Abstract
The authors describe an adapted rolling circle amplification (RCA) method for the determination of human epidermal growth factor receptor 2 (HER2). This method (which is termed immunoRCA) combines an immunoreaction with DNA based signal amplification. Gold nanoparticles (AuNPs) were loaded with antibodies against HER2 and DNA, and then fulfill the functions of recognizing HER2 and achieving signal amplification. The DNA serves as a primer to trigger RCA. This results in formation of a long DNA containing hundreds of copies of circular DNA sequence on the electrode surface. Then, molybdate is added which reacts with the phosphate group of the long DNA to generate the redox-active molybdophosphate. This, in turn, results in an increased current and, thus, in strongly increased sensitivity of the immunoassay. A linear response is linear relationship between the change of current intensity and the logarithm of the concentration in the range from 1 to 200 pg·mL−1 of HER2, and the detection limit is 90 fg·mL−1 (at an S/N ratio of 3). The method was applied to the determination of HER2 in breast cancer patients serum samples, and the results correlated well with those obtained by an ELISA. The method was further successfully applied to the determination of HER2 in HER2-expressed mouse breast cancer 4 T1 cells. Conceivably, this strategy may be adapted to other DNA amplification methods and also may be used for the determination of other proteins and biomarkers by using the appropriate antibodies.

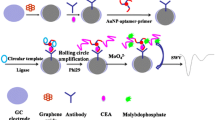
Schematic presentation of an adapted rolling circle amplification (RCA) strategy for the electrochemical detection of human epidermal growth factor receptor 2 (HER2), termed “immunoRCA” utilizing gold nanoparticles (AuNPs). Ab stands for antibody, Phi29 is an E.coli DNA polymerase, dNTP represents deoxynucleotides, and SWV stands for square wave voltammetry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are many biological markers in serum or tissues present at too low a concentration to be detected by conventional immunoassays. Thus, establishing sensitive detection methods and effective signal amplification strategies are of great significance for extremely low level protein detection. Compared to conventional enzyme linked immunosorbent assay (ELISA) [1,3,4,5], fluorescence [4, 6] and chemiluminescence [7, 8], electrochemistry has the advantages of relatively simple instrumentation, high sensitivity and low cost [9].
DNA based signal amplifications have been widely reported and include polymerase chain reaction (PCR) [10, 11], strand displacement amplification (SDA) [12], hybridization chain reaction (HCR) and rolling circle amplification (RCA). Among these methods, RCA is an isothermal technique that does not require expensive and complex equipment for thermal cycling or special conditions to avoid contamination [13,14,15]. Using a single primer, RCA generates hundreds of tandemly linked copies of the circular template DNA within a few minutes.
Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase receptor associated with cancer cell aggressiveness, which is related closely to cancer metastasis [16]. It is often used to determine the prognosis of breast cancer and help doctors to develop accurate treatment plans. HER2 detection is one of the essential testing items for breast cancer patients, because the HER2 positive patients are highly invasive and have higher recurrence rates. HER2 positive patients can employ anti-HER2 therapy by taking target HER2 drug Herceptin to control the tumor growth and improve survival rates effectively [17]. At present, the most commonly used diagnostic methods for HER2 involve fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC) [18, 19]. However, FISH and IHC require invasive biopsy tissue sample, specific instruments and professional technician to complete. In addition, detection of biomarkers in serum and tumor cells has been suggested as prognostic and predictive way for cancer diagnosis and treatment [20]. With this in mind establishing sensitive and accurate method for detection of HER2 in easy access serum and cell is of great significance.
In previous work, we have studied DNA generated electrochemical current based on the reaction of DNA, specifically, the reaction of phosphate groups on DNA with molybdate to form redox molybdophosphate [21, 22]. In this paper, a novel immunoelectrochemical assay is designed combing antibody based immunoreaction with RCA signal amplification, termed “immunoRCA” for detection of HER2. Gold nanoparticles (AuNPs) loaded with antibody and RCA primer are utilized as immunoelectrochemical probe. The assay was established based on a sandwich structure with HER2 specific peptide, HER2 molecules and anti-HER2 antibody loaded AuNPs sequentially assembled onto electrode. In presence of circular DNA, DNA polymerase and nucleotides (DNTPs), RCA was carried out that resulted in a long DNA molecule containing hundreds of copies of the complementary circular DNA sequence. Then, the phosphate groups on the DNA sequence react with molybdate can form redox molybdophosphate precipitate and generate electrochemical current. RCA enhanced the amount of DNA on electrode, and then increased the sensitivity of the assay. After the detection of standard HER2 sample, we applied this immunoelectrochemical assay to detect HER2 in serum samples, which indicated the assay results are well correlated to ELISA kit testing results. In addition, HER2 in 4 T1 cell was also analyzed, and the electrochemical current intensity was increased in accordance with the number of cell tested.
Experimental section
Materials and apparatus
The peptide (CKLRLEWNR) used for capture HER2 was obtained from GL Biochem Ltd. (Shanghai, China, http://www.glbiochem.com/). HER2 protein and HER2-antibody (Ab) were acquired from Abcam Co., Ltd. (Cambridge, MA, https://www.abcam.com/). The primer and padlock probe (the sequences were listed in Supporting Information, Table S1) were synthesized and purified by Sangon Biotech Co., Ltd. (Shanghai, China, http://www.sangon.com/). E.coli DNA Ligase and E.coli DNA Polymerase were purchased from Takara (Dalian, China, http://www.takara.com.cn/). Deoxynucleotides (dNTPs) was purchased from Genview Scientific Inc.(El Monte, CA,http://www.gen-view.com/). Sodium molybdatedihydrate (Na2MoO4·2H2O) and 6-mercapto-1-hexanol (MCH) were boughtd from Sigma-Aldrich (https://www.sigmaaldrich.com/). The human HER2 ELISA kit was obtained from Jining Biotech Co., Ltd. (Shanghai, China, http://shjnsjh.cn.coovee.net/). The human serum samples (six samples from breast cancer patients) were acquired from the Second Xiangya Hospital. All stock solutions were prepared with deionized water.
All electrochemical measurements were performed on CHI-650D electrochemical workstation (Shanghai CH Instruments Co., China). A standard three-electrode system with a working electrode (gold electrode, 2 mm in diameter), a saturated Ag/AgCl reference electrode, and a platinum auxiliary electrode was utilized for electrochemical measurement. Shimadzu UV-2450 spectrophotometer was used to record UV-spectra. FEI Titan G2 60–300 transmission electron microscope was used to examine the morphologies of AuNP and other compounds. Zeta potential was tested on a dynamic light scattering spectrometer (DLS, ZEN3600). For detecting HER2 in cell lysates, the 4 T1 cells were broken with Fisher Model FB120 ultrasonic processor.
Preparation of the gold nanoparticle (AuNP) probe
Preparation of the AuNP probe by modify AuNPs with anti-HER2 antibody and RCA primer. AuNPs were synthesized based on previous literature report [23, 24]. Briefly, 3 mL sodium citrate (w/v, 2%) was added to 100 mL boiling and stirring HAuCl4 (0.01%) solution and kept heated for another 10 min. With continuous stirring until cooled to room temperature, the AuNPs were synthesized. To modify antibody and RCA primer onto AuNP, firstly, antibody was added to AuNP solution to reach a concentration of 500 pg·mL−1 and shaken for 2 h. Then the RCA primer was added to the mixture at a concentration of 1 mM and shaken for 16 h. After that 1 M NaCl was added to the mixture every 10 h successfully for 3 times until the NaCl reached 0.2 M. The solution was aged at room temperature for 24 h and centrifuged (12,000 rpm) for 30 min. Finally, the product was re-dispersed in deionized water. The probe was stored at 4 °C refrigerator before use.
Construction of the immunoelectrochemical assay
The cleaned gold electrode was immersed into 10 μg·mL−1 peptide solution at 4 °C overnight to link HER2 specificity peptide onto the gold electrode. After thorough washing with deionized water and drying with nitrogen, the electrode was blocked by dipped into 1 mM MCH for 50 min to avoid nonspecific adsorption. Then a series of different concentrations of HER2 were dropped onto the electrode for 2 h, followed by adding AuNP probes onto the electrode for another 1 h. Then, to perform RCA, after the electrode was washed, 0.2 μM padlock probes were added onto the electrode for 2 h to hybridize with the primer on the AuNP surface. Next the solution of 2 U E.coli DNA Ligase, 10 × E.coli DNA Ligase buffer (provided along with ligase), 10 × BSA (0.05%) was added onto the electrode at room temperature for 1 h to make the padlock probe form a circular template. Finally, the solution of 1 U E.coli DNA polymerase, 10 × E.coliDNA polymerase buffer (provided along with polymerase) and 1 mM dNTPs were dropped onto electrode for 2 h. After washing again, 5 mM Na2MoO4 solution was dropped onto the electrode and incubated for 25 min before electrochemical detection.
Detection of HER2 in human serum and 4 T1 cell
The serum of breast cancer patients were diluted with water and detected by ELISA kit and our assay.
4 T1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 μg·mL−1 penicillin and 100 μg·mL−1 streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. Then the cultured 4 T1 cells were broken with an ultrasonic processor (the ultrasonic frequency was 20 KHz) for 40 min at an interval of 3 s in ice bath and centrifuged for 10 min. The suspended lysate was collected and diluted to a series of concentrations with saline for the detection.
Results and discussion
Principle of the detection
In this paper, we combined rolling circle amplification (RCA) and traditional immunoreaction, termed “immunoRCA” to build an immunoelectrochemical assay for ultrasensitive detection of HER2. Scheme 1 shows the schematic representation for the synthesis of AuNP based electrochemical probe, for the preparation of the electrochemical assay and voltammetric detection process. Thiolated ss-DNA has a strong affinity for AuNPs and is quickly adsorbed. The antibodies are large molecules and leave enough space on the surface of the AuNPs to allow the subsequent adsorption of RCA primer. Stable AuNP probe was obtained by modifying both HER2 antibody and RCA primer onto AuNPs. The anti-HER2 antibody on AuNP probe was to bind with HER2 that was captured by HER2 specific peptide immobilized on gold electrode surface. Simultaneously, both the 3′-and 5′-ends of the padlock DNA can hybridize to the 3′-end of the primer (The sequences of DNA used in this article were listed in Table S1.), and resulted in a circular template for RCA. The long repeated DNA sequence, as a result of RCA, can react with molybdate, generating electrochemical current and measured by voltammetry.
Immunoelectrochemical detection principle for HER2 with AuNP-based rolling circle amplification reaction. a Modification of AuNPs with HER2 antibody and RCA primer. b Illustration of the preparation process of immunoelectrochemical assay, and detection of HER2 using prolonged DNA, which react with molybdate to generate electrochemical current
Characterization of the AuNP probe synthetic process and the RCA
AuNPs were synthesized by reducing HAuCl4 with trisodium citrate, and then modified with antibody and RCA primer in succession. As shown in Fig. 1a, for UV-Vis characterization, compared to AuNPs (curve a), the modification of HER2 antibody onto AuNPs (curve b) does not bring significant changes to either the intensity or position of the plasma bands. After RCA primer conjugation, the absorption intensity reduced and the absorption position shows a little red shift, from around 518 nm to 524 nm (curve c), indicating AuNPs formed aggregation slightly. This may be attributed to low concentration of antibody and negative charged DNA. The color of solution a is almost the same with solution b, only the color of solution c become a little shallow, which is consistent with the result of UV-Vis spectrum. In addition, we used zeta potential to characterize the synthesis of AuNP probe. It can be seen the AuNPs is negative charged. The modification of low concentration of antibody made the potential of AuNPs become more negative charged. With the conjugation of RCA primer, the negative charge increases obviously (Fig. 1b and Supporting Information, Fig. S1.). The above data suggest the AuNPs was successfully modified with antibody and RCA primer successfully.
Characterization of AuNP probe (a): UV-vis absorption of AuNPs (curve a), AuNPs modified with HER2 antibody (curve b), and AuNPs modified with both antibody and RCA primer (curve c). The insert picture shows the color change of the corresponding solutions under visible light. b: The change of zeta potential during the AuNP modification process
To prove the accomplishment of RCA, we used the formed AuNP probe to initiate RCA in centrifuge tube, and then the morphology and size of the AuNPs were characterized by TEM. As shown in Fig. 2a, AuNPs display uniform sphere structure with size around about 13 nm. After modified by antibody and RCA primer, the size of the AuNPs is increased to around 15 nm (Fig. 2b). As the RCA reaction performed, the diameter of AuNPs is further increased to around 17 nm (Fig. 2c). Electrochemical impedance spectroscopy (EIS) can also provide detailed information about the electrode modification process [25], so we also used EIS to characterize the RCA reaction. In Fig. 2d, we can see that compared to bare gold electrode, the resistance value of the immunoelectrochemical assay for the detection of HER2 is increased (curve a and b) and further enhanced significantly after RCA (curve c), which prove the RCA reaction was performed successfully in both the centrifuge tube and on electrode surface.
Feasibility study and signal amplification by AuNP-based rolling circle amplification (RCA)
According to previous work, phosphate on DNA can react with molybdate and form redox molybdophosphates, which can generate two pairs of redox peaks at around 0.22 V and 0.37 V [26,27,28,29]. To demonstrate the feasibility of the immunoelectrochemical assay for HER2 detection and the efficiency of signal amplification by AuNP-based RCA, the immunoelectrochemical assay was charaterized by cyclic voltammetry (CV) and square wave voltammetry (SWV). As shown in Fig. 3a. There is no redox peak when only sodium molydbate is covered on bare electrode (curve a). However, of the assay for 200 pg·mL−1 HER2 detection, two pairs of relatively weak redox peaks are appeared (curve b), which proved the succesfully capture of the AuNP probe onto the electrode through immunoreaction and the reaction of the primer on AuNP with molydbate. The current intensity is rather weak is because the amount of primer on AuNP is limited. Of the same assay for 200 pg·mL−1 HER2 detection, after RCA, it can be seen the peak current is increased obviously, which is about 4 times higher than that before RCA (curve c). SWV was also utilized to characterize the assay and the results in Fig. 3b are in agreement with the CV results. From the above data, we can conclude that the immunoelectrochemical assay is feasible for detection of HER2, and the RCA can can significantly enhance the sensitiivty of the assay. The reaction time of RCA affects the the performance of RCA, which was optimized to be 2 h (Supporting Information, Fig. S2).
a: CV responses of the immunoelectrochemical assay after react with molybdate: (a) bare electrode; (b) assay for 200 pg·mL−1 HER2 without RCA; (c) assay for 200 pg·mL−1 HER2 with RCA. b: The corresponding SWV responses of the assay. c SWV responses of the assay to different concentrations of HER2, from (a) to (g): 0, 1, 5, 10, 50, 100, 200 pg·mL−1. d The calibration plot. Supporting electrolyte, 0.5 M H2SO4
Analytical performance of immunoelectrochemical assay to HER2
To test the analytical performance of the assay for HER2 detection, a series of different concentrations of HER2 were detected by the immunoelectrochemical assay. As depicted in Fig. 3c, the SWV responses of the assay at 0.2 V is increased with the concentrations of HER2 rising from 0 pg·mL−1 to 200 pg·mL−1. This result is because higher concentration of target HER2 resulted in more AuNP probes captured onto gold electrode through immunoreaction, and then triggered more RCA reaction, leading to higher DNA loading on the electrode. There is a linear relationship between the change of current intensity and logarithm of HER2 concentration in the range from 1 to 200 pg·mL−1 (Fig. 3d with equation of y = 3.9268x + 3.74231, R2 = 0.993). Based on S/N = 3, the detection limit of this immunoelectrochemical assay is calculated as 0.09 pg·mL−1. The sensitivity is high enough for the detection of HER2 in diluted serum samples as normal individuals have a HER2 concentration between 2 and 15 ng·mL−1 in the blood, and breast cancer patients have blood HER2 levels from 15 to 75 ng·mL−1 [21]. On the basis of the above results, the immunoelectrochemical assay demonstrated lower detection limit compared to other assay for HER2 detection reported previously (Table 1).
Selectivity and reproducibility of the immunoelectrochemical assay to HER2
Selectivity and reproducibility are also of great importance for the assay. To test the selectivity of this assay, we selected six proteins including 1 U·mL−1 β-site amyloid precursor protein cleaving enzyme 1 (BACE1), 0.1 ng·mL−1 p53, 0.1 mg·mL−1 glutathione (GSH), 10 μg·mL−1 human immunoglobulin G (human IgG), 2.5 ng·mL−1 carcinoembryonic antigen (CEA) and 1 U·mL−1 protein kinase A (PKA), which exist in human serum. As seen in Fig. 4, compared to 200 pg·mL−1 HER2, the responses of the assay to the above interfering proteins are negligible, which is due to the specific interaction of HER2 with antibody and capture peptide. This result demonstrate that the assay has good selectivity despite the concentration of interferences are much larger than HER2.
In addition, we studied the reproducibility of the assay by testing 10 pg·mL−1 and 50 pg·mL−1 of HER2 independently 3 times. The relative standard deviation (RSD) of the testing results is 3.39% and 2.48%, respectively, indicating the results of assay was quite reproducible.
Detection of HER2 in human serum and 4 T1 cell
To verify the clinical application of our assay, six serum samples were collected from the breast cancer patients and then detected by the assay and commercial HER2 ELISA kit. As seen in Fig. 5a, the linear regression indicates the concentrations of HER2 detected by our assay were in good agreement to the results of ELISA kit. Furthermore, HER2 in 4 T1 cell lysate were also detected by our assay. As shown in Fig. 5b, the current intensity enhanced with the increased of cell number, and the insert photograph proved the change of current is linear with logarithm of cell number within a certain range. These results suggest that the assay can detect HER2 in both serum and cancer cells, indicating the assay has great potential in clinical application.
Conclusion
In summary, we utilized AuNP as the carrier for anti-HER2 antibody and RCA primer to combine RCA with traditional immunodetection to build an immunoelectrochemical assay for ultrasensitive detection of HER2. Based on the AuNP probe, the detection of HER2 was converted to the detection of DNA amount on electrode. Compared to existing methods, this assay has advantages of high sensitivity and low detection limit. With these advantages, the immunoelectrochemical assay can detect very low concentration of HER2 in serum and cancer cell, which has great potential in cancer diagnostics and clinical analysis. However, the RCA process is still a little time consuming. This immnuoRCA strategy can be easily adapted to other DNA signal amplification methods and find wide applications.
References
Li X, Shen C, Yang M, Rasooly A (2018) Polycytosine DNA electric-current-generated Immunosensor for electrochemical detection of human epidermal growth factor receptor 2 (HER2). Anal Chem 90(7):4764–4769
Tallapragada SD, Layek K, Mukherjee R, Mistry KK, Ghosh M (2017) Development of screen-printed electrode based immunosensor for the detection of HER2 antigen in human serum samples. Bioelectrochemistry 118:25–30
Mino-Kenudson M, Chirieac LR, Law K, Hornick JL, Lindeman N, Mark EJ, Cohen DW, Johnson BE, Janne PA, Iafrate AJ, Rodig SJ (2010) A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res 16(5):1561–1571
Stewart RL, Caron JE, Gulbahce EH, Factor RE, Geiersbach KB, Downs-Kelly E (2017) HER2 immunohistochemical and fluorescence in situ hybridization discordances in invasive breast carcinoma with micropapillary features. Mod Pathol 30:1561–1566
Alhalwani AY, Repine JE, Knowles MK, Huffman JA (2018) Development of a sandwich ELISA with potential for selective quantification of human lactoferrin protein nitrated through disease or environmental exposure. Anal Bioanal Chem 410(4):1389–1396
Guo L, Tang T, Hu LS, Yang MH, Chen X (2017) Fluorescence assay of Fe (III) in human serum samples based on pH dependent silver nanoclusters. Sensors Actuators B Chem 241:773–778
He L, Yang H, Xiao P, Singh R, He N, Liu B, Li Z (2017) Highly selective, sensitive and rapid detection of Escherichia coli O157:H7 using duplex PCR and magnetic nanoparticle-based Chemiluminescence assay. J Biomed Nanotechnol 13(10):1243–1252
Ali Z, Wang J, Tang Y, Liu B, He N, Li Z (2016) Simultaneous detection of multiple viruses based on chemiluminescence and magnetic separation. Biomater Sci 5(1):57–66
Zhang B, Liu B, Tang D, Niessner R, Chen G, Knopp D (2012) DNA-based hybridization chain reaction for amplified bioelectronic signal and ultrasensitive detection of proteins. Anal Chem 84:5392–5399
Das J, Ivanov I, Sargent EH, Kelley SO (2016) DNA clutch probes for circulating tumor DNA analysis. J Am Chem Soc 138(34):11009–11016
Gevensleben H, Garcia-Murillas I, Graeser MK, Schiavon G, Osin P, Parton M, Smith IE, Ashworth A, Turner NC (2013) Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin Cancer Res 19(12):3276–3284
He J-L, Wu Z-S, Zhou H, Wang H-Q, Jiang J-H, Shen G-L, Yu R-Q (2010) Fluorescence aptameric sensor for Strand displacement amplification detection of cocaine. Anal Chem 82:1358–1364
Lee J, Icoz K, Roberts A, Ellington AD, Savran CA (2010) Diffractometric detection of proteins using microbead-based rolling circle amplification. Anal Chem 82:197–202
Schweitzer B, Wiltshire S, Lambert J, O'Malley S, Kukanskis K, Zhu Z, Kingsmore SF, Lizardi PM, Ward DC (2000) Immunoassays with rolling circle DNA amplification: a versatile platform for ultrasensitive antigen detection. Proc Natl Acad Sci U S A 97(18):10113–10119
Lizardi PM, Huang X, Zhu Z, Bray-Ward P, Thomas DC, Ward D (1998) Mutation detection and single-molecule counting using isothermal rolling-circle amplificatio. Nat Genet 19:225–232
Okochi M, Koike S, Tanaka M, Honda H (2017) Detection of Her2-overexpressing cancer cells using keyhole shaped chamber array employing a magnetic droplet-handling system. Biosens Bioelectron 93:32–39
Figura NB, Long W, Yu M, Robinson TJ, Mokhtari S, Etame AB, Tran ND, Diaz R, Soliman H, Han HS, Sahebjam S, Forsyth PA, Ahmed KA (2018) Intrathecal trastuzumab in the management of HER2+ breast leptomeningeal disease: a single institution experience. Breast Cancer Res Treat 169(2):391–396
Giovanni P, Suganda D, HongMei R, Lilllian R, HongJun P, Ram S, Dennis JS (2000) Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast Cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 18(21):3651–3664
Agersborg S, Mixon C, Nguyen T, Aithal S, Sudarsanam S, Blocker F, Weiss L, Gasparini R, Jiang S, Chen W, Hess G, Albitar M (2018) Immunohistochemistry and alternative FISH testing in breast cancer with HER2 equivocal amplification. Breast Cancer Res Treat 170(2):321–328
Ding C, Zhang C, Yin X, Cao X, Cai M, Xian Y (2018) Near-infrared fluorescent Ag2S Nanodot-based signal amplification for efficient detection of circulating tumor cells. Anal Chem 90(11):6702–6709
Hu L, Hu S, Guo L, Shen C, Yang M, Rasooly A (2017) DNA generated electric current biosensor. Anal Chem 89(4):2547–2552
Shen C, Zeng K, Luo J, Li X, Yang M, Rasooly A (2017) Self-assembled DNA generated electric current biosensor for HER2 analysis. Anal Chem 89(19):10264–10269
Ji X, Song X, Li J, Bai Y, Yang W, Peng X (2007) Size control of gold nanocrystals in citrate reduction the third role of citrate. J Am Chem Soc 129(45):13939–13948
Nam JM, Thaxton CS, Mirkin CA (2003) Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 301(5641):1884–1886
Arya SK, Zhurauski P, Jolly P, Batistuti MR, Mulato M, Estrela P (2018) Capacitive aptasensor based on interdigitated electrode for breast cancer detection in undiluted human serum. Biosens Bioelectron 102:106–112
Feng K, Liu J, Deng L, Yu H, Yang M (2018) Amperometric detection of microRNA based on DNA-controlled current of a molybdophosphate redox probe and amplification via hybridization chain reaction. Microchim Acta 185(1):28
Xiang W, Wang G, Cao S, Wang Q, Xiao X, Li Ting, Yang M (2018) Coupling antibody based recognition with DNA based signal amplification using an electrochemical probe modified with MnO2 nanosheets and gold nanoclusters: Application to the sensitive voltammetric determination of the cancer biomarker alpha fetoprotein. Microchim Acta 185 335
Xie B, Zhou N, Ding R, Zhao Y, Zhang B, Li T, Yang M (2017) Dual signal amplification strategy for electrochemical detection of platelet-derived growth factor BB. Anal Methods 9(46):6569–6573
Jiang W, Liu L, Zhang L, Guo Q, Cui Y, Yang M (2017) Sensitive immunosensing of the carcinoembryonic antigen utilizing aptamer-based in-situ formation of a redox-active heteropolyacid and rolling circle amplification. Microchim Acta 184(12):4757–4763
Shamsipur M, Emami M, Farzin L, Saber R (2018) A sandwich-type electrochemical immunosensor based on in situ silver deposition for determination of serum level of HER2 in breast cancer patients. Biosens Bioelectron 103:54–61
Sharma S, Zapatero-Rodríguez J, Saxena R, O’Kennedy R, Srivastava S (2018) Ultrasensitive direct impedimetric immunosensor for detection of serum HER2. Biosens Bioelectron 106:78–85
Tian S, Zeng K, Yang A, Wang Q, Yang M (2017) A copper based enzyme-free fluorescence ELISA for HER2 detection. J Immunol Methods 451:78–82
Capobianco JA, Shih WY, Yuan QA, Adams GP, Shih WH (2008) Label-free, all-electrical, in situ human epidermal growth receptor 2 detection. Rev Sci Instrum 79(7):076101
Gohring JT, Dale PS, Fan X (2010) Detection of HER2 breast cancer biomarker using the opto-fluidic ring resonator biosensor. Sensors Actuators B Chem 146(1):226–230
Niazi JH, Verma SK, Niazi S, Qureshia A (2015) In vitro HER2 protein-induced affinity dissociation of carbon nanotube-wrapped anti-HER2 aptamers for HER2 protein detection. Analyst 140:243–249
Acknowledgments
The authors thank the support of this work by the National Key Basic Research Program of China (2014CB744502), the National Natural Science Foundation of China (No. 21575165) and the Hunan Provincial Science and Technology Plan Project, China (no.2016TP1007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 136 kb)
Rights and permissions
About this article
Cite this article
Shen, C., Liu, S., Li, X. et al. Immunoelectrochemical detection of the human epidermal growth factor receptor 2 (HER2) via gold nanoparticle-based rolling circle amplification. Microchim Acta 185, 547 (2018). https://doi.org/10.1007/s00604-018-3086-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3086-x