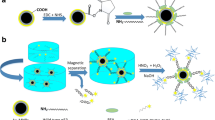
Abstract
A method is presented for chemiluminescence resonance energy transfer (CRET) using APTES-Fe3O4 as a highly efficient energy acceptor with strong magnetic effectiveness over extended distances, while an Au@BSA-luminol composite acts as the donor. In order to boost the chemiluminescence reactions, CuO nanoparticles were successfully employed. The distance between the donor and acceptor is a crucial factor in the occurrence of the CRET phenomenon. A sensitive and high-throughput sandwich chemiluminescence immunosensor has been developed accordingly with a linear range of 1.0 × 10–7 g/L to 6.0 × 10–5 g/L and a limit of detection of 0.8 × 10–7 g/L. The CRET-based sandwich immunosensor has the potential to be implemented to early cancer diagnosis because of its high sensitivity in detecting Nanog, fast analysis (30 min), and simplicity. Furthermore, this approach has the potential to be adapted for the recognition of other antigen–antibody immune complexes by utilizing the corresponding antigens and their selective antibodies.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-radiative (dipole–dipole) energy transfer between a luminescent doner and acceptor is the basis of resonance energy transfer (RET) [1]. These processes are generally categorized as fluorescence RET (FRET), bioluminescence RET (BRET), or chemiluminescence RET (CRET), depending on the nature of the donor (either a fluorophore, bioluminescent protein, or chemiluminescent compound) [2]. The distance between the donor and acceptor plays a crucial role in facilitating chemiluminescent resonance energy transfer (CRET) [3]. CRET-based assays demonstrate high sensitivity and the absence of an external excitation by light results in low background noise of these assays. During the past few decades, there has been increasing focus on chemiluminescence immunoassay (CLIA) as an analytical technique across various fields such as environmental [4], clinical [5], pharmaceutical [6], food [7], and agricultural [8] analysis. This is primarily due to the exceptional sensitivity, ease of use, and broad linear range of these methods [9].
Nanog Homeobox is a gene with a significant role in the maintenance of pluripotency in embryonic stem cells [10]. It is a key transcription factor that helps regulate gene expression pertaining to cell differentiation and self-renewal. Nanog Homeobox is imperative in keeping stem cells in the undifferentiated state and is considered a master regulator of pluripotency. Nanog is the product of the nanog gene situated on chromosome 12 which consists of four exons and three introns [11, 12]. The human Nanog protein is comprised of 305 amino acids with a homeodomain. The estimated molecular weight of Nanog is around 35 kDa [13]. Recent research has established a connection between elevated levels of Nanog in different types of cancers and the regulation of various molecules. This reciprocal relationship has been proven to contribute to the promotion of proliferation, metastasis, invasion, and chemoresistance. Consequently, Nanog acts as an oncogene by inducing stem cell circuitries and fostering heterogeneity within cancer cells [14]. Cancer stem cells (CSCs) or tumor-initiating cells, based on the type of cancer, exhibit varying levels of expression of transcription factors. Elevated levels of these stemness genes are seen in CSCs across various tumor types, playing a crucial role in sustaining the stem cell program [15].
Developing CRET-based detection systems have pivoted around nanomaterials in the recent years including magnetic iron oxide nanoparticles (MNPs), gold nanoparticles (AuNPs) and copper oxide nanoparticles (CuONPs) as the carriers, donors or acceptors [16,17,18,19] and enhancers [20]. Studies have explored the use of magnetic beads (MBs) for developing immunoassays based on CRET for the detection of antigens [21].
Herein, a CRET-based immunoassay using MBs for the detection of Nanog was developed. For the first time, CRET-based (3-aminopropyl) triethoxysilane (APTES)-Fe3O4 nanoparticles and luminol/H2O2/CuONPs were fabricated for the sensitive detection of Nanog antigen. The CRET phenomenon was triggered by close proximity of the Au@BSA-luminol-Ab2 (Nanog antibody) nanocomposites, as the chemiluminescence (CL) light source, and the APTES-Fe3O4-Ab1 (Nanog antibody) in immune complexes formed on the MBs surface, which activates the luminescence of APTES-Fe3O4. The APTES compound is a crucial silane coupling agent extensively employed as a grafting agent to enhance the interfacial behavior of various inorganic oxides such as silica, ceramics, titania, and magnetic iron oxide nanoparticles (MNPs) [22]. MNPs with APTES surface functionalization have particularly been prominent in many biological applications involving antibodies [23]. A less mentioned feature for APTES is its intrinsic fluorescence property [24]. Absorption and emission spectrum studies for APTES and APTES-Fe3O4 showed that not only APTES has intrinsic fluorescence emission, but also the APTES-Fe3O4 nanoparticles have absorption and fluorescence emission. The interaction of APTES with Fe3O4 nanocrystals was assessed by investigating the absorption and emission spectra. The overlap of the absorption spectrum of APTES-Fe3O4NPs with the emission spectrum of luminol-H2O2 led to design a CRET-based diagnostic system.
The CRET energy transfer distance between the donor (Au@BSA-luminol-Ab2-H2O2-CuONPs CL system) and acceptor (APTES-Fe3O4NPs-Ab1) occurs after the sandwich immunocomplex is formed. The Au@BSA nanoparticles have numerous binding sites to accommodate a large number of Ab binding, and they also demonstrate a strong adsorption capacity for luminol molecules. The immunocomplex formation between the antigen (Ag) and the carboxyl-modified APTES-Fe3O4 antibody in the presence of Au@BSA-luminol-Ab2 after magnetic separation was the basis of the determination step. CuONPs acts as the oxidation catalyzer of luminol by H2O2 to yield CL. CuONPs are highly stable and exhibit strong peroxidase-like activity with consistent catalysis under various pH and temperature conditions [25]. CuONPs have been known to highly enhance the CL intensity in luminol-H2O2 systems [26]. Nanog detection was carried out based on the sensitive CL immunoassay using APTES-Fe3O4, luminol and nanomaterials conjugates. Therefore, the target Nanog was sensitively and selectively detected.
Experimental section
Chemicals and materials
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and bovine serum albumin (BSA), PBS buffer (0.1 M, pH 7.4), 3-aminopropyltriethoxysilane (APTES)99%, Ferrous chloridetetrahydrate (FeCl2.4H2O), ammonium hydroxide (NH4OH), copper (II)chloride hexahydrate (Cl2CuH12O6), gold (III) chloride hydrate (HAuCl4⋅4H2O, 99%), luminol (C8H7N3O2) and hydrogen peroxide (H2O2) were attained from Sigma Aldrich. Sodium citrate, glacial acetic acid(C2H4O2), sodium borohydride (NaBH4), and glutaraldehyde (25%) (GA) were purchased from Merck Chemical Co.
The monoclonal antibody anti-Nanog (made in rabbit, 1.3 g/L) (Ab1 and Ab2), and Nanog antigen (Nanog Ag), human epidermal growth factor receptor 2 (HER2), receptor tyrosine kinase-like orphan receptor 1 (ROR1) and prostate-specific antigen (PSA) were attained from Padzacompany (Iran). PBS buffer (0.1 M buffer, pH 7.4) was used for the preparation and dilution of antigens and antibodies.
Apparatus
A LS-55 luminescence spectrometer (Perkin-Elmer, USA) was used for CL spectra measurement. Absorption spectra were acquired using a Perkin Elmer lambda25 spectrometer. The UV–visible spectra were attained using a Perkin Elmer Lambda 35 spectrophotometer. AuNPs were characterized using a transmission electron microscope (TEM) (Zeiss, EM10C, 80 kV, Germany). The TEM samples were prepared by letting a drop of a colloidal solution dry at room temperature on carbon-coated copper grid. A Field emission scanning electron microscope (FE-SEM) (TESCAN, MIRA3, Czech) was used to observe the morphology of the APTES-Fe3O4 nanoparticles samples.
Preparation of Au@BSA nanomaterials and synthesis of luminol and Ab2 labeled Au@BSA (luminol-Au@BSA-Ab2)
The synthesis process utilized AuNPs that were obtained using a previously published method, with small modifications [27,28,29,30]. Briefly, 5 mL trisodium citrate solution (38 mM) was mixed with a 25 mL boiling solution of HAuCl4 (1 mM) under constant stirring. A color change to red wine was witnessed within about 8–10 min as a sign of the formation of the gold seeds [31, 32]. Following that, BSA was introduced which adhered to the NPs via thiol groups and led to the formation of functional groups. For this, sodium borohydride (6 mg) was used to denature a freshly prepared BSA solution (24 mg) and kept for overnight under vigorous stirring. This process reduces the disulfide bonds to generate thiol groups and also adds extra thiol groups onto the surface of BSA [33]. We thus centrifuged and washed the resulting product with double-distilled water and ethanol several times. All compounds were freeze-dried and stored at 4 ◦C until further use.
The chemical bonding approach was used to create the luminol and Ab2 modified Au@BSA study. Via ultrasonication, we dissolved 2 mg of Au@BSA in 1 mL double-distilled water and thus mixed it with 400 µL of 12.5% GA. To ensure the adherence of GA to the Au@BSA surface, the resulting solution was shaken for 3 h. The unreacted chemicals were then removed by centrifugation and several washing steps from the GA-linked Au@BSA before being dissolved in 1 mL PBS (0.1 M, pH 7.4). The above solution was then mixed with a 0.1 M luminol solution shaken for 4 h. In order to activate the carboxylic groups on the surface of BSA, the generated Au@BSA-luminol nanocomposites underwent centrifugation and were then washed in 2 mL 0.1 M PBS (pH 7.4) which contained 2 mg EDC and 2 mg NHS with a 2 h shaking periods. Finally, 80 μL Ab2 antibodies (1 g/L) was mixed with the solution and slowly stirred in an ice bath for 8 h to ensure the luminol-Au@BSA and Ab2 conjugation. All remaining reagents were removed by centrifuging the resulting luminol-Au@BSA-Ab2 nanocomposites at 6000 rpm and washing them with double-distilled water. The resulting nanocomposites were ultimately dispersed in 2 mL PBS (0.1 M, pH 7.4) until further use.
Synthesis of APTES-Fe3O4 nanoparticles and preparation of antibody immobilized on MBs
In this method, FeCl2.4H2O (0.3125 g) was dissolved in 10 mL DDW and stirred in order to attain a homogeneous mixture. Ammonia solution (25%) (1.6 mL) was mixed under constant stirring which enabled the oxidation of Fe (II). The solution was left to stir for 10 min. APTES (0.625 mL) was thus mixed and again the mixture was stirred for an extra 10 min. The reaction mixture was autoclaved in a 50 mL volume sealed pressure vessel at 134 °C for 3 h and then cooled at room temperature. This resulted in black precipitates, which were purified through several washing steps with water and ethanol to ensure the elimination of excess reactants. Magnetic separation was used for the final separation of the resulting compound which was dried at 80 °C for 7–8 h using a hot air oven [22]. Surface functionalization of the MNPs was carried out to generate amine groups (-NH2) on their surface for their covalent bonding to carboxyl groups (-COOH) on the antibodies. Accordingly, APTES-Fe3O4 was used. Covalent coupling of the antibody to the amino-functionalized MNPs was carried out based on the classical EDC/NHS blocking chemistry [34]. Briefly, to activate the antibody, NHS (9 mg) and EDC (5 mg) were dissolved in 1 mL PBS buffer (0.1 M, pH 7.4) and added to MNPs (1 mL, 0.1 g/L) in PBS (0.1 M, pH 7.4). Finally, we introduced 80 μL of Nanog antibodies (1 g/L) to the mixture which was stirred slowly in an ice bath for 8 h for the effective attachment of the Ab2 to the surface of the APTES-Fe3O4 [35].
Synthesis of cupric oxide nanoparticles
The sol–gel method was implemented for the synthesis of the CuO nanopowders. In a sterile round bottom flask, an aqueous solution of CuCl2.6H2O (0.2 M) was first prepared; 1 mL of glacial acetic acid was introduced to the prepared solution and thus heated to 100 ℃ under continuous stirring. In order to attain a pH of 7, 8 M of NaOH solution was added. This resulted in the immediate color change from blue to black and the formation of large amounts of black precipitate at the bottom of the flask. The precipitates were gathered through centrifugation and washed 3–4 times with deionized water. The obtained precipitates were then dried in air for 24 h [36].
Determination of Nanog
As shown in Scheme 1, a biosensing platform for Nanog Ag detection was developed using the CRET-based immunosensor. 200 μL of the Au@BSA-luminol-Ab2 suspension was mixed with 50 μL of 0.1 × 10–6 g/L Nanog solution, and the final mixture was incubated for 15 min in an ice bath to allow the Au@BSA-luminol-Ab2-Nanog immune complexes to form. Then, a 200 μL volume of APTES-Fe3O4-Ab1 was also mixed for 15 min in the ice bath. After that, we separated the suspension magnetically and resuspended it in 200 μL of PBS (0.1 M, pH 7.4). The final mixture was transferred to a 1 cm path length quartz cell. Then 50 μL of the CuONPs was added to the suspension, followed by the immediate introduction of H2O2 (1.0 × 10–3 M) to the quartz cell. The CL spectrum was recorded immediately with a LS-55 luminescence spectrometer.
Results and discussion
Characterization of Au@BSA nanoparticles
TEM was used to analyze the morphology and structure of the Au@BSA nanoparticles. As depicted in Fig. S1a, the nanoparticles exhibited spherical-shaped particles coated with a BSA layer with an average size of approximately 15 nm. The proposed immunosensor benefited from the presence of Au@BSA nanoparticles, which was made of several self-assembled Au nanoparticles. These nanoparticles exhibited a large surface area with high electroconductivity, leading to enhanced electron transfer and improved assay sensitivity [37]. The BSA layer coating the Au@BSA nanoparticles provided a versatile platform, allowing for easy conjugation of a significant quantity of luminol and anti-Nanog antibodies. In this case, the beneficial functional groups (-COOH, -NH2, and -SH) found on the external BSA layer facilitate the cross-linking of numerous Anti-Nanog and luminol molecules [38]. Our team's earlier publications (Hosseini et al. [39, 40]) have validated the satisfactory outcomes of Energy Dispersive Spectroscopy (EDS) for the subsequent assessment of the synthesized Au@BSA nanoparticles, which further confirmed the presence of gold, carbon, nitrogen, and oxygen elements corroborating the successful synthesis of the Au@BSA nanoparticles.
The FTIR spectrum, as depicted in Fig. S1b, displayed the common stretching vibrations of –OH resulting in the wide peaks at 3446 cm−1, 3382 cm−1, 3131 cm−1 and the -NH vibrations which translated to a peak at 1403 cm−1. The absorption band at 2361 cm−1 can be assigned to alkyl groups [–(CH2)–] which are characteristic of the organic coupling agent. Moreover, the characteristic peaks at 1702, 993 and 1135 cm−1 resulted from the –COO group vibrations, further showing the variety of functional groups provided by the synthesized Au@BSA@anti-Ab2, including –COOH and –NH2 groups.
Characterization of APTES-Fe3O4 nanoparticles
The UV–Visible spectrum of 1 g/L solutions of Fe3O4 NPs and APTES-Fe3O4 NPs was recorded and is shown in Fig. 1A. A peak was observed at 406 ± 0.88 nm for APTES-Fe3O4 NPs [25]. As these nanoparticles are magnetic in nature (Fig. S2), coating them with non-magnetic material, such as APTES, helps minimize the magnetic forces between the particles. Furthermore, to understand the structure of APTES-Fe3O4 NPs, FTIR spectrum was recorded (Fig. 1C), which confirmed the conjugation of the antibodies to the MNPs. Characteristic Fe–O bonds were seen at 524 cm−1 pertaining to stretching vibrations, and at 859 cm−1 showing the stretching of Fe–O–H. The vibrations of OH on the surface of Fe3O4 are corroborated at 1077 cm−1 and the peak at 1160 cm−1 indicated the asymmetric stretching vibrations of the Si–O groups. The other characteristic broad peak at 3200–3600 cm−1 can be assigned to hydroxyl and amine groups. At around 1650 cm−1 a peak is seen which depicts the C = O bond of the antibody amide I. The one at 1403 cm−1 shows the N–H bond of antibody amide II. The absorption band at 2438 cm−1 is due to alkyl groups [–(CH2)–] that are characteristic of the organic coupling agent. The reaction of silane molecules on the surface of Fe3O4 nanoparticles and covalent attachment of the antibodies to the MNP surface is perfectly confirmed by the FTIR spectrum. FE-SEM was used to assess the surface morphology of APTES-Fe3O4 NPs which showed uniform shape (spherical) and size of the particles. The image of APTES-Fe3O4 NPs is shown in Fig. 1B.
Characterization of cupric oxide nanoparticles
Figure 2A displays the UV–Visible spectrum of 1 mM concentration solutions of CuO NPs. The size of the nanoparticles was estimated from the FE-SEM micrograph (Fig. 2B), revealing that the majority of the particles are less than 200 nm in size. These findings correlate with the data obtained from the particle size analyzer. The FE-SEM graph and Size Distribution Report by Intensity graph also illustrates that the copper oxide nanoparticles have a spherical shape and exhibit a nearly uniform size (Fig. 2C). The results display the relationship between intensity and Zeta Potential. It reveals a maximum value of -36.8 mV. The negative zeta potential indicates that the copper oxide particles are negatively charged and exhibit moderate stability [36, 41, 42]. The negative zeta potential of CuO nanoparticles prevents them from agglomerating, resulting in excellent dispersion stability. The well-dispersed and stable nature of CuO nanoparticles make them highly effective catalysts in chemical reactions. The catalytic properties of CuO have been tested in various chemical reactions, confirming its efficacy as a catalyst [43].
Optimization for the assay
In this work, CuONPs were used as an enhancer in the luminol-H2O2 CL system. The optimized CL reaction conditions and the assessment of the enhancing characteristics of CuONPs in the luminol-H2O2 CL system have been described. As depicted in Fig. S3, the best conditions for the luminol-H2O2-CuONPs CL system were 5.0 mM H2O2 aqueous solution (Fig. S3A) and 3.0 × 10–3 g/L CuONPs solution (Fig. S3B). Also, the optimal time for CL reaction was 6 s (Fig. S3C). In all experiments, the concentration of APTES-Fe3O4 remained constant at 1 g/L, with the addition of 200 μL of the Au@BSA-luminol-Ab2 suspension.
CRET mechanism
The CRET-based immunoassay was constructed with luminol-H2O2, which acted as the donor and APTES-Fe3O4 as the acceptor. CRET was based on an immuno-interaction of Au@BSA-Ab2 and APTES-Fe3O4-Ab1 (anti-Nanog) in the H2O2 and CuONPs chemiluminescence reaction in the presence of target (Nanog Ag). When luminol-Au@BSA-Ab2 is exposed to H2O2 and excited at 435 nm, the resulting excitation is weak. Therefore, the use of a catalyst, such as CuONPs, becomes necessary. The investigation focused on the catalytic effects of CuONPs on the luminol-H2O2 chemiluminescent system. Figure S4 demonstrates that, in alkaline media, the oxidation of luminol by H2O2 produces a weak chemiluminescent (CL) signal. However, with the addition of CuO nanoparticles, the intensity of the CL signal can be significantly enhanced by approximately 13.0 folds.
The absorption and emission spectrum studies for APTES and APTES-Fe3O4 showed that APTES has intrinsic fluorescence emission (Fig. S5). The fluorescence spectrum and UV–visible of APTES-Fe3O4 NPs showed that these nanoparticles have luminescence emission in the range of 620 nm and absorption in the region of 435 nm (Fig. S6 and Fig. S7). The overlapping absorption spectrum of APTES-Fe3O4 NPs with the emission spectrum of luminol-H2O2 facilitated the design of a CRET-based diagnostic system (Fig. 3). To investigate the target detection capability of the biosensor, the complex was reacted with different concentrations of Nanog from 1.0 × 10–8 g/L to 6.0 × 10–5 g/L. There was a uniform increasing trend in the CRET intensity with increasing target concentration, indicating the detection capability of this platform (Fig. 4A). With regard to the obtained results, the regression equation is y = 11.848x + 101.74, and the determination coefficient R2 = 0.9767 suggests a linear relationship between Nanog Ag concentration of 1.0 × 10–7 g/L to 6.0 × 10–5 g/L and CL intensity and with the limit of detection for the assay was 0.8 × 10 −7 g/L, which was calculated according to the Eq. 3 σ / m, where m represents the slope of the calibration curve and σ is the standard deviation of the blank samples, as shown in Fig. 4B. The CRET based immunosensor performance for antigen detection was compared in Table S1 with similar publications, which includes the limits of detection (LOD), linear dynamic range, and donor/acceptor information obtained from the literature [30,31,32,33].
A The overlapping fluorescence spectrum of APTES-Fe3O4 NPs with the emission spectrum of luminol-H2O2 (a) CL of luminol-H2O2 (b) fluorescence of APTES-Fe3O4 NPs at 435nm excitation (PBS 0.1 M, pH 7.4); and B) The overlap of CL spectrum of luminol (a) and UV–visible absorption spectrum of APTES-Fe3O4 NPs (b)
The in vitro detection of Nanog by CRET-based APTES-Fe3O4(MNPs): A CRET responses of Nanog at different concentrations; and (B) Linearity of CL signals versus Nanog concentrations (The concentration of Nanog was 0.1–60 × 10 −6 g/L). Inset: plot of the CL intensity (the concentration of Nanog was 0.1–1.5 × 10 −6 g/L)
Selectivity
The critical parameter of selectivity plays a significant role in evaluating the performance of luminescence immunosensors. To further explore the selectivity of the described sensing platform, we conducted additional investigations. Specifically, we examined the CRET responses of the probe antibody towards potential interfering antigens including HER2 and PSA and ROR1. Various potential interfering antigens such as HER2, PSA, and ROR1 were evaluated at a concentration of 200 × 10 −6 g/L, while the concentration of Nanog was 10 × 10 −6 g/L. Despite the 20-fold concentration of the interfering antigens, the overall signal intensity in the presence of Nanog antigen remained notably higher compared to the other antigens. Results showed that their CRET intensities were significantly lower when compared to the Nanog sample, proving that the method was only specific for Nanog Ag (Fig. 5).
Performance test of the CRET-based immunoassay
The developed CRET-based immunoassay was employed to detect Nanog under ideal conditions. To better assess the analytical performance of the immunoassay system, a recovery test was conducted. To assess the analytical recovery, normal urine was spiked with a known concentration of Nanog and measured in 10 replicates within one analytical run. Recovery and RSD for real samples varied from 103.5% to 106.4% and from 3.3% to 4.4%, respectively. This proves that the proposed method can effectively detect the target in biological samples.
Conclusions
In this study, a magnetic-based CRET immunoassay was developed for the detection of Nanog antigens. Notably, the acceptor itself serves as a magnetic component, offering a dual functionality. Nanog was chosen as a model analyte to depict the overall performance of the proposed strategy. Despite the requirement for magnetic separation, this method was remarkably time-efficient, with a measurement completion time of approximately 30 min, making it much faster and simpler than the conventional ELISA method. Additionally, this method demonstrated exceptional sensitivity, reaching ultrahigh levels.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
References
Jia BJ, He X, Cui PL, Liu JX, Wang JP (2019) Detection of chloramphenicol in meat with a chemiluminescence resonance energy transfer platform based on molecularly imprinted graphene. Anal Chim Acta 1063:136–143. https://doi.org/10.1016/j.aca.2019.02.044
Dou L, Pan Y, Ma M, Zhang S, Shen J, Wang Z, Yu W (2021) Antibody engineering-driven controllable chemiluminescence resonance energy transfer for immunoassay with tunable dynamic range. Anal Chim Acta 1152:338231. https://doi.org/10.1016/j.aca.2021.338231
Yan Y, Wang X, Hai X, Song W, Ding C, Cao J, Bi S (2020) Chemiluminescence resonance energy transfer: From mechanisms to analytical applications. TrAC Trends Anal Chem 123:115755
Gu L, Zou Y, Li Y, Zeng K, Zhu N, Zhu F (2020) High-throughput chemiluminescence immunoassay based on Co2+/hemin synergistic catalysis for sensitive detection tetrabromobisphenol A bis (2-hydroxyethyl) ether in the environments. Sci Total Environ 714:136880
Salvagno GL, Gianfilippi G, Fiorio G, Pighi L, De Nitto S, Henry BM, Lippi G (2021) Clinical assessment of the DiaSorin LIAISON SARS-CoV-2 Ag chemiluminescence immunoassay. Ejifcc 32(2):216
Franzin M, Ruoso R, Concato M, Radaelli D, D’Errico S, Addobbati R (2024) Applicability of a chemiluminescence immunoassay to screen postmortem bile specimens and its agreement with confirmation analysis. Int J Mol Sci 25(7):3825
Li ZB, Cui PL, Liu J, Liu JX, Wang JP (2020) Production of generic monoclonal antibody and development of chemiluminescence immunoassay for determination of 32 sulfonamides in chicken muscle. Food Chem 311:125966
Qiu Y, Li P, Liu B, Liu Y, Wang Y, Tao T, Xu J, Hammock BD, Liu X, Guan R, Zhang C (2019) Phage-displayed nanobody based double antibody sandwich chemiluminescent immunoassay for the detection of Cry2A toxin in cereals. Food Hydrocolloids 30(1):924–936
Chen J, Qiu H, Zhao S (2020) Fabrication of chemiluminescence resonance energy transfer platform based on nanomaterial and its application in optical sensing, biological imaging and photodynamic therapy. TrAC Trends in Anal Chem 122:115747
Nsengimana B, Khan FA, Awan UA, Wang D, Fang N, Wei W, Zhang W, Ji S (2022) Pseudogenes and liquid phase separation in epigenetic expression. Front Oncol 12:912282
Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113(5):643–655
Ibrahim EE, Babaei-Jadidi R, Saadeddin A, Spencer-Dene B, Hossaini S, Abuzinadah M (2012) Embryonic NANOG activity defines colorectal cancer stem cells and modulates through AP1-and TCF-dependent mechanisms. Stem cells 30(10):2076–2087
Liu B, Badeaux MD, Choy G, Chandra D, Shen I, Jeter CR, Rycaj K, Lee CF, Person MD (2014) Nanog1 in NTERA-2 and recombinant NanogP8 from somatic cancer cells adopt multiple protein conformations and migrate at multiple MW species. PLoS one 9(3):e90615
Kang KT, Shin MJ, Moon HJ, Choi KU, Suh DS, Kim JH (2023) TRRAP enhances cancer stem cell characteristics by regulating NANOG protein stability in colon cancer cells. Int J Mol Sci 24(7):6260. https://doi.org/10.3390/ijms24076260
Sneha S, Nagare RP, Manasa P, Vasudevan S, Shabna A, Ganesan TS (2019) Analysis of human stem cell transcription factors. Cell Reprogram 21(4):171–180
Zhang L, Hou Y, Lv C, Liu W, Zhang Z, Peng X (2020) Copper-based metal–organic xerogels on paper for chemiluminescence detection of dopamine. Anal Methods 12(34):4191–4198
Yang N, Huang Y, Ding G, Fan A (2019) In situ generation of prussian blue with potassium ferrocyanide to improve the sensitivity of chemiluminescence immunoassay using magnetic nanoparticles as label. Anal Chem 91(7):4906–4912
Rezaei Z, Ranjbar B (2017) Ultra-sensitive, rapid gold nanoparticle-quantum dot plexcitonic self-assembled aptamer-based nanobiosensor for the detection of human cardiac troponin I. Eng Life Sci 17(2):165–174
Shahbazi N, Hosseinkhani S, Ranjbar B (2017) A facile and rapid aptasensor based on split peroxidase DNAzyme for visual detection of carcinoembryonic antigen in saliva. Sens Actuators B: Chem 253:794–803
Chen W, Hong L, Liu AL, Liu JQ, Lin XH, Xia XH (2012) Enhanced chemiluminescence of the luminol-hydrogen peroxide system by colloidal cupric oxide nanoparticles as peroxidase mimic. Talanta 99:643–648. https://doi.org/10.1016/j.talanta.2012.06.061
Qin G, Zhao S, Huang Y, Jiang J, Ye F (2012) Magnetic bead-sensing-platform-based chemiluminescence resonance energy transfer and its immunoassay application. Anal Chem 84(6):2708–2712. https://doi.org/10.1021/ac202959d
Yadav N, Singh A, Kaushik M (2020) Hydrothermal synthesis and characterization of magnetic Fe3O4 and APTS coated Fe3O4 nanoparticles: physicochemical investigations of interaction with DNA. J Mater Sci: Mater Med 31:1–11
Ling W, Wang M, Xiong C, Xie D, Chen Q, Chu X, Qiu X, Li Y, Xiao X (2019) Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. J Mater Res 34(11):1828–1844
Saravanan P, Jayamoorthy K, Anandakumar S (2016) Fluorescence quenching of APTES by Fe2O3 nanoparticles–Sensor and antibacterial applications. J Lumin 178:241–248
Chen W, Chen J, Liu AL, Wang LM, Li GW, Lin XH (2011) Peroxidase-like activity of cupric oxide nanoparticle. ChemCatChem 3(7):1151–1154
Ehsani M, Chaichi MJ, Nezammeddin Hosseini S (2017) Comparison of CuO nanoparticle and CuO/MWCNT nanocomposite for amplification of chemiluminescence immunoassay for detection of the hepatitis B surface antigen in biological samples. Sensors Actuators B: Chem 247:319–328. https://doi.org/10.1016/j.snb.2017.02.019
Shahsavar K, Shokri E, Hosseini M (2022) Sensitive colorimetric detection of miRNA-155 via G-quadruplex DNAzyme decorated spherical nucleic acid. Microchim Acta 189(9):357
Naderi M, Hosseini M, Ganjali MR (2018) Naked-eye detection of potassium ions in a novel gold nanoparticle aggregation-based aptasensor. Spectrochim Acta A: Mol Biomol Spectrosc 195:75–83
Amani F, TohidiMoghadam T, Farahani N, Ranjbar B (2020) Design and Fabrication of an Aptasensor for Facile and Rapid Detection of Carcinoembryonic Antigen based on Gold-nanoparticles. Modares J Biotechnol 11(2):201–207
Mohammadi S, Khajeh K, Taghdir M, Ranjbar B (2021) An experimental investigation on the influence of various buffer concentrations, osmolytes and gold nanoparticles on lysozyme: spectroscopic and calorimetric study. Int J Biol Macromol 172:162–169
Barkheh H, Zeinoddini M, Ranjbar B, Xodadadi N (2021) A novel strategy for trinitrotoluene detection using functionalized gold nanoparticles. J Anal Chem 76:459–465
Mirsadoughi E, Nemati F, Oroojalian F, Hosseini M (2022) Turn–on FRET-based cysteine sensor by sulfur-doped carbon dots and Au nanoparticles decorated WS2 nanosheet. Spectrochim Acta A: Mol Biomol Spectrosc 272:120903
Zhang A, Xiang H, Zhang X, Guo W, Yuan E, Huang C, Jia N (2016) A novel sandwich electrochemiluminescence immunosensor for ultrasensitive detection of carbohydrate antigen 19–9 based on immobilizing luminol on Ag@ BSA core/shell microspheres. Biosens Bioelectron 75:206–212
Sam S, Touahir L, Salvador Andresa J, Allongue P, Chazalviel JN, Gouget-Laemmel AC (2010) Semiquantitative study of the EDC/NHS activation of acid terminal groups at modified porous silicon surfaces. Langmuir 26(2):809–814
Haghighi AH, Khorasani MT, Faghih Z, Farjadian F (2020) Effects of different quantities of antibody conjugated with magnetic nanoparticles on cell separation efficiency. Heliyon 6(4)
Aparna Y, Rao KV, Subbarao PS (2012) Preparation and characterization of CuO Nanoparticles by novel sol-gel technique. J Nano- Electron Phys 4(3):3005–3011
Ning X, Bao H, Liu X, Fu H, Wang W, Huang J, Zhang Z (2019) Long-term in vivo CT tracking of mesenchymal stem cells labeled with Au@ BSA@ PLL nanotracers. Nanoscale 11(43):20932–20941
Zhang A, Huang C, Shi H, Guo W, Zhang X, Xiang H, Jia T, Miao F, Jia N (2017) Electrochemiluminescence immunosensor for sensitive determination of tumor biomarker CEA based on multifunctionalized Flower-like Au@ BSA nanoparticles. Sens Actuators B: Chem 238:24–31
Firoozbakhtian A, Hosseini M, Sheikholeslami MN, Salehnia F, Xu G, Rabbani H, Sobhanie E (2022) Detection of COVID-19: a smartphone-based machine-learning-assisted ECL immunoassay approach with the ability of RT-PCR CT value prediction. Anal Chem 94(47):16361–16368
Hosseini M, Sobhanie E, Salehnia F, Xu G, Rabbani H, Sheikholeslami MN, Firoozbakhtian A, Sadeghi N, Farajollah MH, Ganjali MR, Vosough H (2022) Development of sandwich electrochemiluminescence immunosensor for COVID-19 diagnosis by SARS-CoV-2 spike protein detection based on Au@ BSA-luminol nanocomposites. Bioelectrochemistry 147:108161
Fuerstenau D (2005) Zeta potentials in the flotation of oxide and silicate minerals. Adv Colloid Interface Sci 114:9–26
Chang M-H, Liu H-S, Tai CY (2011) Preparation of copper oxide nanoparticles and its application in nanofluid. Powder Technol 207:378–386
Aparna Y, Rao KE, Subbarao PS (2012) Synthesis and characterization of CuO nano particles by novel sol-gel method. In Proc 2nd Int Conf Environ Sci Biotechnol 48:156–160 IACSIT Press Singapore
Acknowledgements
The authors are grateful to the Research Council of Tarbiat Modares University and University of Tehran for the financial support of this work.
Funding
This study was funded by the Council of Tarbiat Modares University and University of Tehran.
Author information
Authors and Affiliations
Contributions
Fatemeh Mehrabi: Formal analysis, Visualization, Data curation, Writing-Original draft. Bijan Ranjbar: Conceptualization, Methodology, Designing the analysis, Writing-review & editing. Morteza Hosseini: Conceptualization, Methodology, Project administration, Funding acquisition, Writing-review & editing. Niloufar Sadeghi: Conceptualization, Methodology, Designing the analysis, Resource Javad Mohammadi: Methodology, writing-review & editing Mohammad Reza Ganjali: writing-review & editing.
All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The work has been done in accordance with the recommendations of the National Institute of Genetic Engineering and Biotechnology (NIGEB) and the Human Ethical Committee of the University of Tarbiat Modares.
Conflicts of interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehrabi, F., Ranjbar, B., Hosseini, M. et al. CRET-based immunoassay on magnetic beads for selective and sensitive detection of Nanog antigen as a key cancer stem cell marker. Microchim Acta 191, 419 (2024). https://doi.org/10.1007/s00604-024-06505-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06505-y