Abstract
A “turn on” time-resolved fluorometric aptasensor is described for the simultaneous detection of zearalenone (ZEN), trichothecenes A (T-2), and aflatoxin B1 (AFB1). Multicolor-emissive nanoparticles doped with lanthanide ions (Dy3+, Tb3+, Eu3+) were functionalized with respective aptamers and applied as a bioprobe, and tungsten disulfide (WS2) nanosheets are used as a quencher of time-resolved fluorescence. The assay exploits the quenching efficiency of WS2 and the interactions between WS2 and the respective DNA aptamers. The simultaneous recognition of the three mycotoxins can be performed in a single solution. In the absence of targets, WS2 is easily adsorbed by the mixed bioprobes via van der Waals forces between nucleobases and the WS2 basal plane. This brings the bioprobe and WS2 into close proximity and results in quenched fluorescence. In the presence of targets, the fluorescence of the bioprobes is restored because the analytes react with DNA probe and modify their molecular conformation to weaken the interaction between the DNAs and WS2. Under the optimum conditions and at an excitation wavelength of 273 nm, the time-resolved fluorescence intensities (peaking at 488, 544 and 618 nm and corresponding to emissions of Dy3+, Tb3+ and Eu3+) were used to quantify ZEN, T-2 and AFB1, respectively, with detection limits of 0.51, 0.33 and 0.40 pg mL−1 and a linear range from 0.001 to 100 ng mL−1. The three mycotoxins can be detected simultaneously without mutual interference. The assay was applied to the quantification of ZEN, T-2 and AFB1 in (spiked) maize samples. This homogeneous aptamer based assay can be performed within 1 h. Conceivably, it can become an alternative to other heterogeneous methods such as the respective enzyme-linked immunosorbent assays.

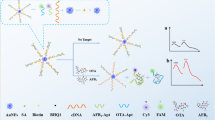
Schematic presentation of an aptasensor for simultaneous detection of zearalenone, trichothecenes A and aflatoxin B1 using aptamer modified time-resolved fluorescence nanoparticles as signalling probes and tungsten disulfide as the quencher. This assay shows lower detection limit and requires no washing steps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycotoxins are naturally occurring secondary metabolites that are produced by fungi during their growth [1]. The most common mycotoxins found in maize include ochratoxin A, aflatoxins (especially aflatoxin B1), T-2 toxin, and zearalenone [2]. In this study, ZEN, T-2 and AFB1 were chosen as targets to monitor in maize due to their severe damage and toxicity to human health. ZEN can affect the mammal’s reproductive system in terms of decreased fertility and abortions [3]. T-2 toxins are toxic to the immune system and also cause cytotoxic and immunosuppressive injury by preventing RNA and DNA synthesis [4]. AFB1 are the most toxic and predominant mycotoxins which are classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) due to their carcinogenic, teratogenic and mutagenic potential [5]. Consequently, the development of a sensitive, easy-to-use and rapid assay for the quantification of multiple mycotoxins in cereals is highly desirable in agro-food analysis.
Conventional methods including high-performance liquid chromatography, gas chromatography-mass spectrometry and liquid chromatography tandem mass spectrometry [6, 7] have been established for screening of mycotoxins. Although these techniques offer sufficient accuracy, sensitivity and good repeatability, laborious sampling and trained personnel are required for measurements. Antibodies-based immunoassays i.e. microarray-based immunoassay [2], enzyme-linked immunosorbent assay [8], time-resolved fluoroimmunoassay [9], time-resolved fluorescent immunochromatography analysis [10] have been used as simple, fast and effective methods for detection of mycotoxin. However, antibodies are obtained from an expensive and long procedure and also antibodies are vulnerable to the environment, leading to poor detection performances. Thus, it is a great challenge to accomplish sensitive and rapid simultaneous recognition of mycotoxins in real samples.
Lanthanide doped inorganic nanoparticles have been recommended as an encouraging innovative type of fluorescent probes. The Ln3+-doped time-resolved fluorescence nanoparticles (TRFNPs) possess following advantages, such as favorable biocompatibility, resistance to photobleaching, high chemical stability, low cytotoxicity, multicolor doping, controlled and simple synthesis, large Stokes shifts, and extensive fluorescence lifetimes [11]. These characteristics can elude the overlap between the emission and excitation spectra of the fluorophore; therefore, can be used to develop a new fluorescence bioprobes for food safety assays. Moreover, using the long life time fluorescence emission of Ln3+ ion compounds can diminish the background noises efficiently. Thus, as compared to conventional fluorescent assays, time-resolved fluorescent assay offers high signal-to-noise ratio in fluorescence based biodetection. Furthermore, aptamers comparable to antibodies can also offer features such as high binding affinity to targets, easier synthesis, lower immunogenicity, smaller size, versatile chemistry and better thermal stability [12]. As compare to biosensors using natural receptors i.e. enzymes and antibodies, the aptamer based biosensor have following benefits i.e. high specificity, chemical stability, high affinity to targets, high purity and reproducibility through commercial synthesis and great flexibility [13]. In combination with aptamers, lanthanides and their complexes were used as signaling units. They have been used in the aptasensors to detect Staphylococcus aureus enterotoxins [14], ricin [15] and Salmonella typhimurium [16]. Conversely, its grouping with aptamers for detection of mycotoxin has not been studied so far.
WS2 nanomaterial, which is 2D layered and has S-W-S sandwich structures, is one of the newly emergent transition metal dichalcogenides (TMDC) [17]. Up to now, only few WS2 nanosheet-based bioassays have been reported [18, 19], mostly related with quenching of dye by WS2 [18, 20, 21]. As reported by the previous works, WS2 has been applied in fluorescence based assays due to its differential affinity towards single stranded and double-stranded DNA (ssDNA and dsDNA) [21, 22], but the applications in time-resolved fluorescence aptasensor is not very common. Thus, there is an urgent necessity to discover the new use of WS2 as fluorescence quencher for time-resolved fluorescence in the area of biosensor. Moreover, WS2 presented extraordinarily high fluorescence quenching efficiency over grapheme oxide, which is very important for the sensitivity of quenching and recovery based biosensor [21]. Consequently, inspired by the above findings, we believe that WS2 can be applied as fluorescence quencher for quenching and restoration based biosensor for the detection of ZEN, T-2 and AFB1.
In this study, a new “turn on” aptasensor was designed (Fig. 1) for sensitive and rapid simultaneous recognition of three mycotoxins (ZEN, T-2 and AFB1). Herein, simultaneous detection were carried out using aptamers as specific detection element conjugated to Ln3+ (Ln3+ = Dy3+, Tb3+, Eu3+) doped TRFNPs and WS2 as fluorescence quencher. The presented aptasensor indicate the novel use of strong fluorescence and long lifetime of TRFNPs and also unique quenching efficiency of WS2 for the quantitative analysis of mycotoxins. Besides, the method can quantify ZEN, T-2 and AFB1 in real sample. The aptasensor has been confirmed to display high sensitivity, high-throughput and appropriate simultaneous recognition of three mycotoxins.
Materials and methods
Reagents
YCl3.6H2O was obtained from Aladdin chemistry Co. Ltd. (Shanghai, China) (http://www.aladdin-e.com). EuCl3.6H2O, TbCl3.6H2O and DyCl3.6H2O were bought from Sigma-aldrich (www.sigmaaldrich.com). Polyethylenimine (PEI, ca. 30% in water) was obtained from TCI (Shanghai, China) Development Co., Ltd. (http://www.tcic hemicals.com). The glutaraldehyde solution (25% in V/V), ethylene glycol, KCl, NH4F, ethanol and all the chemicals used for solutions and buffers preparation were purchased from Sinopahrm chemical reagent Co., Ltd. (China) (http://www.sinoreagent.com). The tungsten disulfide (WS2) nanosheet was bought from Nanjing XF Nano Material Tech Co., Ltd. (Nanjing, China). Avidin was purchased from Sangon Biotech Company (China) (http://www.sangon.com). The ZEN, T-2 and AFB1 aptamers [23,24,25] were manufactured by Sangon Biotech Company (China) (http://www.sangon.com). The sequences of the ZEN, T-2 and AFB1 aptamers were 5′-biotin-TCA TCT ATC TAT GGT ACA TTA CTA TCT GTA ATG TGA TAT G-3′, 5′-biotin-CAG CTC AGA AGC TTG ATC CTG TAT ATC AAG CAT CGC GTG TTT ACA CAT GCG AGA GGT GAA GAC TCG AAG TCG TGC ATC TG-3′, and 5′-biotin AGC AGC ACA GAG GTC AGA TGG TGC TAT CAT GCG CTC AAT GGG AGA CTT TAG CTG CCC CCA CCT ATG CGT GCT ACC GTG AA-3′, respectively.
Instrumentation
Powder XRD pattern was measured by a D8 Avance (Bruker AXS Ltd., Germany) with Cu-Kα radiation (λ = 0.1540 nm). TEM images of NPs were obtained by a JEOL model 2100 HR instrument (TEM, JEOL, Ltd., Japan) working at 200 kV accelerating voltage. The time-resolved fluorescence spectra (TRF) were measured by using a Synergy H1 multi-mode microplate reader (BioTek Instruments, Inc., Highland Park, USD) in time-resolved mode spectra scan with an excitation wavelength of 273 nm. UV-Vis absorption spectra were measurement by UV-1800 spectrophotometer (Shimadzu Co., Japan). The surface examination of WS2 nanosheet was done by using a Dimension Icon Atomic Force Microscope System (AFM, Bruker Co., Germany).
Preparation of amine functionalized Ln3+ doped KYF4 NPs
Ln3+ (Ln3+ = Dy3+, Tb3+ and Eu3+) doped KYF4 NPs were prepared by one-step solothermal procedure [26] with some changes. The detail method is described in supporting information.
Preparation of aptamer modified KYF4: Ln3+ multicolor bioprobe
First of all, avidin-modified KYF4: Ln3+ NPs were obtained via earlier published classical glutaraldehyde procedure [14]. The avidin-functionalized KYF4: Ln3+ NPs and 5′-Biotin-labeled aptamers were bounded through high affinity avidin-biotin system. The detail method is described in supporting information.
Procedure for simultaneous detection of mycotoxins
In the following experiment, ZEN aptamer modified KYF4: Dy3+, T-2 aptamer modified KYF4: Tb3+ and AFB1 aptamer modified KYF4: Eu3+ TR-FNPs (final concentration 1 mg mL−1) were mixed with PBS (10 mmol mL−1 Na2HPO4, 137 mmol mL−1 NaCl, 2.7 mmol mL−1 KCl and 2 mmol mL−1 KH2PO4) followed by the addition of 0.3 mg mL−1 of WS2 nanosheet solution at room temperature for 20 min. Then, various concentrations of targets solutions were added into above mixture to give the final volume of 200 μL to each well. The whole mixture was incubated for 40 min at 37 °C and then TRF emission spectra were measured using microplate reader. The conditions were set as follows; Synergy H1 multi-mode micro-plate reader in time-resolved mode at emission wavelengths of 488, 544 and 618 nm corresponded with Dy3+, Tb3+ and Eu3+, respectively, and an excitation wavelength of 273 nm, while the delay and gate times were set to be 100 μs and 1000 μs, respectively. All the intensity measurements were measured in triplicate.
Sample preparation
5 g of ground maize samples and 5 g of NaCl were put into a flask of 100 mL and then extracting solution (methanol + water; 7:3 (v:v)) was added into the flask upto the mark. The mixture was homogenized, stirred and extracted for 2 min. Next, the mixture was filtered and 10 mL of the filtrate was added into another flask (50 mL), followed by addition of water to the mark and mixed the whole mixture properly. The final mixture was filtered again using filter paper (glass fiber) to get very clear solution. The samples were used for standard addition and recovery experiment. The standard solutions of ZEN, T-2 and AFB1 were added to the sample before adding the extracting solution.
Results and discussion
Detection scheme
Figure 1 illustrate the principle of this “turn on” fluorometric strategy. A sensitive aptasensor is presented for the simultaneous determination of ZEN, T-2 and AFB1 based on the fluorescence quenching-restoration principle. The ZEN aptamer modified KYF4: Dy3+, T-2 aptamer modified KYF4: Tb3+ and AFB1 aptamer modified KYF4: Eu3+ TRFNPs exhibited high fluorescence at 273 nm excitation wavelength. In the absence of targets, WS2 is easily adsorbed by the mixing bioprobe via van der Waals forces between nucleobases and the WS2 basal plane [22]. This brings the bioprobe and WS2 into close proximity and results in quenched fluorescence. In the presence of targets, fluorescence of bioprobe restored because the targets react with DNA probe and modify their molecular conformation that weakened the interaction between the DNA and WS2. Moreover, the weak affinity of WS2 towards dsDNA leading the bioprobe to switch away from the surface of WS2 resulted in strong fluorescence signal. Thus, a highly sensitive and specific recognition of ZEN, T-2 and AFB1 were attained by measuring the fluorescence signals of the reaction system.
Characterization of Ln3+ doped KYF4 NPs and WS2
Ln3+ (Ln3+ = Dy3+, Tb3+, Eu3+) doped KYF4 NPs were prepared by one-step solothermal method with PEI as capping material. PEI was used to control the growth of the NPs and render them water soluble and surface functionalized for further linkage of biomolecule by bio-conjugate chemistry. TEM images (Fig. 2a) demonstrate monodispersity and uniformity of the NPs with an average diameter of about 25 nm. The XRD patterns (Fig. S1a) of NPs exhibit peaks position and intensities that can be well indexed to the pure cubic KYF4 crystal [27] and no impurities or traces of other phases were identified, indicating high crystallinity of the NPs. Furthermore, the fluorescence emission spectra of separate (Fig. S1b, c & d) and combined (Fig. 2c) Ln3+ doped KYF4 NPs were measured at 273 nm excitation wavelength. The Ln3+ (Ln3+ = Dy3+, Tb3+, Eu3+) doped KYF4 NPs display different emissions at 488 nm (4F9/2 to 6H15/2) for Dy3+, 544 nm (5D4 to 7F5) for Tb3+ and 618 nm (5D0 to 7F2) for Eu3+.
The WS2 nanosheets were characterized by TEM (Fig. 2b) and AFM (Fig. S2). The TEM and AFM images demonstrate the triangular shape and thickness of about 1.3 nm of WS2, indicating the few-layer structure of nanosheet. Besides, there is a good overlap (Fig. 2d) between the broad absorption spectra of WS2 nanosheet and emission spectra of multicolor TRF-NPs which present the good conditions for fluorescence quenching of TRF-NPs by WS2 nanosheet.
Characterization of NPs conjugated to avidin and aptamers
The TRF-NPs were conjugated with avidin and aptamers, respectively. The UV-vis spectrophotometer was used to describe the avidin conjugated NPs. Fig. S3 shows the decrease in peak intensities at 280 nm after functionalized with avidin, indicating a successful attachment of avidin to NPs. Fig. S4 exhibits the weak peak intensities at 260 nm after functionalized with aptamers, representing a successful attachment of aptamers to NPs by avidin-biotin system.
Optimization of the aptasensor
The WS2 nanosheet concentration has a significant effect on the fluorescence quenching. Therefore, the effect of WS2 nanosheet concentration on time-resolved fluorescence intensity (TR-FI) was studied. As shown in the Fig. S5, different concentrations of WS2 nanosheet ranging from 0.0 to 0.5 mg mL−1 were used. The time-resolved fluorescence intensity (TR-FI) decreased gradually with the increase in the concentration of WS2 from 0.01 to 0.2 mg mL−1 and then became stable from 0.3 to 0.5 mg mL−1 which shows the FI of aptasensor was quenched by the WS2. Therefore, 0.3 mg mL−1 was used as optimized WS2 concentration for following experiments.
In order to verify the quenching efficiency of WS2 nanosheet, the WS2 was incubated with three different fluorescent probes and the incubation time was investigated. As shown in the Fig. S6, the fluorescence intensity for KYF4: Dy3+ bioprobe incubated with WS2 was quenched after 15 min while the fluorescence intensities for KYF4: Tb3+ and KYF4: Eu3+ bioprobes incubated with WS2 were quenched after 20 min. Considering the three mycotoxins detected in the same system, 20 min was selected as optimized incubation time for fluorescence quenching by WS2 for the subsequent experiments.
Furthermore, the interference tests for ZEN, T-2 and AFB1 were performed separately using multicolor aptasensor. Firstly, the proper ratio of the three color bioprobes (Dy3+, Tb3+, Eu3+ = 3: 1: 2) was adjusted to make multicolor bioprobe. The multicolor bioprobe was incubated with WS2 for 20 min and then 1 ng mL−1 of each mycotoxins was added separately. The whole mixture was incubated for 40 min at 37 °C. Then TRF emission spectra were measured. As shown in the Fig. 3a–c, in the absence of ZEN, T-2 and AFB1 the original FI of mixing bioprobes was significantly quenched by WS2 (red curve). When ZEN was added separately to the sensing probe, the FI of the respective probe (at 488 nm) was restored (Fig. 3a), while FI of other probes were almost quenched. Similarly, when T-2 and AFB1 were added separately to the sensing probe, the FI of their respective probes at 544 nm (Fig. 3b) and 618 nm (Fig. 3c) respectively, were restored while FI of other probes were still quenched. The result indicated that there is a clear difference between the emission spectra of bioprobes incubated with respective target and bioprobes incubated with WS2. It has been confirmed that the bioprobes with NPs of multi-color fluorescence emission can distinguish among the three mycotoxins and can detected three mycotoxins simultaneously without interfering each other. Consequently, the aptasensor has potential to detect ZEN, T-2 and AFB1 individually and simultaneously.
The measurement of fluorescence emission spectra of multi-color bioprobe for ZEN (a) T-2 (b) and AFB1 (c), separately and emission spectra of mixed multi-color bioprobes (d) at different conditions; bioprobes (curve a), bioprobes incubated with targets (curve b), bioprobes incubated with WS2 and also with targets (curve c) and bioprobes only incubated with WS2 (curve d), respectively
Moreover, the feasibility of the aptasensor was proved by examining the fluorescence spectra of detection solutions at different conditions as shown in Fig. 3d. The strong fluorescence of bioprobes (curve a) was quenched by WS2 nanosheet (curve d). However, the fluorescence signals were restored after the addition of targets due to the removal of bond between DNA and WS2 (curve c). Besides, in the absence of WS2, the presence of target slightly affects the fluorescence (curve b) indicating that the target itself does not have significant effect on the fluorescence of bioprobe. The above results verified the feasibility of the aptasensor.
Analytical performance
In our control experiment, three emissions at 488, 544 and 618 nm for Dy3+, Tb3+ and Eu3+ were selected for simultaneous quantification of ZEN, T-2 and AFB1, respectively. Based on the optimized conditions, various concentrations (0.001–100 ng mL−1) of ZEN, T-2 and AFB1 were detected. As shown in the Fig. 4a, increase in the TRF intensities was proportional to the increase in the concentrations of mycotoxins because more target bind with TRFNPs-Apt, more TRFNPs-Apt switched away from the surface of WS2 nanosheet resulted in enhanced FI. Figure 4b shows a linear relationship between the TRF intensity (F.I) and log concentration of targets. A strong linear correlation (R2 = 0.9947, y = 7920.41 + 1969.39*x) between the F.I at 488 nm and log concentration of ZEN was observed. There is also a good linear relationship between the F.I at 544 nm (R2 = 0.9972, y = 21,690.45 + 5304.80*x) and 618 nm (R2 = 0.9909, y = 18,516.77 + 4625.50*x) and log concentration of T-2 and AFB1, respectively were noted. The results suggested that aptasensor can be applied for simultaneous detection of ZEN, T-2 and AFB1.
The sensitivity of the aptasensor was also observed. Statistical results exhibited that the limit of detection (LOD) of ZEN, T-2 and AFB1 were 0.51 pg mL−1, 0.33 pg mL−1 and 0.40 pg mL−1, respectively, which is lower than others reported methods. A comparison table (Table 1) is given between our aptasensor and earlier methods for ZEN, T-2 and AFB1 detection. It demonstrates that our method for simultaneous recognition of ZEN, T-2 and AFB1 has wider working range and lower LOD than others methods. The above findings proved that our method is highly efficient and sensitive.
Selectivity of assay
In order to test the selectivity of the aptasensor, the five mycotoxins including OTA, FB1, AFB2, DON and Patulin were added to the sample solution as interferences. In our research, specificity of aptasensor is highly depends on aptamers. The change in the fluorescence intensity (ΔF.I) was measured by following the same method as used for simultaneous detection of ZEN, T-2 and AFB1. Herein, ΔF.I = F–F0 where F and F0 were the fluorescence intensities at 488, 544 and 618 nm in the presence and absence (Probe + WS2) of targets. The fix concentration of 0.1 ng mL−1 for all the mycotoxins was interrogated. Figure 5 shows that no obvious change in the fluorescent response was detected when the interferences were in the sample. In contrast, very strong fluorescence intensities were detected when ZEN, T-2 or AFB1 were in the sample. These results clearly indicated that the aptasensor has suitable selectivity for ZEN, T-2 and AFB1 detection which was credited to the characteristic and high specificity of the aptamers toward ZEN, T-2 and AFB1.
Real food sample analysis
To explore the effectiveness of the aptasensor, tests for ZEN, T-2 and AFB1 were performed in maize sample to measure the recoveries by standard addition method. The samples were tested by ZEN, T-2 and AFB1 ELISA test kits to confirm that maize samples were not contaminated with ZEN, T-2 and AFB1. The samples were spiked with 0.05, 0.5, 5 and 50 ng mL−1 ZEN, T-2 and AFB1. The spiked samples were examined by new method and ELISA method to detect ZEN, T-2 and AFB1. The recovery results are summarized in Table S1. The recoveries obtained from our method for ZEN, T-2 and AFB1 were in the range of 94.4–98.0%, 90.0–92.0% and 87.0–96.0%, respectively. The recoveries obtained from ELISA for ZEN, T-2 and AFB1 were in the range of 98.0–102%, 92.2–100% and 96.0–112%, respectively. These results confirm the fluorescent assay as a suitable recognition method. Moreover, the results shown in Fig.S7 indicate that there is no significant difference between two methods and they are particularly correlated. The results indicate that the aptasensor can be applied to the determination of ZEN, T-2 and AFB1 in agricultural products.
Conclusion
We report that the unique interaction between WS2 and DNA can be used to construct time-resolved fluorescence nanoparticles based aptasensor for simultaneous recognition of ZEN, T-2 and AFB1. The present method has several significant features. First, it uses the strong fluorescence emission and long luminescence lifetimes of time-resolved fluorescence nanoparticles. Second, it uses extraordinarily high fluorescence quenching efficiency of WS2 which is very important for the sensitivity of quenching and recovery based biosensor. This aptasensor showed high selectivity, sensitivity and stability owing to the unique WS2 to DNA interaction and the definite aptamer to target recognition. Moreover, the detection limit of present aptasensor was much lower than most other reported assays and also homogeneous assay design made the method convenient without washing and separation steps. However, the method requires several operational steps and important nanotechnology skills in order to be reproduced by others. Conversely, it would be easy to simplify this strategy to detect the multiple targets using different aptamers.
References
Grenier B, Oswald I (2011) Mycotoxin co-contamination of food and feed: meta-analysis of publications describing toxicological interactions. World Mycotoxin J 4(3):285–313. https://doi.org/10.3920/WMJ2011.1281
Chen Y, Meng X, Zhu Y, Shen M, Lu Y, Cheng J, Xu Y (2018) Rapid detection of four mycotoxins in corn using a microfluidics and microarray-based immunoassay system. Talanta 186:299–305. https://doi.org/10.1016/j.talanta.2018.04.064
Sun Y, Hu X, Zhang Y, Yang J, Wang F, Wang Y, Deng R, Zhang G (2014) Development of an immunochromatographic strip test for the rapid detection of zearalenone in corn. J Agric Food Chem 62(46):11116–11121. https://doi.org/10.1021/jf503092j
Gao X, Cao W, Chen M, Xiong H, Zhang X, Wang S (2014) A high sensitivity electrochemical sensor based on Fe3+-ion molecularly imprinted film for the detection of T-2 toxin. Electroanal 26(12):2739–2746. https://doi.org/10.1002/elan.201400237
Ma Y, Mao Y, Huang D, He Z, Yan J, Tian T, Shi Y, Song Y, Li X, Zhu Z (2016) Portable visual quantitative detection of aflatoxin B 1 using a target-responsive hydrogel and a distance-readout microfluidic chip. Lab Chip 16(16):3097–3104 https://pubs.rsc.org/en/content/articlehtml/2016/lc/c6lc00474a
Solfrizzo M, Gambacorta L, Lattanzio VM, Powers S, Visconti A (2011) Simultaneous LC–MS/MS determination of aflatoxin M 1, ochratoxin a, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B 1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem 401(9):2831. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s00216-011-5354-z–2841
Tokuşoǧlu Ö, Ünal MK, Yemiş F (2005) Determination of the phytoalexin resveratrol (3, 5, 4 ‘-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography− mass spectrometry (GC-MS). J Agric Food Chem 53(12):5003–5009. https://doi.org/10.1021/jf050496+
Deng Q, Qiu M, Wang Y, Lv P, Wu C, Sun L, Ye R, Xu D, Liu Y, Gooneratne R (2017) A sensitive and validated immunomagnetic-bead based enzyme-linked immunosorbent assay for analyzing total T-2 (free and modified) toxins in shrimp tissues. Ecotoxicol Environ Saf 142:441–447 https://www.sciencedirect.com/science/article/pii/S0147651317302476
Zhang Z, Wang D, Li J, Zhang Q, Li P (2015) Monoclonal antibody–europium conjugate-based lateral flow time-resolved fluoroimmunoassay for quantitative determination of T-2 toxin in cereals and feed. Anal Methods 7(6):2822–2829 https://pubs.rsc.org/en/content/articlehtml/2015/ay/c5ay00100e
Wang D, Zhang Z, Li P, Zhang Q, Zhang W (2016) Time-resolved fluorescent immunochromatography of aflatoxin b1 in soybean sauce: a rapid and sensitive quantitative analysis. Sens 16(7):1094 https://www.mdpi.com/1424-8220/16/7/1094
Liu Y, Tu D, Zhu H, Chen X (2013) Lanthanide-doped luminescent nanoprobes: controlled synthesis, optical spectroscopy, and bioapplications. Chem Soc Rev 42(16):6924–6958 https://pubs.rsc.org/en/content/articlehtml/2013/cs/c3cs60060b
Li F, Zhang H, Wang Z, Newbigging AM, Reid MS, Li X-F, Le XC (2014) Aptamers facilitating amplified detection of biomolecules. Anal Chem 87(1):274–292. https://doi.org/10.1021/ac5037236
Zhou W, Huang P-JJ, Ding J, Liu J (2014) Aptamer-based biosensors for biomedical diagnostics. Anal 139(11):2627–2640 https://pubs.rsc.org/en/content/articlehtml/2014/an/c4an00132j
Huang Y, Zhang H, Chen X, Wang X, Duan N, Wu S, Xu B, Wang Z (2015) A multicolor time-resolved fluorescence aptasensor for the simultaneous detection of multiplex Staphylococcus aureus enterotoxins in the milk. Biosens Bioelectron 74:170–176. https://doi.org/10.1016/j.bios.2015.06.046
Huang Y, Chen X, Wu S, Duan N, Yu Y, Wang Z (2015) Homogeneous time-resolved fluorescence assay for the detection of ricin using an aptamer immobilized on europium-doped KGdF4 nanoparticles and graphene oxide as a quencher. Microchim Acta 182(5–6):1035–1043. https://doi.org/10.1007/s00604-014-1422-3
Wang X, Niazi S, Yukun H, Sun W, Wu S, Duan N, Hun X, Wang Z (2017) Homogeneous time-resolved FRET assay for the detection of Salmonella typhimurium using aptamer-modified NaYF4: Ce/Tb nanoparticles and a fluorescent DNA label. Microchim Acta 184(10):4021–4027. https://doi.org/10.1007/s00604-017-2399-5
Voiry D, Yamaguchi H, Li J, Silva R, Alves DC, Fujita T, Chen M, Asefa T, Shenoy VB, Eda G (2013) Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat Mater 12(9):850. https://www.nature.com/articles/nmat3700–855
Qin Y, Ma Y, Jin X, Zhang L, Ye G, Zhao S (2015) A sensitive fluorescence turn-on assay of bleomycin and nuclease using WS2 nanosheet as an effective sensing platform. Anal Chim Acta 866:84–89. https://doi.org/10.1016/j.aca.2015.01.049
Lin T, Zhong L, Song Z, Guo L, Wu H, Guo Q, Chen Y, Fu F, Chen G (2014) Visual detection of blood glucose based on peroxidase-like activity of WS2 nanosheets. Biosens Bioelectron 62:302–307. https://doi.org/10.1016/j.bios.2014.07.001
Ge J, Du Y-H, Chen J-J, Zhang L, Bai D-M, Ji D-Y, Hu Y-L, Li Z-H (2017) Highly sensitive fluorescence detection of mercury (II) ions based on WS2 nanosheets and T7 exonuclease assisted cyclic enzymatic amplification. Sensors Actuators B Chem 249:189–194. https://doi.org/10.1016/j.snb.2017.04.094
Zuo X, Zhang H, Zhu Q, Wang W, Feng J, Chen X (2016) A dual-color fluorescent biosensing platform based on WS2 nanosheet for detection of Hg2+ and Ag+. Biosens Bioelectron 85:464–470. https://doi.org/10.1016/j.bios.2016.05.044
Xi Q, Zhou D-M, Kan Y-Y, Ge J, Wu Z-K, Yu R-Q, Jiang J-H (2014) Highly sensitive and selective strategy for microRNA detection based on WS2 nanosheet mediated fluorescence quenching and duplex-specific nuclease signal amplification. Anal Chem 86(3):1361–1365. https://doi.org/10.1021/ac403944c
Chen X, Huang Y, Duan N, Wu S, Ma X, Xia Y, Zhu C, Jiang Y, Wang Z (2013) Selection and identification of ssDNA aptamers recognizing zearalenone. Anal Bioanal Chem 405(20):6573–6581. https://doi.org/10.1007/s00216-013-7085-9
Chen X, Huang Y, Duan N, Wu S, Xia Y, Ma X, Zhu C, Jiang Y, Wang Z (2014) Screening and identification of DNA aptamers against T-2 toxin assisted by graphene oxide. J Agric Food Chem 62(42):10368–10374. https://doi.org/10.1021/jf5032058
Ma X, Wang W, Chen X, Xia Y, Wu S, Duan N, Wang Z (2014) Selection, identification, and application of aflatoxin B1 aptamer. Eur Food Res Technol 238(6):919–925. https://doi.org/10.1007/s00217-014-2176-1
Ju Q, Tu D, Liu Y, Li R, Zhu H, Chen J, Chen Z, Huang M, Chen X (2011) Amine-functionalized lanthanide-doped KGdF4 nanocrystals as potential optical/magnetic multimodal bioprobes. J Am Chem Soc 134(2):1323–1330. https://doi.org/10.1021/ja2102604
Schäfer H, Ptacek P, Zerzouf O, Haase M (2008) Synthesis and optical properties of KYF4/Yb, Er nanocrystals, and their surface modification with undoped KYF4. Adv Funct Mater 18(19):2913–2918. https://doi.org/10.1002/adfm.200800368
Wu Z, Xu E, Chughtai MF, Jin Z, Irudayaraj J (2017) Highly sensitive fluorescence sensing of zearalenone using a novel aptasensor based on upconverting nanoparticles. Food Chem 230:673–680. https://doi.org/10.1016/j.foodchem.2017.03.100
Niazi S, Wang X, Pasha I, Khan IM, Zhao S, Shoaib M, Wu S, Wang Z (2018) A novel bioassay based on aptamer-functionalized magnetic nanoparticle for the detection of zearalenone using time resolved-fluorescence NaYF4: Ce/Tb nanoparticles as signal probe. Talanta 186:97–103. https://doi.org/10.1016/j.talanta.2018.04.013
Khan IM, Zhao S, Niazi S, Mohsin A, Shoaib M, Duan N, Wu S, Wang Z (2018) Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin. Sensors Actuators B Chem 277:328–335. https://doi.org/10.1016/j.snb.2018.09.021
Wang D, Zhang Z, Li P, Zhang Q, Ding X, Zhang W (2015) Europium nanospheres-based time-resolved fluorescence for rapid and ultrasensitive determination of total aflatoxin in feed. J Agric Food Chem 63(47):10313–10318. https://doi.org/10.1021/acs.jafc.5b03746
Zhang Z, Tang X, Wang D, Zhang Q, Li P, Ding X (2015) Rapid on-site sensing aflatoxin B1 in food and feed via a chromatographic time-resolved fluoroimmunoassay. PLoS One 10(4):e0123266. https://doi.org/10.1371/journal.pone.0123266
Sun L, Wu L, Zhao Q (2017) Aptamer based surface plasmon resonance sensor for aflatoxin B1. Microchim Acta 184(8):2605–2610. https://doi.org/10.1007/s00604-017-2265-5
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182(3–4):571–578. https://doi.org/10.1007/s00604-014-1360-0
Tang X, Li P, Zhang Q, Zhang Z, Zhang W, Jiang J (2017) Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal Chem 89(21):11520–11528. https://doi.org/10.1021/acs.analchem.7b02794
Acknowledgements
This work was partly funded by the National Natural Science Foundation of China (31871881), Zhangjiagang Science and Technology Support plan (Social development) (ZKS 1803), Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) (CX (18)2025), S&T Support Program of Jiangsu Province (BE2017623), the National First-class Discipline Program of Food Science and Technology (JUFSTR20180303), JUSRP51714B and the Distinguished Professor Program of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3.72 mb)
Rights and permissions
About this article
Cite this article
Niazi, S., Khan, I.M., Yu, Y. et al. A “turnon” aptasensor for simultaneous and time-resolved fluorometric determination of zearalenone, trichothecenes A and aflatoxin B1 using WS2 as a quencher. Microchim Acta 186, 575 (2019). https://doi.org/10.1007/s00604-019-3570-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3570-y