isozymes in plant biology. Dioscorides Press, Portland, pp 87–105
Hamrick JL (1993) Genetic diversity and conservation in tropical forest. In: Proceeding of the ASEAN-Canada Symposium on Genetic Conservation and Production of Tropical Tree Seed (eds Drysdale RM, John SET, Yapa AC). ASEAN-Canada Forest Tree Center, Saraburi, Thailand, pp 1–9
Hamrick JL, Godt MJW (1989) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics, breeding and genetic resources. Sinauer Associates Inc., Sunderland, pp 43–63
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Phil Trans R Soc Lond B Biol Sci 351:1291–1298
Hazler Pilepić K, Maleš Ž, Plazibat M (2008) Genetic structure in Hypericum perforatum L. population. Period biol 110(4):367–371
Hu Y, Wang L, Xie X, Yang J, Li Y, Zhang H (2010) Genetic diversity of wild populations of Rheum tanguticum endemic to China as revealed by ISSR analysis. Biochem Syst Ecol 38(3):264–274
Joshi K, Chavan P, Warude D, Patwardhan B (2004) Molecular markers in herbal drug technology. Curr Sci 87:159–165
Koch MA, Scheriau C, Betzin A, Hohmann N, Sharbel TF (2013) Evolution of cryptic gene pools in Hypericum perforatum: the influence of reproductive system and gene flow. Ann Bot 111(6):1083–1094
Kumar A, Mishra P, Singh SC, Sundaresan V (2014) Efficiency of ISSR and RAPD markers in genetic divergence analysis and conservation management of Justicia adhatoda L., a medicinal plant. Plant Syst Evol 300(6):1409–1420
Lebaschi MH, Matin A, Sharifi Ashourabadi E (2003) Comparison of hypericin between natural and agroecosystem. Pajouhesh va Sazandegi In Nat Resour 16(4):48–54
Li S, Cai Y, Qian Z, Zhao G (2003) Researches on the relationship between the morphological and genetic variations of Dipteronia dyeriana Henry (Aceraceae). Acta Ecol Sin 24(5):925–931
Mártonfi P, Brutovská R, Ĉellárová E, Repĉák M (1996) Apomixis and hybridity in Hypericum perforatum. Folia Geobot 31(3):389–396
McDermott JM, McDonald BA (1993) Gene flow in plant pathosystems. Annu Rev Phytopathol 31(1):353–373
Meruelo D, Lavie G, Lavie D (1988) Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci 85(14):5230–5234
Morgante M, Olivieri A (1993) PCR-amplified microsatellites as markers in plant genetics. Plant J 3(1):175–182
Morshedloo M, Ebadi A, Fatahi Moghadam M, Yazdani D (2012) Evaluation of essential oil composition in tree species of Hypericum from Iran. J Med Plants 11(9):23–31
Nei M (1972) Genetic distance between populations. Am Nat 106(949):283–292
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci 70(12):3321–3323
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89(3):583–590
Orčić DZ, Mimica-Dukić NM, Francišković MM, Petrović SS, Jovin EĐ (2011) Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem Cent J 5(1):1–8
Pandey A, Tomer AK, Bhandari D, Pareek S (2008) Towards collection of wild relatives of crop plants in India. Genet Resour Crop Evol 55(2):187–202
Pasqualone A, Caponio F, Blanco A (2001) Inter simple sequence repeat DNA markers for identification of drupes from different Olea europaea L. cultivars. Eur Food Res Technol 213(3):240–243
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6(1):288–295
Percifield RJ, Hawkins JS, McCoy JA, Widrlechner MP, Wendel JF (2007) Genetic diversity in Hypericum and AFLP Markers for species-specific identification of H. perforatum L. Planta Med 73(15):1614–1621
Prevost A, Wilkinson M (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98(1):107–112
Radusiene J, Judzentiene A, Bernotiene G (2005) Essential oil composition and variability of Hypericum perforatum L. growing in Lithuania. Biochem Syst Ecol 33(2):113–124
Reddy MP, Sarla N, Siddiq E (2002) Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 128(1):9–17
Robson N (1981) Studies in the genus Hypericum L. (Guttiferae). 2. Characters of the genus. Bull Br Mus Nat Hist 8:55–226
Roldàn-Ruiz I, Dendauw J, Van Bockstaele E, Depicker A, De Loose M (2000) AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol Breed 6(2):125–134
Sarla N, Neeraja C, Siddiq E (2005) Use of anchored (AG), and (GA), primers to assess genetic diversity of Indian landraces. Curr Sci 89(8):1371–1381
Semagn K, Bjørnstad Å, Ndjiondjop M (2006) An overview of molecular marker methods for plants. Afr J Biotechnol 5(25):2540–2568
Sheath P, Sokal R (1973) Numerical taxonomy-the principles and practice of numerical classification. WH Freeman & Co, San Francisco, p 573
Singh A, Smartt J, Simpson C, Raina S (1998) Genetic variation vis-a-vis molecular polymorphism in groundnut, Arachis hypogaea L. Genet Resour Crop Evol 45(2):119–126
Singh S, Panda MK, Nayak S (2012) Evaluation of genetic diversity in turmeric (Curcuma longa L.) using RAPD and ISSR markers. Ind Crops Prod 37(1):284–291
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Tonk FA, Giachino RRA, Sonmez C, Yuce S, Bayram E, Telci I, Furan M (2011) Characterization of various Hypericum perforatum clones by hypericin and RAPD analyses. Int J Agric Biol 13(1):31–37
Verotta L (2002) Are acylphloroglucinols lead structures for the treatment of degenerative diseases. Phytochem Rev 1(3):389–407
Wang X, Zhao F, Hu Z, Critchley AT, Morrell SL, Duan D (2008) Inter–simple sequence repeat (ISSR) analysis of genetic variation of Chondrus crispus populations from North Atlantic. Aq Bot 88(2):154–159
Zhao Y, Chen XY, Wang XR, Pian RQ (2007) ISSR analysis of genetic diversity among Lespedeza bicolor populations. J Plant Genet Resour 8:195–199
Zong M, Liu HL, Qiu YX, Yang SZ, Zhao MS, Fu CX (2008) Genetic diversity and geographic differentiation in the threatened species Dysosma pleiantha in China as revealed by ISSR analysis. Biochem Genet 46(3–4):180–196
Acknowledgments
We are very grateful to Dr. Akramian (University of Arak, medicinal plant department) for his assistance in collection and identification of samples. This research work was supported by grants from Iran national science foundation which is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morshedloo, M.R., Moghadam, M.R.F., Ebadi, A. et al. Genetic relationships of Iranian Hypericum perforatum L. wild populations as evaluated by ISSR markers. Plant Syst Evol 301, 657–665 (2015). https://doi.org/10.1007/s00606-014-1103-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1103-z