Abstract
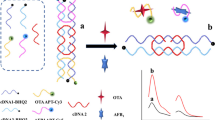
Herein, a dual-signal output fluorescent aptamer sensor was constructed for the simultaneous detection of aflatoxin B1 (AFB1) and ochratoxin A (OTA) using the specific recognition ability of aptamers and the programmability of DNA. A functional capture probe (cDNA) was designed with the black hole quenching motif BHQ1 labeled at the 5′ end and biotin (bio) labeled at the 3′ end. The fluorescent dye Cy3-labeled aflatoxin B1 aptamer (AFB1-Apt) and the carboxyfluorescein FAM-labeled ochratoxin A aptamer (OTA-Apt) were used as two fluorescent probes. The cDNA is anchored to the quenching material gold nanoflowers (AuNFs) by the action of streptavidin (SA) and biotin. Its ends can be complementarily paired with two fluorescent probe bases to form a double-stranded structure. The fluorescence of Cy3 was quenched by AuNFs, and the fluorescence of FAM was quenched by BHQ1 through the fluorescence energy resonance transfer (FRET) effect, forming a fluorescence quenching system. Due to the high affinity of the target and the aptamer, the structure of the aptamer probe changes and detaches from the sensor when AFB1 and OTA are present, resulting in enhanced fluorescence. Under optimal conditions, the linear range of AFB1 was 0.1–100 ng/mL (R2 = 0.996), the limit of detection (LOD) was as low as 0.014 ng/mL, and the limit of quantification (LOQ) was 0.046 ng/mL. The linear range of OTA was 0.1–100 ng/mL (R2 = 0.995), the limit of detection (LOD) was as low as 0.027 ng/mL, and the limit of quantification (LOQ) was 0.089 ng/mL. The sensor had high accuracy in detecting both AFB1 and OTA in real sample analysis. The results of the t test show that there is no significant difference between the results of this study and the high-performance liquid phase (HPLC) method, indicating that the prepared sensor can be used as a potential platform for multiple mycotoxins detection.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycotoxins are toxic secondary metabolites produced by fungal species that often contaminate feed and grains during processing and storage. More importantly, the majority of them are extremely heat-stable and difficult to remove once they have contaminated the food chain [1, 2]. The main mycotoxins in food are aflatoxin B1 (AFB1) and ochratoxin A (OTA). AFB1 is the most toxic of the various mycotoxins and is defined as a class I carcinogen by the International Agency for Research on Cancer (IARC). OTA is closely related to nephrotoxicity and teratogenic effects and is classified as a class II-B carcinogen by IARC [3, 4]. There is definite evidence that both can co-exist in agricultural products and can have synergistic toxic effects, even carcinogenic to humans [5, 6]. Thus, it is important to establish analytical methods for the simultaneous detection of multiple mycotoxins compared to the detection of single mycotoxins.
In recent years, the commonly used detection methods include high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), high-performance liquid chromatography-tandem mass spectrometry (HPLC–MS), and enzyme-linked immunoassay methods [7,8,9,10,11]. Although the sensitivity and accuracy of these methods are good, a cumbersome sample pretreatment process, expensive instruments, difficult antibody preparation, and a high level of expertise are required for laboratory personnel. They cannot meet the requirements of rapid and on-site detection and have certain limitations. Based on this, it is necessary to develop a simple and economical method for mycotoxin detection.
Aptamers are synthetic DNA or RNA oligonucleotides isolated from random sequence libraries with high specificity and affinity using an exponential enrichment (SELEX) process [12]. Compared to traditional immune antibodies, aptamers have low synthetic costs, better chemical stability, and simple structures that can be easily modified [13]. Aptamers are promising sensing elements that can be used to develop important biosensors. There is a wide variety of biosensors that use aptamers to detect fungal toxins, such as electrochemical aptamer sensors, surface-enhanced Raman scattering aptamer sensors, colorimetric aptamer sensors, and fluorescent aptamer sensors [14,15,16,17]. Fluorescent aptamer sensor has high sensitivity, wide detection range, rapid signal response, strong selectivity, so it has received wide attention in the field of food detection.
Nanomaterials have been introduced into the design of biosensors due to their high quenching efficiency and specific surface area, including gold nanomaterials, composite nanomaterials, and carbon nanomaterials [18]. In fluorescent aptamer sensors, gold nanomaterials are often used as quenching materials and to improve sensitivity. In recent years, with the in-depth research on gold nanomaterials, more and more gold composites have attracted widespread attention, such as gold nanorods (GNRs), gold nanobipyramids (AuNBPs), gold nanoflowers (AuNFs), silver/gold bimetallic nanoparticles (Ag/AuNPs), AuNFs consist of a central core and many protruding tips, which can form a strong electric field effect compared to the prominent branching structure of AuNPs exhibiting a strong optical extinction coefficient [19, 20]. In addition, this special structure also increases the adsorption sites on its surface and reduces the spatial resistance during labeling, effectively increasing the quenching efficiency and thus improving the sensitivity of the sensor. Li et al. [21] reported a method based on AuNFs as quenching materials to detect small molecule thiols in apoptotic cells using fluorescence. Sun et al. [22] established an ultrasensitive sensing platform based on the FRET effect of the “AuNF@graphene quantum dot” probe for the detection of miRNA-34a. These results indicate that branched gold nanocrystals have ideal fluorescence quenching capabilities. In fluorescent aptamer sensors, the distance between the quenching material and the fluorescent moiety is the main factor affecting fluorescence resonance energy transfer (FRET). It has been shown that the tip structure of AuNFs is more likely to be close to the probe to achieve good quenching, and the quenching effect of AuNFs is gradually enhanced with the increase of protruding tips during the growth process [23, 24].
Herein, a dual-signal output fluorescent aptamer sensor was constructed for the simultaneous detection of AFB1 and OTA using the high affinity of aptamer and target, the high fluorescence quenching efficiency of AuNFs, and the programmability of DNA. A complementary strand was designed as a capture probe (cDNA) that can bind to two different aptamer strands simultaneously for dual-target detection. The sensor uses AuNFs and the quenching group BHQ1 in the capture probe as energy receptors and Cy3 and FAM in the fluorescent probe as energy receptors. The cDNA binds to the aptamer strand by base complementary pairing, and the proximity of FAM to the quenching group BHQ1 as well as Cy3 to the AuNFs produces a FRET effect, leading to quenching of the fluorescence of Cy3 and FAM. When the target is present, the fluorescent probe detaches from the sensor due to the high affinity of the target and the aptamer, resulting in a diminished FRET effect and enhanced fluorescence. The sensor is easy to operate and enables high-precision detection of AFB1 and OTA in food.
Experimental sections
Materials and instruments
AFB1-Apt, OTA-Apt, and complementary strands were synthesized, modified, and purified by Sangon Biotechnology Co., Ltd. (Shanghai, China). Detail sequences are given in Table S1. Both DNA dilution and sensor preparation were performed with 50 mM Tris–HCl buffer (50 mM Tris, 0.2 M NaCl, 1 mM EDTA, pH 7.4). Sodium chloride (NaCl), hydrochloric acid (HCl), and 1,4-hydroquinone were purchased from Tianjin Kemeiou Chemical Reagent Co., Ltd. Tetrachloroauric acid (HAuCl4) was obtained from Tianjin Chemical Reagent Co., Ltd. Streptavidin (SA) was purchased from Beijing Soleibo Technology Co., Ltd. AFB1, OTA, zearalenone (ZEN), deoxynivalenol (DON), and T-2 toxin were all from Sigma-Aldrich. Ochratoxin B(OTB), aflatoxins M1 (AFM1), and aflatoxins B2 (AFB2) were provided by ANPEL Laboratory Technologies (Shanghai, China) Inc. Fumonisin B1 (FB1) was supplied by Acros Organics. White peony powder is white peony herb-based, processed into powder form. It can be used as a Chinese medicinal material. The white peony powder used in our research is a certified standard substance. The actual samples were bought from a local supermarket in Zhengzhou, China. All chemicals are analytically pure, and all aqueous solutions are prepared with ultrapure water (18.2 MΩ).
Scanning electron microscope (SEM, JSM-7610F JEOL, Japan), transmission electron microscope (TEM, FEI Tecnai G2 F20 S-TWIN), and high-resolution transmission electron microscope (HRTEM, JEM-F200 JEOL, Japan) were used to observe the surface morphology and internal structure of AuNFs. The crystal structure of the gold nanoparticles was determined using a D8 Advance x-ray powder diffractometer (XRD, Bruker, Germany). An ultraviolet spectrophotometer (UV, 6100S, Shanghai, China) was used to measure the ultraviolet absorption spectrum. The fluorescent emission spectra were recorded by using a Cary Eclipse Fluorescence Spectrophotometer (Varian, USA).
Preparation of gold nanoflowers
First, colloidal gold seeds were prepared by the classical hydrothermal reduction method [25], and the preparation process is shown in supporting information. The obtained colloidal gold seed solution was stored at room temperature with cooling at 4 °C for backup. AuNFs were prepared by the seed growth method using sodium citrate dihydrate as a reducing agent and HAuCl4 as a precursor using a previously reported method [26]. Three hundred seventy-five microliter μL of 1% (w/v) HAuCl4, 220 μL of 1% sodium citrate, and 2 mL of 20 nm colloidal gold seed solution were added to 100 mL of ultrapure water with vigorous stirring. Then 1 mL of 0.03 mol/L 1,4-benzenediol solution was added to the mixture and incubated for 30 min at room temperature to obtain a clear, dark blue AuNFs solution.
Preparation of AuNFs@SA/cDNA/Apt sensor
First, 20 μL of AuNFs solution was thoroughly mixed with 80 μL of SA solution, and the mixed solution was incubated for 2 h at room temperature to obtain an AuNFs @SA solution. Mix the cDNA with AuNFs@SA solution, and incubate for 1 h to obtain AuNFs@SA/cDNA. Finally, AFB1-Apt and OTA-Apt were added to the above solution and incubated for 1 h to obtain AuNFs@SA/cDNA/Apt sensor.
Simultaneous detection of AFB1 and OTA by the sensor
AFB1 and OTA standard solutions were added to the above-prepared system and incubated for 40 min at 37 °C. Finally, the volume of the final reaction system was replenished to 200 μL with Tris–HCl solution. The measurements were then performed at room temperature using a fluorescence spectrophotometer. The excitation and emission slit widths were set to 10 nm, the voltage was 630 V, and the excitation wavelengths were 535 nm (Cy3) and 490 nm (FAM), respectively.
Detection of actual samples
In order to study the detection performance of the sensor in actual samples, the actual test samples were purchased from a local supermarket in Zhengzhou, China. The feasibility of the designed sensor was evaluated.
Pretreatment of solid samples [27]: the samples were ground into powders with an electric mill. One milliliter of different concentrations of AFB1 and OTA standard solutions was added to 0.5 g of the powdered sample, respectively. After drying at room temperature, 5 mL of extraction solvent (methanol:water = 7:3 (v/v)) was added and placed in an oscillator for 30 min. Then the extracts were centrifuged at 12,000 r/min for 10 min. The supernatant was obtained by filtration with 0.22 μm organic filter membrane, and the filtrate was diluted tenfold with Tris–HCl buffer. According to the above pretreatment method, 0 ng/mL, 0.1 ng/mL, 1 ng/mL, and 10 ng/mL of AFB1 and OTA spiked sample extracts were prepared, respectively.
Pretreatment of red wine samples: 5.0 mL of red wine was mixed directly with different concentrations of AFB1 and OTA standard solutions. The spiked samples of 0 ng/mL, 0.1 ng/mL, 1 ng/mL, and 10 ng/mL were prepared by filtering them with a 0.22-μm filter membrane and diluting them 10 times with Tris–HCl buffer.
Results and discussion
The structural characteristics of AuNPs and AuNFs
The shape and size of the AuNPs can be observed by TEM (Fig. 1A), and it can be seen that the gold nanoparticles are well dispersed and have a smooth surface. The average particle size is around 20 ± 2 nm, indicating that the AuNPs were successfully prepared. The structure of the crystalline AuNPs was analyzed by X-ray diffraction (XRD) (Fig. S1). The diffraction peak corresponding to (111) is significantly stronger than the other peaks, and the absence of other impurity peaks indicates the high purity of the prepared AuNPs. The size and morphology of the obtained AuNFs were characterized using TEM and HRTEM. As can be seen in Fig. 1B, the obtained AuNFs are branching structures with a diameter of 75 ± 5 nm. The HRTEM image (Fig. 1C) shows that the AuNFs have periodic 0.23 nm streaks that are close to the eigenvalues of the Au (111) lattice surface [28]. The scanning electron microscope (SEM) image of AuNFs (Fig. 1D) shows the morphology of the AuNF surface in a flower shape, which further verifies the successful preparation of AuNFs. The UV scans of AuNPs and AuNFs were performed (Fig. S2), and the extinction coefficient of AuNFs calculated from the UV absorption spectra was 1.98 times higher than that of AuNPs, indicating that the fluorescence quenching ability of AuNFs was stronger.
The principle of simultaneous detection of AFB1 and OTA by the aptamer sensor
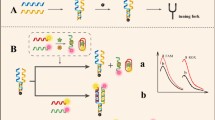
Based on the quenching properties of AuNFs and the high affinity of aptamers, a fluorescent aptamer sensor was constructed for the simultaneous detection of AFB1 and OTA, and its detection principle is shown in Scheme 1. The cDNA is firstly sequestered onto AuNFs by the action of bio and SA to form a capture probe of AuNFs@SA/cDNA structure. When AFB1 and OTA are not present in the system, AFB1-Apt and OTA-Apt, which are fluorescent probes, bind to cDNA hybridization. At this time, the distance between Cy3 and AuNFs and between FAM and BHQ1 is close enough to produce the FRET effect. Therefore, the sensor has a low fluorescence signal (a). When AFB1 and OTA are present in the system, AFB1-Apt binds preferentially to AFB1 and OTA-Apt binds preferentially to OTA and is released from the sensor structure due to the high target selectivity of the aptamer. As the distance between Cy3 and AuNFs, FAM and BHQ1 becomes larger, the FRET effect becomes lower, leading to an increase in fluorescence intensity (b).
Proof of principle for simultaneous detection of AFB1 and OTA by the sensor
The feasibility of this fluorescence sensor was verified using the fluorescence spectra generated in the presence or absence of AFB1 and OTA. Due to the FRET between AuNFs and Cy3, BHQ1 and FAM, Cy3 and FAM showed weak fluorescence when a blank buffer solution without AFB1 and OTA was added, with fluorescence intensities of 303 and 153, respectively (Fig. 2a). When both AFB1 and OTA were present in the system, the aptamer separated from the complementary chain structure due to the specific binding of AFB1 and OTA to their respective aptamers. The fluorescence intensity of Cy3 and FAM recovered to 395 and 298, respectively, with a significant increase (Fig. 2b). The results showed that the fluorescent sensor successfully achieved the simultaneous detection of AFB1 and OTA.
3.0% agarose gel electrophoresis was used to characterize the successful construction of this aptasensor. As shown in Fig. S3, the first lane is the DNA molecular weight standard Marker, and bands 2–4 represent the migration positions of AFB1-Apt, OTA-Apt, and cDNA, respectively. Lane 5 represents the DNA duplex assembled by AFB1-Apt/OTA-Apt/cDNA. Due to the self-assembly forming the DNA duplex structure, the molecular weight becomes larger and the migration rate is the slowest, and the duplex bases can embed more dye, resulting in the brightest bands. Lane 6 is a mixture of AFB1-Apt/ OTA-Apt/ cDNA/AFB1/OTA. The double-stranded structure is dissociated due to the specific binding of the target and the aptamer strand. Therefore, the brightness of the bands in lane 6 is significantly darker compared to lane 5. The above gel electrophoresis characterization results indicate that the sensor was prepared successfully.
Experimental parameter optimization
The concentration of SA was optimized (Fig. 3A and Fig. 3B). The relative fluorescence intensity (ΔF) increased gradually with the increase of SA concentration. The cDNA was maximally bound to SA when the SA concentration reached 10 μg/mL. Afterward, when AFB1-Apt was added to complementary binding with cDNA, the fluorescence of Cy3 was quenched by AuNFs, and ΔF was maximum when AFB1 was added. As SA continues to increase, there will be excess SA free in the system, at which time some cDNA will bind to the free SA and the ΔF obtained is smaller. Therefore, 10 μg/mL was used as the optimal concentration of SA.
Experimental parameter optimization. Fluorescence spectra (A) and fluorescence intensity changes (B) under different SA concentrations. Fluorescence spectra (C) and fluorescence intensity changes (D) of AuNFs@SA with different volumes. Fluorescence spectra (E) and fluorescence intensity changes (F) at different concentration ratios of AFB1-Apt and cDNA. Fluorescence spectra (G) and fluorescence intensity changes (H) of OTA-Apt and cDNA at different concentration ratios. [CAFB1 = COTA = 100 ng/mL]
Six to 25 μL of AuNFs@SA was used to construct the aptamer sensor to obtain the optimal AuNFs@SA dosage (Fig. 3C and Fig. 3D). As the amount of AuNFs@SA increased, ΔF also increased significantly, because more cDNA strands could be bound and immobilized on the surface of AuNFs@SA to participate in the construction of the sensor. When the amount of AuNFs@SA reached 10 μL, the ΔF reached the maximum value. With the increasing amount of AuNFs@SA, the degree of c of AuNFs@SA was further increased, resulting in a lower ΔF. Therefore, 10 μL was chosen as the parameter for the subsequent reaction.
The ratio of the complementary strand to AFB1-Apt (Fig. 3E and Fig. 3F) and OTA-Apt (Fig. 3G and Fig. 3H) was optimized. ΔF was lower when the concentrations of AFB1-Apt and OTA-Apt were lower. When cDNA: AFB1-Apt: OTA-Apt = 1:1:1, the fluorescence recovery value reaches the maximum. Continuing to increase the concentration of AFB1-Apt and OTA-Apt, there will be excess AFB1-Apt and OTA-Apt free in the system, and some of the targets will bind to the free AFB1-Apt and OTA-Apt in the system, resulting in a smaller ΔF. Therefore, 1:1:1 was used as the optimal ratio of cDNA to AFB1-Apt and OTA-Apt.
Optimization of base complementation pairs of cDNAs with AFB1-Apt (Fig. S4A and Fig. S4B) and OTA-Apt (Fig. S4C and Fig. S4D) was studied. When the number of aptamer chain clamps with complementary chains is too many or too few, it will affect the binding of the target to the aptamer chain. It is not favorable for sensor construction. The ΔF reaches a maximum when AFB1-Apt complements the cDNA by 19 bases and OTA-Apt complements the cDNA by 28 fragments. Therefore, we chose cDNA as the last experimental parameter.
Simultaneous detection AFB1 and OTA
The spectral results of the prepared aptamer sensors after incubation with different concentrations of AFB1 and OTA are shown in Fig. 4A. The fluorescence signal increased gradually with the increase of AFB1 and OTA concentrations. The difference in fluorescence intensity of Cy3 was linearly related to the logarithm of AFB1 concentration in the concentration range of 0.1–100 ng/mL (Fig. 4B) with the linear equation ΔF = 48.601 + 33.175LgCAFB1 (R2 = 0.995). The difference in FAM fluorescence intensity was linearly related to the logarithm of OTA concentration over the concentration range of 0.1–100 ng/mL (Fig. 4C) with the linear equation ΔF = 57.571 + 41.719LgCOTA (R2 = 0.996). The detection limits for AFB1 and OTA were 0.014 ng/mL and 0.027 ng/mL, respectively (3σ principle), and the quantification limits are 0.046 ng/mL and 0.089 ng/mL, respectively. The designed fluorescent aptamer sensor was compared with other detection methods, and the results are shown in Table 1. It can be seen that the designed fluorescent aptamer sensor has the advantages of rapidity, wider linear range, and lower detection limit.
A Fluorescence spectra of the prepared aptasensor incubated with different concentrations of AFB1 and OTA (0, 0.1, 0.5, 1, 10, 50, 100, 200, 500 ng/mL). B Fluorescence signal changes of the prepared aptasensor incubated with different concentrations of AFB1 (0.1, 0.5, 1, 10, 50, 100, 200, 500 ng/mL). Inset: linear relationship between fluorescence recovery and Lg (CAFB1). C Fluorescence signal changes of the prepared aptasensor after incubation with different concentrations of OTA (0.1, 0.5, 1, 5, 10, 50, 100, 200,500 ng/mL). Inset: linear relationship between fluorescence recovery value and Lg (COTA)
Specificity, repeatability, and reproducibility of the sensor
To evaluate the specificity of this fluorescent sensor for AFB1 and OTA, the fluorescence signal changes of the fluorescent sensor in the presence of some interfering toxins (DON, FB1, T-2, ZEN, OTB, AFB2, AFM1) were measured (Fig. 5A). The relative fluorescence intensities obtained when the sensor was incubated with DON, FB1, T-2, ZEN, OTB, AFB2, and AFM1 (tenfold AFB1 and OTA concentrations) alone were similar to the blank values, indicating that none of these interfering toxins caused significant changes in fluorescence intensity. When the seven toxins were mixed with AFB1 and OTA and then incubated with the sensor, the relative fluorescence intensities obtained were significantly higher compared to the blank group and were not significantly different from those in the presence of only the target. This indicates that the sensor has a high specificity for the detection of AFB1 and OTA. Six measurements of AFB1 and OTA were performed using the same sensor (Fig. 5B) with RSDs of 3.6% and 5.1%, respectively, indicating that the sensor prepared by this method has good repeatability. AFB1 and OTA were detected using six identical sensors (Fig. 5C) with RSDs of 3.9% and 2.5%, respectively, indicating that the sensors prepared by this method have acceptable reproducibility.
A Selective analysis of AFB1 and OTA by adapted sensors. The mixture contains DON, FB1, T-2, ZEN, OTB, AFB2, AFB1 and OTA. [CAFB1 = COTA = 100 ng/mL; CDON = CFB1 = CT-2 = CZEN = COTB = CAFB2 = CAFM1 = 1000 ng/mL]. B Six replicates of AFB1 and OTA tests were analyzed using the same sensor. C Reproducibility analysis using six identical sensors. [CAFB1 = COTA = 100 ng/mL]
Detection of actual samples
To investigate the feasibility of the sensor for AFB1 and OTA detection in real samples, corn, red wine, wheat, and peanut purchased from the Zhengzhou area were selected as the actual samples. The results of the spiking experiments are shown in Table 2. The spiked amounts of AFB1 and OTA were 0.1, 1.0, and 10 ng/mL, respectively. The recoveries of AFB1 and OTA in the spiked samples ranged from 94.1% to 111.7% and 98.7% to 112.2%, respectively. It was shown that the method has good analytical ability in real samples. In order to further evaluate the applicability of the constructed fluorescence sensor, quality control samples certified to contain AFB1 white peony powder (Chinese medicinal medicine) were assayed and analyzed. The results are shown in Table 3. The calculated values of t in the quality control sample of white peony powder were 1.732 and 0.616, which were less than 4.303 for t (0.05 n=3) with 95% confidence level, and the values obtained by the sensors were consistent with the certified values. The HPLC method was used as a standard method to assess the accuracy of the method for OTA levels in corn flour, and the results are shown in Table 4. The analysis was performed using a T test and all t values in corn flour were less than t (0.05 n=3) of 4.303, indicating no significant difference. It can be seen that the prepared fluorescent aptamer sensor has a certain accuracy with 95% confidence level. It indicates that the aptamer sensor has a strong potential for practical applications.
Conclusion
In summary, we successfully constructed a fluorescent aptamer sensor for the simultaneous detection of AFB1 and OTA. The excellent optical properties and large specific surface area of the quenching material AuNFs greatly improved the performance of the sensor. A functional capture probe was also designed using the programmability of DNA, enabling its two ends to bind to both fluorescent probes simultaneously. The nanogap based on AuNFs and Cy3 as well as BHQ and FAM generates a FRET effect. The sensor has the advantage of multi-target detection capability and high sensitivity. The linear range of AFB1 and OTA is 0.1–100 ng/mL, the detection limits are 0.014 ng/mL and 0.027 ng/mL, respectively, and the quantification limits are 0.046 ng/mL and 0.089 ng/mL, respectively. In addition, good recoveries were demonstrated in the spiked measurements of maize, red wine, wheat, and peanut samples, confirming the reliability and practicality of the strategy. The rational design of this fluorescent aptamer sensor promotes the application of aptamers in sensors while providing a promising method for the detection of multiple fungal toxins.
References
Zhang D, Cai L, Bian F, Kong T, Zhao Y. Label-free quantifications of multiplexed mycotoxins by G-quadruplex based on photonic barcodes. Anal Chem. 2020;92:2891–5. https://doi.org/10.1021/acs.analchem.9b05213.
Li Q, Liang B, Li W, Li W, Sun J, Jiao S, Wang S, Jin Y, Zheng T, Li J. A capillary device made by aptamer-functionalized silica photonic crystal microspheres for the point-of-care detection of ochratoxin A. Sensors Actuators B Chem. 2021;330:129367. https://doi.org/10.1016/j.snb.2020.129367.
Sun S, Zhao R, Xie Y, Liu Y. Photocatalytic degradation of aflatoxin B by activated carbon supported TiO2 catalyst. Food Control. 2019;100:183–8. https://doi.org/10.1016/j.foodcont.2019.01.014.
Qian J, Cui H, Lu X, Wang C, An K, Hao N, Wang K. Bi-color FRET from two nano-donors to a single nano-acceptor: a universal aptasensing platform for simultaneous determination of dual targets. Chem Eng J. 2020;401:126017. https://doi.org/10.1016/j.cej.2020.126017.
Yang C, Song G, Lim W. Effects of mycotoxin-contaminated feed on farm animals. J Hazard Mater. 2020;389:122087. https://doi.org/10.1016/j.jhazmat.2020.122087.
Serrano AB, Font G, Ruiz MJ, Ferrer E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chem. 2012;135(2):423–9. https://doi.org/10.1016/j.foodchem.2012.03.064.
Xu J, Liu T, Chi J, Zhang W, Lin C, Lin X, Xie Z. Online high-efficient specific detection of zearalenone in rice by using high-loading aptamer affinity hydrophilic monolithic column coupled with HPLC. Talanta. 2020;219:121309. https://doi.org/10.1016/j.talanta.2020.121309.
Feizollahi E, Arshad M, Yadav B, Ullah A, Roopesh MS. Degradation of deoxynivalenol by atmospheric-pressure cold plasma and sequential treatments with heat and UV light. Food Eng Rev. 2020;13(3):696–705. https://doi.org/10.1007/s12393-020-09241-0.
Pernica M, Kyralova B, Svoboda Z, Bosko R, Brozkova I, Ceslova L, Benesova K, Cervenka L, Belakova S. Levels of T-2 toxin and its metabolites, and the occurrence of Fusarium fungi in spring barley in the Czech Republic. Food Microbiol. 2021;102:103875. https://doi.org/10.1016/j.fm.2021.103875.
Lai W, Wei Q, Xu M, Zhuang J, Tang D. Enzyme-controlled dissolution of MnO2 nanoflakes with enzyme cascade amplification for colorimetric immunoassay. Biosens Bioelectron. 2017;89:645–51. https://doi.org/10.1016/j.bios.2015.12.035.
Sun S, Zhao R, Xie Y, Liu Y. Reduction of aflatoxin B1 by magnetic graphene oxide/TiO2 nanocomposite and its effect on quality of corn oil. Food Chem. 2021;343:128521. https://doi.org/10.1016/j.foodchem.2020.128521.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–22. https://doi.org/10.1038/346818a0.
Lv S, Zhang K, Zhu L, Tang D. ZIF-8-assisted NaYF4:Yb, Tm@ZnO converter with exonuclease III-powered DNA walker for near-infrared light responsive biosensor. Anal Chem. 2020;92(1):1470–6. https://doi.org/10.1021/acs.analchem.9b04710.
Wei M, Yue S, Zhang W, Li X. Development of an electrochemical aptasensor using Au octahedra and graphene for signal amplification. Anal Methods. 2020;12(3):317–23. https://doi.org/10.1039/c9ay01981b.
Ma X, Shao B, Wang Z. Gold@silver nanodumbbell based inter-nanogap aptasensor for the surface enhanced Raman spectroscopy determination of ochratoxin A. Anal Chim Acta. 2021;1188:339189. https://doi.org/10.1016/j.aca.2021.339189.
Lerdsri J, Chananchana W, Upan J, Sridara T, Jakmunee J. Label-free colorimetric aptasensor for rapid detection of aflatoxin B1 by utilizing cationic perylene probe and localized surface plasmon resonance of gold nanoparticles. Sensors Actuators B Chem. 2020;320:128356. https://doi.org/10.1016/j.snb.2020.128356.
Qian J, Ren C, Wang C, Chen W, Lu X, Li H, Liu Q, Hao N, Li H, Wang K. Magnetically controlled fluorescence aptasensor for simultaneous determination of ochratoxin A and aflatoxin B1. Anal Chim Acta. 2018;1019:119–27. https://doi.org/10.1016/j.aca.2018.02.063.
Sun Y, Zhao J, Liang L. Recent development of antibiotic detection in food and environment: the combination of sensors and nanomaterials. Mikrochim Acta. 2021;188(1):21. https://doi.org/10.1007/s00604-020-04671-3.
Dondapati S, Sau T, Hrelescu C, Klar T, Stefani F, Feldmann J. Label-free biosensing based on single gold nanostars as plasmonic transducers. ACS Nano. 2010;4(11):6318–22. https://doi.org/10.1021/nn100760f.
Shende P, Kasture P, Gaud R. Nanoflowers: the future trend of nanotechnology for multi-applications. Artif Cell Nanomed B. 2018;46:S413–22. https://doi.org/10.1080/21691401.2018.1428812.
Li C, Chen P, Khan I, Wang Z, Zhang Y, Ma X. Fluorescence-Raman dual-mode quantitative detection and imaging of small-molecule thiols in cell apoptosis with DNA-modified gold nanoflowers. J Mater Chem B. 2021;10(4):571–81. https://doi.org/10.1039/d1tb02437j.
Sun J, Cui F, Zhang R, Gao Z, Ji, Ren Y, Pi F, Zhang Y, Sun X. Comet-like heterodimers “gold nanoflower @Graphene quantum dots” probe with FRET “off” to dna circuit signal “on” for sensing and imaging microRNA in vitro and in vivo. Anal Chem. 2018;90(19):11538–11547. https://doi.org/10.1021/acs.analchem.8b02854.
Ahmad N, Wang G, Nelayah J, Ricolleau C, Alloyeau D. Exploring the formation of symmetric gold nanostars by liquid-cell transmission electron microscopy. Nano Lett. 2017;17(7):4194–201. https://doi.org/10.1021/acs.nanolett.7b01013.
Rahman DS, Chatterjee H, Ghosh SK. Excess surface energy at the tips of gold nanospikes: from experiment to modeling. J Phys Chem C. 2015;119(25):14326–37. https://doi.org/10.1021/acs.jpcc.5b03944.
Zhou Y, Huang X, Zhang W, Xiong Y, Chen R, Xiong Y. Multi-branched gold nanoflower-embedded iron porphyrin for colorimetric immunosensor. Biosens Bioelectron. 2018;102:9–16. https://doi.org/10.1016/j.bios.2017.10.046.
Xu P, Li J, Huang X, Duan H, Ji Y, Xiong Y. Effect of the tip length of multi-branched AuNFs on the detection performance of immunochromatographic assays. Anal Methods. 2016;8(16):3316–24. https://doi.org/10.1039/c5ay03274a.
Xiong Z, Wang Q, Xie Y, Li N, Yun W, Yang L. Simultaneous detection of aflatoxin B1 and ochratoxin A in food samples by dual DNA tweezers nanomachine. Food Chem. 2020;338:128122. https://doi.org/10.1016/j.foodchem.2020.128122.
Li Z, Qian W, Guo H, Long X, Tang Y, Zheng J. Electrostatic self-assembled bracelet-like Au@Pt nanoparticles: an efficient electrocatalyst for highly sensitive Non-Enzymatic hydrogen peroxide sensing. ChemElectroChem. 2020;7(7):1581–9. https://doi.org/10.1002/celc.202000019.
Lu D, Wang X, Su R, Cheng Y, Wang H, Luo L, Xiao Z. Preparation of an Immunoaffinity column based on bispecific monoclonal antibody for Aflatoxin B-1 and ochratoxin A detection combined with ic-ELISA. Foods. 2022;11(3):2–16. https://doi.org/10.3390/foods11030335.
Ma Q, Nie D, Sun X, Xu Y, He J, Yang L, Yang L. A versatile Y shaped DNA nanostructure for simple, rapid and one-step detection of mycotoxins. Spectrochim Acta A Mol Biomol. 2022;281:121634. https://doi.org/10.1016/j.saa.2022.121634.
Qi X, Lv L, Wei D, Lee J, Niu M, Cui C, Guo Z. Detection of aflatoxin B-1 with a new label-free fluorescence aptasensor based on PVP-coated single-walled carbon nanohorns and SYBR gold. Anal Bioanal Chem. 2022;414(9):3087–94. https://doi.org/10.1007/s00216-022-03938-3.
Zhao L, Mao J, Hu L, Zhang S, Yang X. Self-replicating catalyzed hairpin assembly for rapid aflatoxin B1 detection. Anal Methods. 2021;13(2):222–6. https://doi.org/10.1039/d0ay01827a.
Jia Y, Zhou G, Wang X, Zhang Y, Li Z, Liu P, Yu B, Zhang J. A metal-organic framework/aptamer system as a fluorescent biosensor for determination of aflatoxin B1 in food samples. Talanta. 2020;219:121342. https://doi.org/10.1016/j.talanta.2020.121342.
Zhu W, Li L, Zhou Z, Yang X, Hao N, Guo Y, Wang K. A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem. 2020;319:126544. https://doi.org/10.1016/j.foodchem.2020.126544.
Li J, Cai T, Li W, Li W, Song L, Li Q, Lv G, Sun J, Jiao S, Wang S, Jin Y, Zheng T. Highly sensitive simultaneous detection of multiple mycotoxins using a protein microarray on a TiO2-modified porous silicon surface. J Agric Food Chem. 2021;69(1):528–36. https://doi.org/10.1021/acs.jafc.0c06859.
Guo H, Ma P, Li K, Zhang S, Zhang Y, Guo H, Wang Z. A novel ratiometric aptasensor based on dual-emission fluorescent signals and the conformation of G-quadruplex for OTA detection. Sensors Actuators B Chem. 2022;358:131484. https://doi.org/10.1016/j.snb.2022.131484.
Guo Z, Tian J, Cui C, Wang Y, Yang H, Yuan M, Yu H. A label-free aptasensor for turn-on fluorescent detection of ochratoxin a based on SYBR gold and single walled carbon nanohorns. Food Control. 2021;123:107741. https://doi.org/10.1016/j.foodcont.2020.107741.
Han B, Fang C, Sha L, Jalalah M, Al-Assiri M, Harraz F, Cao Y. Cascade strand displacement reaction-assisted aptamer-based highly sensitive detection of ochratoxin A. Food Chem. 2020;338:127827. https://doi.org/10.1016/j.foodchem.2020.127827.
Funding
This study was funded by the Key Scientific and Technological Project of Henan Province (212102310001), Special Project for Collaborative Innovation of Zhengzhou (21ZZXTCX15), and the Innovative Funds Plan of Henan University of Technology (2021ZKCJ13).
Author information
Authors and Affiliations
Contributions
Mengxiang Qiao: Conceptualization; experimentation; writing, original draft; formal analysis; investigation. Yong Liu: Material characterization, data analysis. Min Wei: Conceptualization, supervision, funding acquisition, resources, writing—review and editing.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, M., Liu, Y. & Wei, M. Dual-signal output fluorescent aptasensor based on DNA programmability and gold nanoflowers for multiple mycotoxins detection. Anal Bioanal Chem 415, 277–288 (2023). https://doi.org/10.1007/s00216-022-04403-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04403-x