Abstract
An electrochemical magnetic immunosensing strategy was developed for the determination of HER2-ECD, a breast cancer biomarker, and breast cancer cells in human serum. A sandwich assay was performed on carboxylic acid-functionalized magnetic beads (MBs) using a screen-printed carbon electrode (SPCE) as transducer surface. The affinity process was detected using electroactive labels; core/shell streptavidin-modified CdSe@ZnS Quantum Dots (QDs). Cd2+ ions, released from the QDs, were determined by differential pulse anodic stripping voltammetry (DPASV). An assay time of 90 min, with an actual hands-on time of about 20 min, a linear range between 0.50–50 ng·mL−1 of HER2-ECD and a limit of detection of 0.29 ng·mL−1 were achieved. Analysis of live breast cancer cells was also performed using the optimized assay. Breast cancer cell lines SK-BR-3 (a HER2-positive cell line), MDA-MB-231 (a HER2-negative cell line) and MCF-7 (a cell line with low HER2 expression) were tested. The selectivity of the assay towards SK-BR-3 cells was confirmed. A concentration-dependent signal that was 12.5× higher than the signal obtained for the HER2-negative cells (MDA-MB-231) and a limit of detection of 2 cells·mL−1 was obtained.

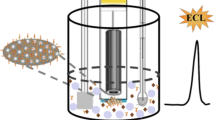
Schematic representation of the electrochemical immunomagnetic assay for the determination of the breast cancer biomarker HER2-ECD and cancer cells using magnetic beads (MBs), a screen-printed carbon electrode (SPCE) as transducer surface and quantum dots (QD) as electroactive labels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer screening programs are implemented at the national level to reduce mortality rates [1]. Nevertheless, the limited sensitivity and the lack of specific results in the traditional diagnosis can significantly lead to unfavourable clinical outcomes [2]. The absence of non-invasive methodologies for accurate determination of carcinogenic biomarkers in the bloodstream is a real concern. Human epidermal growth factor receptor 2 (HER2) is a cancer biomarker overexpressed in primary cancer cases [3]. The tumour expression is dichotomized into HER2-positive and HER2-negative groups [4]. In this field, nanotechnological devices can be used to measure the protein expression in biological fluids (serum, plasma) from screening to follow-up. The assessment of the concentration of the extracellular domain of HER2 (HER2-ECD) in serum samples is a prominent alternative since this domain can be cleaved from the surface of cancer cells by matrix metalloproteases and released into the bloodstream [5]. In addition, the assessment of HER2-positive circulating tumour cells (CTCs) is fundamental for patients that can benefit from personalized therapies and adjuvant treatment [6].
Electrochemical strategies for the determination of HER2 in human serum have been reported using distinct and elaborate electrode surface modifications based on sumptuous structures (nano, micro- and/or magnetic-based materials) to improve the transducer’s performance [7]. Nevertheless, the synthesis of these materials and their functionalization require extensive and time-consuming procedures. In contrast, less innovation has been focused on the detection strategy. Distinct probes or labels to amplify the electrochemical signal are required since the redox pair [Fe(CN)6]3−/4− is still one of the most reported [8,9,10,11,12]. Although silver [13,14,15,16], 1-Naphtol (1-NP) [17], 3,3′,5,5’-Tetramethyl[1,1′-biphenyl]-4,4′-diamine (TMB) [18] and molybdate [19, 20] were employed for the determination of HER2, the use of nanomaterials has not extensively been studied.
Nanoparticle-based signal amplification has attracted considerable attention in immunosensing strategies, since higher sensitivity and lower limits of detection can be achieved [21]. Quantum dots (QDs) are crystalline nanoparticles that revealed to be promising candidates as electrochemical detection labels in breast cancer assays due to the good electroactivity of the employed metals [22,23,24]. QDs can be analysed by stripping voltammetry after preconcentration of the metal on the electrode surface. The obtained signal can then be related to the analyte concentration [25, 26].
Bioassays using QDs as semiconductor nanolabels and magnetic particles as a platform for the targeted immobilization of antibodies constitute a versatile solution for the analysis of biomarkers in clinical practice. In the present work a magnetic immunoassay was developed using carboxylic acid-functionalized magnetic beads (COOH-MBs) onto which capture antibodies were covalently bound, allowing their oriented immobilization. This was followed by the addition of a solution containing the analyte (HER2-ECD or cancer cells) and a biotinylated detection antibody. Then streptavidin-coated core/shell CdSe@ZnS QDs were linked to the previously formed immunocomplex, and the determination was performed by differential pulse anodic stripping voltammetry (DPASV) on a screen-printed carbon electrode (SPCE). This is the first electrochemical magnetic bioassay for the analysis of HER2-ECD using a MB platform and core/shell CdSe@ZnS QDs as electroactive labels. The electrochemical analysis of HER2-positive (SK-BR-3), HER2-negative (MDA-MB-231) and low-expression HER2 cancer cells (MCF-7) using the present methodology is also new.
Experimental
Apparatus
The SEM images were obtained using FEI Quanta 400FEG ESEM/EDAX Genesis X4 M equipment at the “Centro de Materiais da Universidade do Porto (CEMUP)”. Cancer cells were imaged by a Nikon TMS microscope and counted using an automated cell counter (Countess™, ThermoFisher Scientific - Invitrogen, USA, www.thermofisher.com/invitrogen). Screen-printed carbon electrodes (with a 4-mm working electrode (WE) (electroactive area: 0.079 cm2), a silver pseudoreference electrode and a carbon counter electrode (SPCE, DRP-110)) and the specific connector (DRP-CAC) were supplied by Metrohm DropSens (Spain, www.dropsens.com). Electrochemical measurements were carried out with a potentiostat/galvanostat (Autolab PGSTAT204, Metrohm Autolab, The Netherlands, www.metrohm-autolab.com) controlled by the NOVA software package v.1.10 (Metrohm Autolab). Magnetic separations were carried out using a DynaMag™-2 magnet (ThermoFisher Scientific, USA, www.thermofisher.com). Ultra-pure water (resistivity = 18.2 MΩ.cm) used throughout the work was obtained from a Millipore water purification system (Simplicity 185) (Germany, www.merckmillipore.com).
Reagents and solutions
The chemical and biological reagents are indicated in the Electronic Supporting Material (ESM, Section S1). Buffers and working solutions of the antibodies, antigens and QD-Strep were prepared daily. The following buffers were used: MES buffer (0.1 M, pH 6) for the ECD/NHS (200 mM/50 mM) and the capture antibody (Ab-C, various concentrations) solutions, PBS buffer (0.02 M, pH 8) for the EA (1 M) solution, Tris buffer (0.1 M, pH 7.4) for the QD-strep (various concentrations), containing BSA (0.5% m/V), solutions, and acetate buffer (0.1 M, pH 4.5) to prepare the Bi(III) (1.0 mg·L−1) solution. Tween 20 (0.01%) was added to the distinct buffers to be used in the washing steps.
Cell culture and CTC analysis
Breast cancer cell lines MDA-MB-231, MCF-7 and SK-BR-3 were cultured in RPMI medium supplemented with 10 and 15% inactivated FBS, respectively, and 1% antibiotic/antimycotic solution. Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2–95% air. The culture medium was changed every 2–3 days, and the culture was split every 7 days. For analysis, cells were seeded on 21-cm2 plastic cell culture dishes (TPP®, Trasadingen) and were used after 2–5 days in culture (90–100% confluence). On the day of the experiment, the cells were harvested with Trypsin-EDTA 0.25% and counted using the automated cell counter. A trypan-blue exclusion assay was performed to confirm cell viability, which was between 87 and 96% for all the cell lines. The distinct solutions of the cells (1 × 102–1 × 105 cells·mL−1) were prepared in human serum and analysed using the optimized immunoassay.
Electrochemical immunomagnetic bioassay for the analysis of HER2-ECD and living cells
A schematic representation of the sandwich immunoassay (Scheme 1) elucidates the distinct steps of the experimental procedure (note that steps (i) to (v) were performed in 0.5-mL microtubes). All the steps of the assay were performed protected from light at room temperature using vortex stirring (950 rpm). Magnetic separations were carried out using the DynaMag™-2 magnet.
Schematic representation of the magnetic immunoassay. 1 The MBs are first biomodified (i activation with EDC/NHS, ii addition of capture antibody, iii blocking with EA). 2 The magnetic assay is performed through the addition of iv a mixture containing the antigen (HER2-ECD or cancer cells) and the biotinylated antibody, followed by the addition of v a QD-strep solution. The magnetic beads are magnetically attracted to the working electrode. 3 Electrochemical determination of the released Cd2+ is performed. Voltammograms of increasing HER2-ECD concentrations are exemplified.
Prior to the biomodification, the MBs stock solution was washed with MES-T for 2 min.
- (1)
The biomodification consisted of the following steps: (i) a 1.5-μL aliquot of the MBs solution (containing 7.5 μg of MBs) was resuspended in the EDC/NHS solution (100 μL, 15 min) and (ii) incubated with Ab-C (25 μg·mL−1, 100 μL, 1 h). After a washing step with MES-T and PBS-T (2 min each), the particles’ free surfaces were (iii) blocked with EA (100 μL, 10 min). The biomodified particles were resuspended in 100 μL PBS-T and kept at 4 °C until use.
- (2)
The magnetic immunoassay consisted of the following: (iv) The analyte solution (protein biomarker or cancer cells) was previously mixed with Ab-D (2.0 μg·mL−1) in human serum (10 min before use). The prepared mixture was then added to the biomodified MBs (100 μL, 1 h). Afterwards, a washing step with Tris-T was performed and (v) the MBs were incubated with a QD-strep solution (5.0 nM, 70 μL, 30 min).
- (3)
Electrochemical analysis: prior to the measurements, a washing step was performed with ultra-pure water. Then, a 10-μL aliquot of the final solution was placed on the WE of an SPCE under which a magnet (d = 4 mm) was placed. The SPCE was totally dried with a nitrogen flow, and 5 μL of the HCl (1 M) and 40 μL of the Bi(III) (1.0 mg·L−1) solutions were added to the electrode surface to release Cd2+ from the QDs. DPASV voltammograms were recorded using the following conditions: constant potential of + 1.00 V during 60 s, followed by a potential of − 1.10 V for 300 s (in this phase the released cadmium ions were pre-concentrated and a bismuth film was formed). The potential was swept from − 1.00 V to − 0.70 V to strip the cadmium into the solution, recording the electrochemical signal. (DPV parameters, pulse amplitude, 0.05 V; step potential, 0.01 V; modulation time, 0.01 s; interval time, 0.1 s).
Results and discussion
Electrochemical behaviour of the quantum dots on distinctly modified SPCE surfaces
To elaborate the immunomagnetic assay, prior measurements were performed to study the electrochemical behaviour of cadmium (released from the QDs) using DPASV. In the tested procedure, cadmium was deposited simultaneously with a bismuth film which is considered an adequate alternative to the traditional mercury film [27]. Distinct carboxylic acid-functionalized materials were tested using an SPCE as transducer: multi-walled carbon nanotubes (HOOC-MWCNT, 1 μg·mL−1), PEG gold-coated magnetic nanoparticles (HOOC-Fe3O4@AuNP, 30 μg) and magnetic beads (HOOC-MBs, 30 μg). The sensing platforms were employed to analyse a 50-ng·mL−1 HER2-ECD solution, and the obtained signals were compared with the ones obtained with a bare SPCE, using concentrations of Ab-C 25 μg·mL−1, Ab-D 2.0 μg·mL−1, QD 10 nM, HCl 1 M and Bi(III) 1.0 mg·L−1. The respective SEM images and obtained voltammograms are presented in Fig. 1. The functionalization of nano- and micromaterials with carboxylic acid groups commonly leads to an efficient and well-organized attachment of the antibody, allowing the construction of a stable and functional immunoassay [7, 28]. However, when the SPCE is modified with MWCNTs, the obtained peak current intensity was considerably lower than for the unmodified (bare) electrode, as can be seen in Fig. 1a (1 and 2). Despite the wide application of carbon-based nanomaterials in the construction of electrochemical immunosensors, in this work, the modification of the transducer with MWCNT proved to be inappropriate. As described in the literature, the electrode modification with carbon nanomaterials leads to higher adsorption of proteins on the transducer’s surface, increasing the effect of the electrical double layer [29]. On the other hand, the use of both magnetic nano- and microparticles leads to an increase in the current intensity. This is probably due to an efficient immobilization of the capture antibodies on these particles, as well as the specific orientation, which is not as effective on the SPCE-MWCNT surface. Although other studies report the construction of interesting magnetic assays using core/shell nanoparticles, in the present work, the electrochemical signal is noticeably higher when (micro-sized) MBs were used (Fig. 1a (3 and 4, respectively)). In fact, the SEM images (Fig. 1b (3 and 4)) support the results since it is observed that the MBs are arranged in an organized layer that can contribute to a more sensitive method. In contrast, the core/shell nanoparticles are not as homogeneous in size and are dispersed on the electrode’s surface.
Thus, considering that the MBs are the best sensing surface, further studies were carried out to evaluate the possibility of improving the detection strategy. The electrochemical response of the QDs was verified by adding HCl and Bi(III) in four different strategies (ESM, Section S2). The best results were obtained by placing the biomodified-MBs on the WE followed by the addition of the HCl and Bi(III) solutions.
Optimization of the immunosensing strategy
The following experimental parameters that affect the immunoassay’s performance were optimized: (a) assay format, (b) QD concentration, (c) MB amount and (d) Ab concentrations. Respective text and figures regarding the optimizations are included in the ESM (Section S3). The parameters selected to test the immunomagnetic assay for the analysis of HER2-ECD and cancer cells in human serum are presented in Table S1. In short, the following experimental conditions were found to give the best results: MBs 7.5 μg, Ab-C 25 μg·mL−1, Ab-D 2.0 μg·mL−1 and QDs 5.0 nM, using a previously prepared mixture of the antigen and Ab-D (Format B). Under these conditions, the average amount of antibody per MB was 0.188 pg.
Analytical performance and stability of the modified magnetic beads
The optimized method was used to analyse the cancer biomarker in human serum. The precision of the results was evaluated using a 50-ng·mL−1 HER2-ECD solution. Relative standard deviations (RSD) for the repeatability and reproducibility (n = 3) were 2.7 and 3.1%, respectively, demonstrating that the immunomagnetic assay provides precise results.
For calibration purposes, HER2-ECD solutions with increasing concentrations were prepared in human serum (0.50, 1.0, 2.5, 5.0, 10, 25, 50 and 100 ng·mL−1) and analysed with the magnetic immunoassay. The linear range was established between 0.50 and 50 ng·mL−1 (ip = 0.75 ± 0.03 [HER2-ECD] + 4.99 ± 0.58, r = 0.997) with a sensitivity of 9.5 μA·mL·ng−1·cm−2. The calibration plot and examples of differential pulse anodic stripping voltammograms in the linear range are shown in Fig. 2. Limits of detection (LOD = 3 s/m) and quantification (LOQ = 10s/m) were calculated from the calibration straight: “s” is the standard deviation of the blank and “m” is the slope. The values obtained were LOD = 0.29 ng·mL−1 and LOQ = 0.96 ng·mL−1. The obtained LOD was more than 50× lower than the cut-off value (15 ng·mL−1) established for HER2-ECD. The coefficient of variation of the method (Vx0) was 1.27%, demonstrating that the precision of the method is adequate (Vx0 < 5%). Additional figures of merit of the magnetic immunoassay are indicated in Table S2.
Calibration plot for the analysis of HER2-ECD in human serum (ip vs. HER2-ECD concentration) using the magnetic immunoassay. Inset: Differential pulse anodic stripping voltammograms of [HER2-ECD] (a-g); 0, 0.50, 1.0, 2.5, 5.0, 10, 25 and 50 ng·mL−1. (Ab-C 25 μg·mL−1; Ab-D 2.0 μg·mL−1; QDs 5.0 nM. DPASV parameters: preconcentration + 1.00 V for 60 s and − 1.10 V for 300 s; potential scan − 1.00 V to − 0.70 V; pulse amplitude 0.05 V; step potential 0.01 V; modulation time 0.01 s; interval time 0.1 s)
To assess the selectivity of the bioassay, other cancer protein biomarkers were tested, including breast cancer antigen 15–3 (CA15–3, 100 U·mL−1), liver cancer biomarker alpha-Fetoprotein (AFP, 1 μg·mL−1) and a possible serum interferent, human serum albumin (HSA, 100 mg·mL−1). Concentrations were chosen based on values that can be expected in real situations. The results demonstrated the high selectivity of the assay for HER2-ECD since the signals of the tested interferents are similar to the blank signal (Fig. 3a).
To assess the accuracy of the assay’s results both recovery experiments and the use of a reference method (ELISA) were performed. For the recovery tests non-diluted human serum was spiked with distinct HER2-ECD concentrations (1.0, 2.5, 10, 20 and 50 ng·mL−1). Recoveries between 99.8 and 108.0% were obtained (Table 1), indicating that the assay provides accurate results and can successfully be applied to real samples. Experimental specifications of the ELISA are provided in the ESM (Section S.1.2). Spiked serum samples with HER2-ECD concentrations between 0.5 and 50 ng·mL−1 were analysed. A 4-fold dilution was required for the ELISA to ensure that the HER2-ECD was within the linear range. The electrochemical assay did not require any dilution. Table 1 shows the obtained results. The relative deviations were below 10% for concentrations below the cut-off value, which confirms the accuracy of the results. Although the use of the ELISA kit allows the determination of very low concentrations of the biomarker with an elevated precision, a serial dilution is required since direct analysis leads to values outside the range of concentrations in real cases. Furthermore, the ELISA has a very narrow linear range (Fig. S1) and much longer assay (4 h 45 min) and hands-on times (1 h 20 min) than the assay described in this work (1 h 30 min and 20 min, respectively). The results demonstrate that the performance of the immunomagnetic assay can be compared to that of the ELISA, and it provides an important diagnostic tool for cancer biomarker analysis. Because of the huge importance of screening and early diagnosis, the new analytical methodology can be adequate for patient follow-up.
In addition, the stability of the MBs was also studied. The biomodified MBs were stored at 4 °C in PBS-T, and the signal was monitored during several weeks, using 0 and 50 ng·mL−1 HER2-ECD solutions. No significant differences in the analytical signals were observed for at least 60 days; the signal was 99.5% of the initial signal (Fig. 3b).
Analysis of live breast cancer cells
The electrochemical magnetic immunoassay was also used to detect live breast cancer cell-lines: the HER2-positive SK-BR-3, the HER2-negative MDA-MB-231 and the low HER2-expression MCF-7 cell lines. The selectivity of the assay towards the SK-BR-3 cell line was assessed by comparing the signals obtained with the ones obtained for the MDA-MB-231 and MCF-7 cell lines. In Fig. 4a can be observed that SK-BR-3 provided a concentration-dependent signal that was 12.5× higher than the signal obtained for HER2-negative cells MDA-MB-231 and 4× higher than the signal obtained for the MCF-7 cells, confirming the selectivity of the assay for HER2-positive cells. So, the SK-BR-3 cells that overexpress HER2 can easily be captured by the Ab-C. This explains the higher analytical signal because of the higher amount of cells that were captured. The calibration straight for SK-BR-3 cells was established in the range between 1 × 102–5 × 103 cells·mL−1 (ip = 0.15 ± 0.02 log[SK-BR-3] – 0.20 ± 0.06, r = 0.983) (Fig. 4b), obtaining a limit of detection of 2 cells·mL−1. Additional figures of merit of the magnetic immunoassay for the analysis of SK-BR-3 are indicated in Table S2.
Results obtained for cancer cell analysis. a Selectivity study of the magnetic immunoassay in the presence of 1 × 105 cells·mL−1 for SK-BR-3, MDA-MB-231 and MCF-7 cells. b Results obtained for distinct concentrations of HER2-positive cancer cells SK-BR-3 in human serum. Inset: Differential pulse anodic stripping voltammograms of [SK-BR-3] (a–e) 0, 1 × 102, 5 × 102, 1 × 103 and 5 × 103 cells·mL−1. DPASV parameters: preconcentration + 1.00 V for 60 s and − 1.10 V for 300 s, potential scan − 1.00 V to − 0.70 V, pulse amplitude 0.05 V, step potential 0.01 V, modulation time 0.01 s, interval time 0.1 s
The magnetic immunoassay was compared with other electrochemical bioassays for the determination of HER-positive cancer cells [30,31,32,33,34,35] (Table 2). Although an electrochemical nanobiosensor for the detection of SK-BR-3 cell line in a 30-min test has been described in the literature [31], the time required for both the preparation of the transducer and for the modification with the biorecognition element is high (> 16 h), and the use of costly materials leads to an expensive analysis. Comparatively, the present work reports a simplified experimental procedure with a total time for both the sensing surface preparation and the assay of less than 3 h.
One of the limitations of the assay is the use of a heavy metal for detection purposes. Therefore, we minimized the used quantities: 70 μL of a 5.0-nM solution, so only 0.35 pmol of QDs are used in each assay. The generated waste can selectively be collected and sent to a certified entity operating in accordance with European standards regarding waste management. A way of replacing the present strategy by a non-polluting material is by using, for example, enzymes. However, enzymes can lose their bioactivity and the method includes an additional step (the addition of the enzymatic substrate). This leads to a longer assay time. The assay can be optimized further by using a detection antibody that is directly labelled with the QD. Like this one of the steps of the assay can be eliminated, reducing the total assay- and hands-on times.
Conclusion
Determination of HER2-ECD and cancer cells in biological samples is critical for an efficient diagnosis and follow-up of (eventually) HER2-positive breast cancer. With an assay time of 90 min, a sensitive immunomagnetic assay was developed using MBs and SPCE as transducer surface and core/shell CdSe@ZnS QDs as electroactive labels. The Cd2+ ions, released through acid dissolution of the QDs, were analysed by DPASV. Human serum samples were used to test the sensor’s applicability, and the selectivity was confirmed through the analysis of other cancer biomarkers and possible serum interferents. The immunomagnetic assay was further successfully applied to the determination of the HER2-positive SK-BR-3 breast cancer cell line and of HER2-negative (MDA-MB-231) and low-expression HER2 (MCF-7) breast cancer cell lines. The SK-BR-3 cell line provided a concentration-dependent signal that was more than 12.5× higher than the signal obtained for the other cells. The bioassay proved to be a fast, reliable and specific analytical tool for measuring tumour markers in cancer patients’ serum.
References
Basu P, Ponti A, Anttila A, Ronco G, Senore C, Vale DB, Segnan N, Tomatis M, Soerjomataram I, Primic Žakelj M, Dillner J, Elfström KM, Lönnberg S, Sankaranarayanan R (2018) Status of implementation and organization of cancer screening in the European Union member states—summary results from the second European screening report. Int J Cancer 142:44–56. https://doi.org/10.1002/ijc.31043
Mittal S, Kaur H, Gautam N, Mantha AK (2017) Biosensors for breast cancer diagnosis: a review of bioreceptors, biotransducers and signal amplification strategies. Biosens Bioelectron 88:217–231. https://doi.org/10.1016/j.bios.2016.08.028
Sareyeldin RM, Gupta I, Al-Hashimi I et al (2019) Gene expression and miRNAs profiling: function and regulation in human epidermal growth factor receptor 2 (HER2)-positive breast cancer. Cancers 11:646. https://doi.org/10.3390/cancers11050646
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane L, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36:2105–2122. https://doi.org/10.1200/JCO.2018.77.8738
Perrier A, Gligorov J, Lefèvre G, Boissan M (2018) The extracellular domain of Her2 in serum as a biomarker of breast cancer. Lab Investig 98:696–707. https://doi.org/10.1038/labinvest.2012.91
Mamdouhi T, Twomey JD, McSweeney KM, Zhang B (2019) Fugitives on the run: circulating tumor cells (CTCs) in metastatic diseases. Cancer Metastasis Rev 38:297–305. https://doi.org/10.1038/s41374-018-0033-8
Freitas M, Nouws HPA, Delerue-Matos C (2018) Electrochemical biosensing in Cancer diagnostics and follow-up. Electroanalysis 30:1576–1595. https://doi.org/10.1002/elan.201800193
Sharma S, Zapatero-Rodríguez J, Saxena R, O'Kennedy R, Srivastava S (2018) Ultrasensitive direct impedimetric immunosensor for detection of serum HER2. Biosens Bioelectron 106:78–85. https://doi.org/10.1016/j.bios.2018.01.056
Pacheco JG, Rebelo P, Freitas M et al (2018) Breast cancer biomarker (HER2-ECD) detection using a molecularly imprinted electrochemical sensor. Sens Actuator B-Chem 273:1008–1014. https://doi.org/10.1016/j.snb.2018.06.113
Arkan E, Saber R, Karimi Z, Shamsipur M (2015) A novel antibody-antigen based impedimetric immunosensor for low level detection of HER2 in serum samples of breast cancer patients via modification of a gold nanoparticles decorated multiwall carbon nanotube-ionic liquid electrode. Anal Chim Acta 874:66–74. https://doi.org/10.1016/j.aca.2015.03.022
Emami M, Shamsipur M, Saber R, Irajirad R (2014) An electrochemical immunosensor for detection of a breast cancer biomarker based on antiHER2–iron oxide nanoparticle bioconjugates. Analyst 139:2858–2866. https://doi.org/10.1039/c4an00183d
Rostamabadi PF, Heydari-Bafrooei E (2019) Impedimetric aptasensing of the breast cancer biomarker HER2 using a glassy carbon electrode modified with gold nanoparticles in a composite consisting of electrochemically reduced graphene oxide and single-walled carbon nanotubes. Microchim Acta 186:495. https://doi.org/10.1007/s00604-019-3619-y
Freitas M, Nouws HPA, Delerue-Matos C (2019) Electrochemical sensing platforms for HER2-ECD breast Cancer biomarker detection. Electroanalysis 31:121–128. https://doi.org/10.1002/elan.201800537
Chocholova E, Bertok T, Lorencova L et al (2018) Advanced antifouling zwitterionic layer based impedimetric HER2 biosensing in human serum: Glycoprofiling as a novel approach for breast cancer diagnostics. Sens Actuator B-Chem 272:626–633. https://doi.org/10.1016/j.snb.2018.07.029
Shamsipur M, Emami M, Farzin L, Saber R (2018) A sandwich-type electrochemical immunosensor based on in situ silver deposition for determination of serum level of HER2 in breast cancer patients. Biosens Bioelectron 103:54–61. https://doi.org/10.1016/j.bios.2017.12.022
Marques RCB, Viswanathan S, Nouws HPA, Delerue-Matos C, González-García MB (2014) Electrochemical immunosensor for the analysis of the breast cancer biomarker HER2 ECD. Talanta 129:594–599. https://doi.org/10.1016/j.talanta.2014.06.035
Al-Khafaji QAM, Harris M, Tombelli S et al (2012) An electrochemical immunoassay for HER2 detection. Electroanalysis 24:735–742. https://doi.org/10.1002/elan.201100501
Tallapragada SD, Layek K, Mukherjee R, Mistry KK, Ghosh M (2017) Development of screen-printed electrode based immunosensor for the detection of HER2 antigen in human serum samples. Bioelectrochemistry 118:25–30. https://doi.org/10.1016/j.bioelechem.2017.06.009
Shen C, Liu S, Li X, Zhao D, Yang M (2018) Immunoelectrochemical detection of the human epidermal growth factor receptor 2 (HER2) via gold nanoparticle-based rolling circle amplification. Microchim Acta 185:547–548. https://doi.org/10.1007/s00604-018-3086-x
Chai Y, Li X, Yang M (2019) Aptamer based determination of the cancer biomarker HER2 by using phosphate-functionalized MnO 2 nanosheets as the electrochemical probe. Microchim Acta 186:316–316. https://doi.org/10.1007/s00604-019-3412-y
Pastucha M, Farka Z, Lacina K, Mikušová Z, Skládal P (2019) Magnetic nanoparticles for smart electrochemical immunoassays: a review on recent developments. Microchim Acta 186:312–326. https://doi.org/10.1007/s00604-019-3410-0
Wu X, Xiao T, Luo Z, He R, Cao Y, Guo Z, Zhang W, Chen Y (2018) A micro−/nano-chip and quantum dots-based 3D cytosensor for quantitative analysis of circulating tumor cells. J Nanobiotechnol 16:65. https://doi.org/10.1186/s12951-018-0390-x
Boriachek K, Islam MN, Gopalan V et al (2017) Quantum dot-based sensitive detection of disease specific exosome in serum. Analyst 142:2211–2219. https://doi.org/10.1039/c7an00672a
Fernández-Delgado N, Herrera M, Tavabi AH et al (2018) Structural and chemical characterization of CdSe-ZnS core-shell quantum dots. Appl Surf Sci 457:93–97. https://doi.org/10.1016/j.apsusc.2018.06.149
Martín-Yerga D (2019) Electrochemical detection and characterization of nanoparticles with printed devices. Biosens 9:49. https://doi.org/10.3390/bios9020047
Freitas M, Neves MMPS, Nouws HPA, Delerue-Matos C (2020) Quantum dots as nanolabels for breast cancer biomarker HER2-ECD analysis in human serum. Talanta 208:120430. https://doi.org/10.1016/j.talanta.2019.120430
Economou A (2005) Bismuth-film electrodes: recent developments and potentialities for electroanalysis. TrAC - Trends Anal Chem 24:334–340. https://doi.org/10.1016/j.trac.2004.11.006
Campuzano S, Yáñez-Sedeño P, Pingarrón JM (2018) Current trends and challenges in bioelectrochemistry for non-invasive and early diagnosis. Curr Opin Electrochem 12:81–91. https://doi.org/10.1016/j.coelec.2018.04.015
Martín-Yerga D, González-García MB, Costa-García A (2013) Biosensor array based on the in situ detection of quantum dots as electrochemical label. Sens Actuator B-Chem 182:184–189. https://doi.org/10.1016/j.snb.2013.03.004
Freitas M, Nouws HPA, Keating E, Delerue-Matos C (2020) High-performance electrochemical immunomagnetic assay for breast cancer analysis. Sens Actuator B-Chem 308:127667. https://doi.org/10.1016/j.snb.2020.127667
Salahandish R, Ghaffarinejad A, Naghib SM et al (2018) Nano-biosensor for highly sensitive detection of HER2 positive breast cancer. Biosens Bioelectron 117:104–111. https://doi.org/10.1016/j.bios.2018.05.043
Zhu Y, Chandra P, Shim YB (2013) Ultrasensitive and selective electrochemical diagnosis of breast cancer based on a hydrazine-au nanoparticle-aptamer bioconjugate. Anal Chem 85:1058–1064. https://doi.org/10.1021/ac302923k
Mucelli SP, Zamuner M, Tormen M, Stanta G, Ugo P (2008) Nanoelectrode ensembles as recognition platform for electrochemical immunosensors. Biosens Bioelectron 23:1900–1903. https://doi.org/10.1016/j.bios.2008.02.027
Yadav S, Boriachek K, Islam MN et al (2017) An electrochemical method for the detection of disease-specific Exosomes. ChemElectroChem 4:967–971. https://doi.org/10.1002/celc.201600391
Zhou N, Su F, Li Z, Yan X, Zhang C, Hu B, He L, Wang M, Zhang Z (2019) Gold nanoparticles conjugated to bimetallic manganese(II) and iron(II) Prussian blue analogues for aptamer-based impedimetric determination of the human epidermal growth factor receptor-2 and living MCF-7 cells. Microchim Acta 186:75. https://doi.org/10.1007/s00604-018-3184-9
Acknowledgements
The authors are also thankful to Rui Rocha and CEMUP “Centro de Materiais da Universidade do Porto” for the SEM work.
Funding
The authors are grateful for the financial support from the Fundação para a Ciência e a Tecnologia (FCT) / the Ministério da Ciência, Tecnologia e Ensino Superior (MCTES) through national funds (Portugal) (LAQV - UID/QUI/50006/2019 and CINTESIS, R&D -Unit - UID/IC/4255/2019). Maria Freitas and Virgínia Cruz Fernandes are financially supported by FCT through a PhD grant (SFRH/BD/111942/2015) and a postdoc grant (SFRH/BPD/109153/2015), respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on IX NyNA 2019. International Congress on Analytical Nanoscience and Nanotechnology
This work was presented at the IX NyNA 2019, International Congress on Analytical Nanoscience and Nanotechnology at Zaragoza (Spain) from 2 - 4 July, 2019. Chairman: Dr. Juan R. Castillo.
Electronic supplementary material
ESM 1
(DOCX 611 kb)
Rights and permissions
About this article
Cite this article
Freitas, M., Nouws, H.P.A., Keating, E. et al. Immunomagnetic bead-based bioassay for the voltammetric analysis of the breast cancer biomarker HER2-ECD and tumour cells using quantum dots as detection labels. Microchim Acta 187, 184 (2020). https://doi.org/10.1007/s00604-020-4156-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4156-4