Abstract
An innovative approach is presented for portable and sensitive detection of pathogenic bacteria. A novel synthetic hybrid nanocomposite encapsulating platinum nanoparticles, as a highly efficient catalyst, catalyzes the hydrolysis of the ammonia–borane complex to generate hydrogen gas. The nanocomposites are used as a label for immunoassays. A portable hand-held hydrogen detector combined with nanocomposite-induced signal conversion was applied for point-of-care testing of pathogenic bacteria. A hand-held hydrogen detector was used as the transducer. Escherichia coli O157:H7 (E. coli O157: H7), as detection target, formed a sandwich structure with magnetic beads and hybrid nanocomposites. Magnetic beads were used for separation of the sandwich structure, and hybrid nanocomposites as catalysts to catalyze the generation of hydrogen from ammonia−borane. The generated hydrogen was detected by a hydrogen detector using an electrochemical method. E. coli O157:H7 has a detection limit of 10 CFU·mL−1. The immunosensor made the hand-held hydrogen detector a point-of-care meter to be used outdoors for the detection and quantification of targets beyond hydrogen.

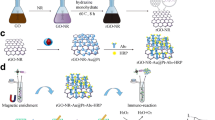
Schematic presentation of one-pot synthetic peptide–Cu3(PO4)2 hybrid nanocomposites embedded PtNPs (PPNs), encapsulating many Pt particles. The PPNs acts as an ideal immunoprobe for hand-held H2 detector signal readouts, by transforming pathogenic bacteria recognition events into H2 signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Point-of-care (POC) immunoassays are beneficial for the detection of pathogenic bacteria or monitoring biomarkers related to human health, and can be used at home or in the field. POC immunosensors are especially suitable for use in resource-limited environments because of their simplicity, accuracy, and low cost [1,2,3]. Although POC testing has grown rapidly over the past few decades [4], only a limited number of products, such as personal glucose meters (PGMs) [5] and pregnancy test strips [6], are commercially available to the public. The portable POC immunosensor has attracted interest from scientists and engineers because it produces real-time signal readouts, especially in low-resource settings [7].
The manufacture of POC devices is challenging. POC testing systems usually comprise a target recognition component, transducer, and signal processor. The transducer plays an important role in POC testing, and converts target molecular recognition events into measured signals. In the last few decades, some portable devices (e.g., PGMs, thermometers, pH meters, and pressure meters) have been developed for POC detection depending on the principle of completely different signals. Multiple new approaches based on converting non-glucose targets into glucose have been developed so that the most widely used PGM is simply used to quantify targets (e.g., platelet-derived growth factor-BB, metal ions, toxins, and pathogens) [8,9,10]. In traditional applications, the pH meter is used for detecting the concentration of hydrogen ions. pH meters also have been successfully used for detection of targets, from pathogens [11], to aflatoxin B1 [12], human oncogenic protein [13], and telomerase activity [14]. Thermometers [15] and pressure meters [16] have been successfully used as the signal readers in bioassays. The use of portable devices that are widely applied in daily life opens a new door for signal transduction strategies. However, these devices for the detection platform also have some shortcomings. For example, the respective interference of endogenous glucose and pH value in PGM and pH meter readouts in clinical samples is inevitable [17]. Pressure reading is sensitive to temperature and elevation changes. Ambient temperature changes affect thermometer readouts.
Hydrogen (H2), as a clean energy source without carbon-based emissions and other pollutants, is a globally accepted clean energy carrier. The requirement for H2 is increasing and H2 is widely used in daily life [18]. For the safety of H2 application, H2 detectors have been universally used, especially portable hand-held devices. The portable hand-held H2 detector has many attractive features, such as ease of use, portability, and accurate, sensitive H2 detection. A major challenge in using a portable H2 detector to assay targets (e.g., pathogenic bacteria) other than H2 is to find an approach that can connect the detection target to a change of H2 so that one simply detects the target and measures its concentration using the detector [15]. The efficient transformation of target recognition into generating H2 is an important step for sensitive detection in such bioassays. In all existing reactions to generate H2, the decomposition of 19.6 wt% high H2 capacity ammonia–borane (NH3-BH3, denoted as AB) has gained a lot of attention. Remarkably, generating H2 gas also has a nontoxic advantage. In theory, under standard conditions, 1 mmol AB is decomposed to produce 67.2 mL H2. If the reaction system is in a sealing device, production of H2 gas can easily be detected by a H2 detector.
A number of metal nanoparticle (MNP) catalysts have shown excellent catalytic activity and recycling performance in AB dehydrogenation [19]. Among them, platinum (Pt) is one of the most widely studied catalysts because it has remarkable activity for hydrolytic dehydrogenation of AB [20]. MNPs need to be coupled with substances, such as antibodies, that have strong affinity to the detection target, and then formed protein–MNP conjugates are used as efficient signal-transduction tags for bioassays. It is important to construct high-performance protein–MNP conjugates in the POC immunosensor. Until now, multiple methods synthesizing high-performance protein–MNP conjugates have been developed. Complex preparation processes often sacrifice the protein recognition function of the protein–MNP conjugates. A type of protein and phosphate (e.g., Cu3(PO4)2, CaHPO4) hybrid nanocomposites has attracted interest because of one-step coprecipitation preparation and large surface–volume ratio. Hybrid nanocomposites have been developed as a matrix to immobilize biological molecules, whose activity and stability are correspondingly enhanced. We have observed that the concanavalin A-Cu3(PO4)2 nanocomposites embedded a large number of hemin particles, which has outstanding peroxidase activity [21].
In the present study, we synthesized peptide–Cu3(PO4)2 hybrid nanocomposites with embedded PtNPs (PPNs), encapsulating many Pt particles, and the PPNs were an ideal immunosensor label for hand-held H2 detector signal readouts, by transforming pathogenic bacteria recognition events into H2 signals. Our adaptation of a hand-held H2 detector for pathogenic bacteria POC detection by combining hybrid nanocomposites consisted of two steps (Scheme 1): 1) the peptide–inorganic hybrid nanocomposites was captured by immunomagnetic bead separation; and 2) the hand-held H2 detector was used as a signal readout. The process of synthesizing hybrid nanocomposites at room temperature mixed antimicrobial peptides (AMPs) and PtNPs into phosphate-buffered saline (PBS) solution containing Cu2+ ions. AMPs have received increasing interest as promising biorecognition molecules for bioassays since they can bind to the surface of bacterial cells with high affinity and specificity. Using AMPs as recognition molecules, several bacterial biosensor platforms have been developed because of their stability, simple acquisition, cost-effectiveness, and standard composite protocols. PPNs had the ability to dehydrogenate AB to generate H2, and can selectively recognize target bacteria through binding of peptide to the bacterial surface. In addition, the uniform size and regular shape of the nanocomposites promoted their consistent attachment to each of the bacterial cells, leading to good linearity in the assay of pathogenic bacteria, namely E. coli O157:H7. Integration of hybrid nanocomposites–dehydrogenated AB and immunomagnetic separation led to a selective and sensitive gas signal method of detecting E. coli O157:H7.
To the best of our knowledge, this study is the first to develop an assay based on hand-held H2 detector readouts and nanocomposites signal amplification for pathogenic bacteria detection. A sensitive and selective method for the detection of E. coli O157:H7 was developed. The limit of detection (LOD) of the POC immunosensor was 10 CFU·mL−1 in a linear range from 10 to 104 CFU·mL−1.
Materials and methods
Reagents
PtNPs were provided by Sigma–Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com/china-mainland.html). NH3-BH3 were obtained from Hwrk Chem Co. Ltd. (Beijing, China, www.hwrkchemical.com). The AMPs (Magainin I: GIGKFLHSAGKFGKAFVGEIMKS) were designed as described previously [22] and obtained from Sangon Biotech Co. Ltd. (Shanghai, China, www.sangon.com). Magnetic beads (MBs) group-modified with streptavidin (average diameter 2.8 μm) were purchased from Invitrogen (Waltham, MA, USA, www.thermofisher.com). E. coli O157:H7 polyclonal antibodies coupled with biotin (AB20640) were obtained from Abcam (Hong Kong, China, www.abcam.cn). CuSO4∙5H2O were purchased from Sangon Biotech (Shanghai, China, www.sangon.com). PBS consisted of 0.01 M NaH2PO4, 0.01 M Na2HPO4 and 0.014 M NaCl (pH 7.4). The hand-held hydrogen detector, which operated on principles of electrochemistry and thermal conductivity (SKY2000-H2), was purchased from Unitec Technology (Shenzhen, China, www.szyuante.com/list-8.html). All chemicals were used without further purification. All aqueous solutions were made from ultrapure water (18.3 MΩ∙ cm, Milli-Q; Millipore, Billerica, MA, USA, www.merckmillipore.com). Strains of Salmonella sp. (CMCC 50115), Listeria monocytogenes (Lis, BNCC 128913), E. coli O157:H7 (CICC 21530) and Staphylococcus aureus (BNCC 186335) were provided by the Academy of Military Medical Sciences (Beijing, China).
Synthesis of hybrid nanocomposites
PPNs with a diameter of about 4 μm were prepared according to the strand method with some modification [23]. Magainin I peptide aqueous solution (0.01 mg·mL−1) and CuSO4∙5H2O aqueous solution (20 μL of 120 mM) were added to 1 mL PBS (pH 7.4, 10 mM). PtNPs (0.9 mg·mL−1) were introduced under mild stirring, and allowed to form the PPNs at room temperature for 24 h. The PPNs were obtained by centrifugation (8000 rpm), rinsed twice and dispersed in 1 mL 0.1 mM PBS solution (pH 6.0). The solution was stored at 4 °C for further use. The structure of the PPNs was determined by scanning electron microscopy (SEM; Hitachi SU8010, Tokyo, Japan).
Detection process of this assay
We designed an immunosensor for E. coli O157:H7, and the complete detection strategy is shown in Scheme 1. MBs labeled with antibody were synthesized according to protocol and used for separation and recognition of E. coli O157:H7 (via streptavidin–biotin interaction); mainly including 0.05 mg·mL−1 of E. coli O157:H7 polyclonal antibodies dissolved in 5 mg·mL−1 MBs. After 45 min incubation at 25 °C, the solution of MBs modified with E. coli O157:H7 antibody was washed three times with PBS to remove the unbound antibody. The antibody−MBs were finally kept at 4 °C until further use. MB-conjugated antibody was added to 10 μL of different concentrations of E. coli O157:H7, for recognition of the target E. coli O157:H7. The mixture was incubated at 35 °C for 80 min and magnetically separated by washing with PBS three times. The supernatants were removed to dispose of the excess nontarget bacteria. PPNs (4 μL) were added to the remaining MBs for 35 min at 35 °C. After addition of the PPNs, the MBs–E. coli O157:H7 bound to the PPNs. The unbound PPNs were removed by magnetic separation. After another four washes with PBS, the mixture was resuspended in 500 μL PBS (0.1 mM, pH 6.0) and placed in a 50-mL centrifuge tube. As shown in Scheme 1, PPNs, after antibody-labeled MBs and the target of E. coli O157:H7, formed an immune−sandwich complex, and the signal output immunosensor can be acquired through this procedure. To exert the maximum catalytic effect of the PPNs, 10 μL NaOH (4 M) was added to destroy the nanocomposites to release the PtNPs. NH3-BH3 (1 mL, 25 mM) was injected through the pipe orifice of the centrifuge tube and the centrifuge tube was closed immediately with a rubber seal (double cover: the first layer was a common centrifugal tube cover with a circular hollow in the middle, and the second layer was a thin rubber layer). The needle of the digital H2 detector was inserted into the rubber-sealed tube (the center of the centrifugal pipe was hollowed out to expose the rubber layer). The generation of H2, which was utilized as a signal readout to detect target bacteria in the sealed centrifuge tube, was accurately measured by a portable H2 detector and the digitally displayed value was read within 3 min. E. coli O157:H7 was quantitatively and qualitatively analyzed. Parts per million (ppm) was the unit of measurement of H2 detection. P/P0 represented the ratio of target E. coli O157:H7 in the presence and absence of 104 CFU·mL−1.
Preparation of milk samples
For processing E. coli O157:H7 in real samples of milk, sterile milk was spiked by target pathogens. E. coli O157:H7 was diluted in PBS to a concentration of 102, 103, and 104 CFU·mL−1. Milk was purchased from a local supermarket. The immune sandwich structure with the target bacteria was used as described above to calculate the recovery (%), on the calibration plot.
Statistical analysis
All data were analyzed using GraphPad Prism 5 and expressed as the mean ± standard deviation. All parameters were measured at least three times to validate repeatability and reproducibility.
Results and discussion
Synthesis and characterization of PPNs
The PPNs were observed using SEM (Fig. 1a, b). According to the SEM characterization (Fig. S1), the PPNs had an average diameter of about 4 μm. This was consistent with the previous traditional organic–inorganic hybrid nanocomposites structure [23, 24].
AB is a promising chemical H2 storage material, which can be released by hydrolysis. The acquired PPNs were tested for catalytic hydrolysis of AB. The reaction was activated by addition of aqueous solution of AB into the reaction tube, comprising the PPN catalyst at room temperature. Figure 2a shows the time-dependent changes in H2 detector and the concentration of H2 produced as a function of target bacterium concentration. This change was directly converted to the H2 detector readout. The linear relation between the volume of PPNs in AB and the final concentration of H2 was apparent (Fig. 2b). This catalytic dissociation activity demonstrated that the synthesized PPNs had excellent AB decomposition efficiency and can be successfully used in H2 detector assays.
Optimization of conditions
Different conditions were investigated: (a) number of PtNPs; (b) concentration of Magainin I; (c) reaction temperature; (d) reaction incubation time; (e) pH value; and (f) concentration of NaOH.
The following experimental conditions were able to achieve the best results: (a) umber of PtNPs, 0.9 mg·mL−1; (b) concentration of Magainin I, 0.01 mg·mL−1; (c) reaction temperature, 35 °C; (d) reaction incubation time, 35 min; (e) pH value, 6.0 and (f) concentration of NaOH, 4 M. Detailed steps and figures can be found in the Electronic Supporting Material.
Sensitivity of the assay
To assess the responses of the immunosensor based on the hand-held H2 detector, various concentrations of E. coli O157:H7 were further tested. The H2 concentration inside the confined space gradually increased due to dehydrogenation of AB, and H2 was accurately measured by the hand-held detector. The H2 concentration increased gradually with concentration of E. coli O157:H7, and there was a linear relationship in the range of 10 to 104 CFU·mL−1 between H2 concentration and target E. coli O157:H7 concentration (Fig. 3). The LOD was 10 CFU·mL−1. The linear regression equation was Y = 0.4412X + 1.112 (R2 = 0.989) where Y is the relative aerogenesis intensity, X represents the common logarithm of the target bacteria concentration, and R2 is the correlation coefficient. The comparative LOD between our established immunosensor and other reported E. coli O157:H7 methods is shown in Table 1. In contrast to other methods, the immunosensors achieved excellent performance for detecting E. coli O157:H7, with an LOD of 10 CFU·mL−1. The lower LOD of the immunosensor may be attributed to the production of more H2 by PtNPs catalyzing AB and high sensitivity of the hand-held H2 detector. Compared with the traditional methods, such as fluorescence, electrochemistry and chemiluminescence, the approach did not rely on professional and expensive instruments.
Specificity
To estimate the specificity of the method, the target E. coli O157:H7 and other nontarget pathogenic bacteria such as Salmonella sp., S. aureus, and L. monocytogenes were compared. The P/P0 ratios of other bacteria were similar to those of the control group, while the P/P0 ratio of E. coli O157:H7 was (Fig. 4). It is obvious from the results of these experiments that the method based on nanocomposites and hand-held H2 detector was specific to E. coli O157:H7.
Reproducibility of this method
To verify applicability and selectivity capability of our method for detection of E. coli O157:H7, we tested the assay in spiked commercial milk samples. Milk samples were mixed with PBS containing different concentration of E. coli O157:H7. The recovery and relative standard deviation (RSD) of E. coli O157:H7 added to milk samples were in the range of 86–126% and 3–27%, respectively (Table S1).
Conclusions
We have demonstrated a specific, sensitive and immunosensor system approach based on H2 generation in PPNs and a hand-held H2 detector for portable detection of pathogenic bacteria. E. coli O157:H7 was detected quantitatively through a direct hand-held H2 detector reading. These results show that the described assay is suitable for detection of a broad range of pathogens in real samples. The outstanding performance of the immunosensor approach was due to the high catalytic activity of PPNs in the dehydrogenation of AB, high H2 capacity of AB, and high sensitivity of the hand-held H2 detector. However, the practical application of this pathogenic bacteria detection strategy is still challenging, and strategies that are more efficient are required to simplify the experimental procedure. Nevertheless, the sensing platform POC detection of pathogenic bacteria based on the hand-held H2 detector has potential for the future in terms of portable application in low-resource settings.
References
Zhao Y, Du D, Lin Y (2015) Glucose encapsulating liposome for signal amplification for quantitative detection of biomarkers with glucometer readout. Biosens Bioelectron 72:348–354
Zhang JJ, Shen Z, Xiang Y, Lu Y (2016) Integration of solution-based assays onto lateral flow device for one-step quantitative point-of-care diagnostics using personal glucose meter. Acs Sensors 1(9):1091–1096
Alamer S, Eissa S, Chinnappan R, Zourob M (2018) A rapid colorimetric immunoassay for the detection of pathogenic bacteria on poultry processing plants using cotton swabs and nanobeads. Microchim Acta 185(3):164
Liu D, Tian T, Chen X, Lei Z, Song Y, Shi Y, Ji T, Zhu Z, Yang L, Yang C (2018) Gas-generating reactions for point-of-care testing. Analyst 143(6):1294–1304
Ye R, Zhu C, Song Y, Song J, Fu S, Lu Q, Yang X, Zhu MJ, Du D, Li H, Lin Y (2016) One-pot bioinspired synthesis of all-inclusive protein-protein nanoflowers for point-of-care bioassay: detection of E. coli O157:H7 from milk. Nanoscale 8(45):18980–18986
Bu SJ, Wang KY, Ju CJ, Han Y, Li ZY, Du P, Hao Z, Li CT, Liu WS, Wan JY (2018) A pregnancy test strip for detection of pathogenic bacteria by using concanavalin A-human chorionic gonadotropin-Cu-3(PO4)(2) hybrid nanoflowers, magnetic separation, and smartphone readout. Microchim Acta 185(10):464
Piro B, Reisberg S (2017) Recent advances in electrochemical immunosensors. Sensors 17(4):794
Das A, Cui XK, Chivukula V, Iyer SS (2018) Detection of enzymes, viruses, and bacteria using glucose meters. Anal Chem 90(19):11589–11598
Huang H, Zhao G, Dou W (2018) Portable and quantitative point-of-care monitoring of Escherichia coli O157:H7 using a personal glucose meter based on immunochromatographic assay. Biosens Bioelectron 107:266–271
Hong L, Zhou F, Shi DM, Zhang XJ, Wang GF (2017) Portable aptamer biosensor of platelet-derived growth factor-BB using a personal glucose meter with triply amplified. Biosens Bioelectron 95:152–159
Ye R, Zhu C, Song Y, Lu Q, Ge X, Yang X, Zhu MJ, Du D, Li H, Lin Y (2016) Bioinspired synthesis of all-in-one organic-inorganic hybrid Nanoflowers combined with a handheld pH meter for on-site detection of food pathogen. Small 12(23):3094–3100
Zhao M, Wang P, Guo Y, Wang L, Luo F, Qiu B, Guo L, Su X, Lin Z, Chen G (2018) Detection of aflatoxin B1 in food samples based on target-responsive aptamer-cross-linked hydrogel using a handheld pH meter as readout. Talanta 176:34–39
Zhang Y, Yang JN, Nie JF, Yang JH, Gao D, Zhang L, Li JP (2016) Enhanced ELISA using a handheld pH meter and enzyme-coated microparticles for the portable, sensitive detection of proteins. Chem Commun 52(17):3474–3477
Wang LX, Chen CQ, Huang HW, Huang D, Luo F, Qiu B, Guo LH, Lin ZY, Yang HH (2018) Sensitive detection of telomerase activity in cancer cells using portable pH meter as readout. Biosens Bioelectron 121:153–158
Zhang JJ, Xing H, Lu Y (2018) Translating molecular detections into a simple temperature test using a target-responsive smart thermometer. Chem Sci 9(16):3906–3910
Zhu Z, Guan ZC, Liu D, Jia SS, Li JX, Lei ZC, Lin SC, Ji TH, Tian ZQ, Yang CYJ (2015) Translating molecular recognition into a pressure signal to enable rapid, sensitive, and portable biomedical analysis. Angew Chem Int Ed 54(36):10448–10453
Zhang JJ, Xiang Y, Wang M, Basu A, Lu Y (2016) Dose-dependent response of personal glucose meters to nicotinamide coenzymes: applications to point-of-care diagnostics of many non-glucose targets in a single step. Angew Chem Int Ed 55(2):732–736
Guo LT, Cai YY, Ge JM, Zhang YN, Gong LH, Li XH, Wang KX, Ren QZ, Su J, Chen JS (2015) Multifunctional Au-Co@CN Nanocatalyst for highly efficient hydrolysis of Ammonia borane. ACS Catal 5(1):388–392
Zhan WW, Zhu QL, Xu Q (2016) Dehydrogenation of Ammonia borane by metal nanoparticle catalysts. ACS Catal 6(10):6892–6905
Zhou QX, Xu CX (2017) Stratified nanoporous PtTi alloys for hydrolysis of ammonia borane. J Colloid Interface Sci 496:235–242
Wang K-Y, Bu S-J, Ju C-J, Li C-T, Li Z-Y, Han Y, Ma C-Y, Wang C-Y, Hao Z, Liu W-S, Wan J-Y (2018) Hemin-incorporated nanoflowers as enzyme mimics for colorimetric detection of foodborne pathogenic bacteria. Bioorg Med Chem Lett 28(23–24):3802–3807
Humblot V, Yala JF, Thebault P, Boukerma K, Hequet A, Berjeaud JM, Pradier CM (2009) The antibacterial activity of Magainin I immobilized onto mixed thiols self-assembled monolayers. Biomaterials 30(21):3503–3512
Ge J, Lei J, Zare RN (2012) Protein-inorganic hybrid nanoflowers. Nat Nanotechnol 7(7):428–432
Hu R, Zhang X, Zhao Z, Zhu G, Chen T, Fu T, Tan W (2014) DNA nanoflowers for multiplexed cellular imaging and traceable targeted drug delivery. Angew Chem Int Ed Eng 53(23):5821–5826
Xu M, Wang R, Li Y (2016) An electrochemical biosensor for rapid detection of E. coli O157:H7 with highly efficient bi-functional glucose oxidase-polydopamine nanocomposites and Prussian blue modified screen-printed interdigitated electrodes. Analyst 141(18):5441–5449
Li YX, Afrasiabi R, Fathi F, Wang N, Xiang CL, Love R, She Z, Kraatz HB (2014) Impedance based detection of pathogenic E-coli O157:H7 using a ferrocene-antimicrobial peptide modified biosensor. Biosens Bioelectron 58:193–199
Zhang H, Shi YP, Lan F, Pan Y, Lin YK, Lv JZ, Zhu ZH, Jiang Q, Yi C (2014) Detection of single-digit foodborne pathogens with the naked eye using carbon nanotube-based multiple cycle signal amplification. Chem Commun 50(15):1848–1850
Akanda MR, Tamilavan V, Park S, Jo K, Hyun MH, Yang H (2013) Hydroquinone diphosphate as a phosphatase substrate in enzymatic amplification combined with electrochemical-chemical-chemical redox cycling for the detection of E. coli O157:H7. Anal Chem 85(3):1631–1636
Dong ZM, Zhao GC (2015) Label-free detection of pathogenic bacteria via immobilized antimicrobial peptides. Talanta 137:55–61
Zhang Y, Tan C, Fei R, Liu X, Zhou Y, Chen J, Chen H, Zhou R, Hu Y (2014) Sensitive chemiluminescence immunoassay for E. coli O157:H7 detection with signal dual-amplification using glucose oxidase and laccase. Anal Chem 86(2):1115–1122
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (No. 2016YFD0501001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no competing financial interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2206 kb)
Rights and permissions
About this article
Cite this article
Bu, SJ., Wang, KY., Bai, HS. et al. Immunoassay for pathogenic bacteria using platinum nanoparticles and a hand-held hydrogen detector as transducer. Application to the detection of Escherichia coli O157:H7. Microchim Acta 186, 296 (2019). https://doi.org/10.1007/s00604-019-3409-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3409-6