Abstract
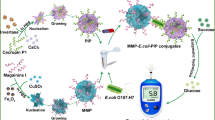
A point-of-care (POC) immunoassay was established for the sensitive and rapid detection of pathogenic Escherichia coli O157:H7, using magnetic Fe3O4 organic-inorganic composites (Ab@Fe3O4) for immunomagnetic separation, nanozyme platinum nanoparticle (PtNp) organic-inorganic composites (Ap@PtNp) for signal amplification, and thermometer readings. Antibodies and Fe3O4 were incubated in Cu2+ phosphate buffer to synthesize the magnetic composite Ab@Fe3O4 with antibodies, to specifically capture E. coli O157:H7. Antimicrobial peptides and PtNp were incubated in Cu2+ phosphate buffer to synthesize the signal composites Ap@PtNp with antimicrobial peptides (magainin I), recognizing and labeling E. coli O157:H7. In the presence of E. coli O157:H7, magnetic microcomposites targeted bacteria and signal microcomposites to form the sandwich structure: Ab@Fe3O4-bacteria-Ap@PtNp for magnetic separation. Ap@PtNp of signal composites catalyzed H2O2 to generate thermo-signals (temperature rise), which were determined by a thermometer. This point-of-care bioassay detected E. coli O157:H7 in the linear range of 101–107 CFU mL−1 and with a detection limit of 14 CFU mL−1.

One-pot process magnetic Fe3O4 organic-inorganic composites (Ab@Fe3O4, magnetic microcomposites, MMC) for immunomagnetic separation and nanozyme platinum nanoparticle (PtNp) organic-inorganic composites (Ap@PtNp, signal microcomposites, SMC) were used as signal amplification and thermometer readings for E. coli O157:H7 detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foodborne diseases caused by pathogenic bacteria have become a global public health problem, posing a significant threat to human health [1]. It is widely recognized that the rapid, sensitive, and specific detection of pathogens is becoming more important in controlling illness outbreaks [2]. However, access to laboratory tests remains limited in some poor countries, and also in some developing countries [3]. The rapid, sensitive, and specific point-of-care (POC) detection of foodborne pathogenic bacteria can effectively reduce food- and water-borne outbreaks, especially in resource-limited settings [4]. Other common methods of detecting pathogenic bacteria use microbiological plating and counting and the polymerase chain reaction (PCR). Although these approaches are specific and sensitive, their applications in such settings are limited as they are easy to contaminate and generate false-positive results [5].
Over recent decades, scientists have successfully developed viable POC bioassays, based on immunoassay to detect foodborne pathogenic bacteria [6]. POC testing has the advantage of greater availability in resource-constrained areas when compared to standard laboratory-based diagnostics. Currently, the main challenge of the POC bioassay is the detection of pathogenic bacteria in food, and the amplification and conversion of associated signals into readable digital outputs on portable devices.
Magnetic nanoparticles (MNPs) have been widely used for the capture and enrichment of pathogenic bacteria in complex food samples [7]. MNPs have unique properties: (1) they are easily manipulated by magnets; (2) they contain rapid solution kinetics and high surface volume ratios to ensure magnetic separation of targets (e.g., pathogenic bacteria); and (3) they have reduced matrix effects [8, 9]. MNPs are excellent candidates for POC immune assays, as they can be developed into multifunctional bioassay materials, with great potential [10]. To label and enrich for target bacteria, MNPs require biomolecular modifications that recognize targets using complex reagents such as antibodies, aptamers, peptides, carbohydrates, and antibiotics [11]. In recent years, a facile approach for preparing organic-inorganic microcomposites with flower-like construction has been reported; it uses phosphate as an inorganic component and protein as an organic component [12]. Due to its high surface volume ratio, facile synthesis, and outstanding protein compatibility, these organic-inorganic microcomposites have attracted great attention [13]. When compared with traditional protein affixation methods, microcomposites can effectively maintain and even improve protein activity [14]. Spurred on by this facile approach, our research group observed that some nanoparticles (e.g., hemin and MnO2) could be embedded into organic-inorganic microcomposites, which are capable of biological recognition and signal amplification for the POC detection of foodborne pathogenic bacteria [15, 16]. In this study, the magnetic nanoparticles Fe3O4 and pathogenic bacterial antibodies were incubated in Cu2+ phosphate buffer to synthesize magnetic organic-inorganic microcomposites for the enrichment and immune-separation of foodborne pathogenic bacteria. To the best of our knowledge, antibody-Cu3(PO4)2 microcomposites loading Fe3O4 nanoparticles (magnetic microcomposites, MMC) have not been reported for the magnetic separation of pathogenic bacteria.
Transducers perform a vital role in the specificity and the signal detection of POC bioassays [17]. Bio-recognition signals from pathogenic bacteria are transformed into quantifiable signals using such transducers [18]. Recently, scientists have developed a variety of transduction methods, applied to the detection of foodborne pathogenic bacteria [19]. Signal transduction modes and physical parameters, such as electrochemical, optical, mass, and pH, are used for signal analysis in bioassays. The development of temperature-sensing technologies has an important practical significance across many fields, such as meteorology, chemistry, pharmacy, biology, and the military [20, 21]. It is encouraging that the emergence of thermo-based bioassays has opened up a new field of bio-sensing systems [22]. Thermometers, as an inexpensive, easy-to-use, fast, and real-time reading independent instrument, provide a powerful, portable, reliable, fast, and cheap detection tool for clinical biomarkers and other analyses [23].
Due to its catalytic efficiencies, excellent stability in harsh environments, ease of production, high efficiencies, and affinity for substrates, nanozyme platinum nanoparticles (PtNp) have been used as substitutes for natural enzymes in immunoassay signal amplification [24]. Our previous research found that PtNp catalyzed the decomposition of H2O2 into O2, which caused a significant increase in temperature across the reaction system [25]. PtNp can transform target biological identification processes into quantifiable temperature signals. However, PtNp must be coupled to target biomolecules via a complex process. In this study, the nanozyme, PtNp, and magainin I were incubated in Cu2+ phosphate buffer to synthesize a nanozyme Ap@PtNp (signal microcomposites, SMC) as a signal-transforming label for foodborne pathogenic bacteria.
We developed a rapid and portable bioassay for detecting foodborne pathogenic bacteria by used Escherichia coli O157:H7 as a model pathogen (Scheme 1). This immunoassay combined magnetic separation using magnetic microcomposites, Ab@Fe3O4, and signal amplification using signal microcomposites Ap@PtNp, with thermo-signal analysis, using a thermometer. In the presence of the target E. coli O157:H7, a sandwich structure Ab@Fe3O4-bacteria-Ap@PtNp was formed (Scheme 1). H2O2 decomposition was catalyzed by the nanozyme platinum of SMC to produce thermo. Finally, a thermometer was used to detect temperature changes for the quantitative determination of the target pathogen, E. coli O157:H7.
Materials and methods
Reagents and setup
Phosphate-buffered saline (PBS, 0.01 M) (0.01 M NaH2PO4 and 0.01 M Na2HPO4, pH 7.4) were procured from Invitrogen, Germany (https://www.thermofisher.com/us/en/home.html). Ferro ferric oxide (Fe3O4), copper sulfate pentahydrate (CuSO4∙5H2O), and the magainin I (GIGKFLHSAGKFGKAFVGEIMKS) peptide were obtained from Sangon Biotech (Shanghai, China, www.sangon.com). PtNp (10 nm) were obtained from Beijing Dk Nanotechnology Co., Ltd. (Beijing, China), and H2O2 (30 wt%) was purchased from Shanghai Ling Feng Chemical Regent Co., Ltd. (Shanghai, China, http://lfhxsj.cn.b2b168.com/). E. coli O157:H7 antibodies were supplied by Abcam (ab252713, Shanghai, China, www.abcam.cn). All other chemicals were from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China, http://www.crc-bj.com/) and were analytical grade. Deionized water was used in all experimental procedures and was ultra-filtered using a Millipore Milli-XQ system (18.3 MΩ cm, Billerica, MA, USA, www.merckmillipore.com). Bacterial strains of Salmonella typhimurium, (Sal, CICC 21484), Staphylococcus aureus (Sta, CICC 10384), Listeria monocytogenes (Lis, CICC 21635), and Escherichia coli O157:H7 (E. coli O157:H7, CICC 24187) were purchased from China Center of Industrial Culture Collection (Beijing, China, http://www.china-cicc.org). A digital thermometer (model number SJPT302), with a temperature detection range of − 50 to + 300 °C (degree precision = 0.1 °C) was obtained from Sensegene flagship store (Hangzhou, China, http://www.sensegene.com/).
Scanning electron microscopy (SEM) images were obtained using a JEOL JEM-2100 (Tokyo, Japan, https://www.jeol.co.jp/). X-ray diffraction (XRD) measurements were determined using an X-ray polycrystal diffractometer (Beijing Purking General Instrument Co., Ltd. XD6, Beijing, China, www.pgeneral.com.cn).
Preparation of Ab@Fe3O4 and Ap@PtNp microcomposites
Ab@Fe3O4 and Ap@PtNp were prepared according to a previously published report, with a slight modification [26]. Details are also specified in the Supporting Material. The capture efficiency of Ab@Fe3O4 was measured by conventional plate method. The capture efficiency (%) = X/Y × 100%, where X are E. coli O157:H7 numbers after magnetic capture, and Y are E. coli O157:H7 numbers before magnetic separation.
The detection of E. coli O157:H7 using a thermometer
Firstly, 100 μL of different E. coli O157:H7 densities (101, 102, 103, 104, 105, 106, 107, 108 CFU mL−1) were added to a 5-μL Ab@Fe3O4 solution, followed by the addition of 10-μL Ap@PtNp microcomposite, to a final volume of 115 μL. The mixture was incubated for 90 min at 35 °C. The reaction mixture was then washed four times in PBS (0.1 mM, pH 7), using a magnetic separation rack to remove unbound microcomposites. Finally, 600-μL H2O2 (30 wt%) was added to generate a decomposition reaction, producing heat. After 7 min, the final reaction temperature was measured. As shown (Fig. S1), the thermo-signal was considerably increased before (left image) and after H2O2 decomposition (right image). The temperature was obtained from this mixture. The major advantage of this assay is the omission of traditional sandwich cleaning steps; a one-step cleaning method is required. When compared with other sandwich methods, our approach had a simpler cleaning procedure and reduced incubation times (Table 1).
Real food assay to determine target E. coli O157:H7
The standard addition method was used to calculate recovery [33]. Briefly, 103, 104, and 105 CFU mL−1 E. coli O157:H7 densities were diluted in PBS (0.1 mM, pH 7). After this, bacteria were added to 1-mL commercial milk, and detected under optimized experimental conditions. Using our linear calibration curves and thermo-signals, target E. coli O157:H7 densities were determined from six independent experiments.
Data analysis
The temperature change (∆T) was represented as the difference between the thermal signals measured by the digital thermometer in the presence and absence of the target bacteria. The experiment date were performed at least three independent experiments (mean ± standard deviation (SD)) and analyzed by GraphPad PRIME (7.0) software.
Results and discussion
Characterization of Ab@Fe3O4 and Ap@PtNp
We report a novel approach that directly synthesizes Ab@Fe3O4 that exhibits magnetic properties and hierarchical structures. The morphology of microcomposite of ferrites were characterized by SEM. As shown (Fig. 1a and b), typical SEM images show a spherical structure, with a coarse surface and an average spherical diameter of approximately 12 μm. These dimensions and characteristics illustrate that Ab@Fe3O4 morphology was similar to previous hybrid nanoflowers [26]. This was in accordance with our SEM characterization results. These microcomposites not only immobilized Fe3O4, but their high ratio to surface areas, and their good space utilization ratios for efficient antibody entrapment effectively captured the target. In addition, the larger specific surface areas of Ab@Fe3O4 microcomposites also facilitated the application of related surface reactions. They also provided intrinsic magnetism, allowing the target molecule to be quickly separated from the complex mixture, by a magnetic field in a short time. Similarly, Ap@PtNp had the properties of the catalytic activity of platinum that could be used for the dissociation of H2O2 molecules. This catalytic process produced heat which was processed as a final signal output. As shown (Fig. 1c and d), SEM images of Ap@PtNp displayed a classic form, similar to the morphology of Ab@Fe3O4, with an average diameter of approximately 3 μm.
The crystal structure and crystallinity of Ab@Fe3O4 and Ap@PtNp were recorded by XRD in the range at 2θ = 5° − 85° on a desktop X-ray diffractometer. Figure 2 a indicates the Ab@Fe3O4 fitted well with Cu3(PO4)2·3H2O (JCPDS file 22-0548) and Fe3O4 (JCPDS file 77-1545), revealing the copper phosphate and ferro ferric oxide in the nanoparticles. These characteristic signals appeared for Ab@Fe3O4, suggesting that the synthesis of Ab@Fe3O4 was crystalline. Similarly, XRD pattern for Ap@PtNp (Fig. 2b) confirmed that microcomposites consisted of crystalline nanoparticles (Cu3(PO4)2·3H2O (JCPDS file 22-0548 and PtNP JCPDS file 65-2868). In addition, Ab@Fe3O4 and Ap@PtNp microcomposite stability was investigated to confirm that the antibody and magainin I still maintained initial activities. As shown (Fig. S2), no thermo-signal changes were observed for E. coli O157: H7 detection up to 60 days at 4 °C, thus confirming microcomposite stability up to 2 months. These results suggested that microcomposites had been successfully generated for further studies.
Optimization conditions
Several different parameters were optimized: Fig. S3 A; antibody immobilization on Ab@Fe3O4, i.e., 8 μg mL−1; (B) Fe3O4 particles in Ab@Fe3O4, 1.2 mg mL−1; Fig. S4; the effects of Ab@Fe3O4 volume composites on capture efficiency, 5 μL; Fig. S5; (A) the concentration of magainin I, 15 μg mL−1; (B) the concentration of PtNp in Ap@PtNp, 0.5 μg mL−1; Fig. S6; (A) reaction temperature, 35 °C; (B) reaction time, 90 min, and (C) pH 7.
Quantitative detection of E. coli O157: H7
Under optimized reaction conditions, E. coli O157: H7, at different cell densities, was added to immunoassays to ascertain detection sensitivities. As shown (Fig. 3), a linear relationship was observed between the thermo-signal and the E. coli O157: H7 cell density logarithmic scale, in the range 101–107 CFU mL−1. A correlation coefficient of 0.975 (Fig. 3b) was also recorded. The linear equation was Y = 1.631*X + 0.1. We also determined the limit of detection (LOD) using the following formula: 3σ/slope [34] (where σ is the standard deviation of blank samples). The LOD was 14 CFU mL−1, which was superior or comparable to other immunoassay detection limits (Table S1). These materials were easy to bioassay and prepare without any particular molecular interactions that supplies the results compare to other antibody/magainin I-based detection method of pathogenic bacteria [35]. The synthetic processing of this material efficiently eliminated the damage of recognizing element (antibody or magainin I) during immobilization. The main advantage of Ab@Fe3O4 and Ap@PtNp materials was that they integrated recognition, separation, and signal release functions to achieve a one-step cleaning detection. Hence, our bioassay had a simpler synthetic procedure and operation steps, when compared with other materials (e.g., metal-organic framework [36]), or common pathogen detection methods (e.g., ELISA [37]). Such sensitive sensing performances may be ascribed to the following: (a) magnetic microcomposites containing millions of Fe3O4 particles and target antibody sites, thus achieving significant target accumulation and magnetic separation, allowing thermal signal responses to be efficiently amplified; (b) signal microcomposites also have millions of material binding sites which can be layer-by-layer deposited by PtNp and peptides. Thus, the dual-microcomposite immunoassay structure exhibited great specificity for the sensitive detection of E. coli O157: H7.
Bioassay selectivity and reproducibility
To further confirm assay selectivity, several common foodborne pathogens were assessed; E. coli O157:H7, Sal, Lis, Sta, and a control (PBS) were investigated. Our data demonstrated that Ab@Fe3O4 and Ap@PtNp selectively captured only E. coli O157:H7 (Fig. 4). Equally, E. coli O157:H7 at densities of 102 CFU mL−1, 103 CFU mL−1, and 104 CFU·mL−1 were used to identify bioassay coefficients of variation (CV). Our data indicated that CV of 2.37%, 2.04%, and 2.24%, respectively, were achieved (in quintuplicate), thereby indicating bioassay reproducibility towards target E. coli O157:H7 detection.
System selectivity. Target bacteria, E. coli O157:H7, and other bacteria (Staphylococcus aureus (Sta); Listeria monocytogenes (Lis); and Salmonella typhimurium (Sal)) were used at a cell density of 107 CFU mL−1. PBS was used as a control at 0.1 mM (pH = 7). All experimental conditions were optimized and three independent experiments were conducted
Detection of E. coli O157: H7 in spiked milk samples
To truly evaluate system long-term stability in complex samples, the system was evaluated to detect different E. coli O157:H7 cell densities spiked into sterile pure milk. Thermo-signals were obtained and the results summarized (Table S2). These data showed that recoveries of 97.2–115.3% were achieved for milk samples by standard addition methods, with a relative standard deviation (RSD) of 3.9–10.8% (n = 6). This method showed an acceptable reproducibility, and has the potential to be used for complex biological samples. These results also demonstrated that the immunoassay possessed improved detection capabilities for pathogenic bacteria, with high sensitivity and accuracy.
Conclusions
In this study, a sensitive, rapid, and portable bioassay was successfully developed for detecting E. coli O157:H7 in complex food samples. The system was based on Ab@Fe3O4 microcomposite immunomagnetic separation, Ap@PtNp microcomposite signal amplification, and thermometer readings. Utilization of these materials of Ab@Fe3O4 and Ap@PtNp efficient guarantee this method’s sensitivity and selectivity. These microcomposites need to be simplified the synthetic steps when it is be appropriate for non-professionals. Nevertheless, this POC bioassay has great potential for screening in-field foodborne pathogenic bacteria.
References
Chlebicz A, Slizewska K (2018) Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: a review. Int J Env Res Public Health 15 (5). doi: ARTN 863 https://doi.org/10.3390/ijerph15050863
Kant K, Shahbazi MA, Dave VP, Ngo TA, Chidambara VA, Than LQ, Bang DD, Wolff A (2018) Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol Adv 36(4):1003–1024. https://doi.org/10.1016/j.biotechadv.2018.03.002
Xianyu YL, Wang QL, Chen YP (2018) Magnetic particles-enabled biosensors for point-of-care testing. Trac-Trends in Analytical Chemistry 106:213–224. https://doi.org/10.1016/j.trac.2018.07.010
Chen J, Andler SM, Goddard JM, Nugen SR, Rotello VM (2017) Integrating recognition elements with nanomaterials for bacteria sensing. Chem Soc Rev 46(5):1272–1283. https://doi.org/10.1039/c6cs00313c
Wu Z, Fu Q, Yu S, Sheng L, Xu M, Yao C, Xiao W, Li X, Tang Y (2016) Pt@AuNPs integrated quantitative capillary-based biosensors for point-of-care testing application. Biosens Bioelectron 85:657–663. https://doi.org/10.1016/j.bios.2016.05.074
Rubab M, Shahbaz HM, Olaimat AN, Oh DH (2018) Biosensors for rapid and sensitive detection of Staphylococcus aureus in food. Biosens Bioelectron 105:49–57. https://doi.org/10.1016/j.bios.2018.01.023
El-Boubbou K, Gruden C, Huang X (2007) Magnetic glyco-nanoparticles: a unique tool for rapid pathogen detection, decontamination, and strain differentiation. J Am Chem Soc 129(44):13392–13393. https://doi.org/10.1021/ja076086e
Xianyu Y, Chen Y, Jiang X (2015) Horseradish peroxidase-mediated, iodide-catalyzed cascade reaction for plasmonic immunoassays. Anal Chem 87(21):10688–10692. https://doi.org/10.1021/acs.analchem.5b03522
Chen YP, Xianyu YL, Wu J, Yin BF, Jiang XY (2016) Click chemistry-mediated nanosensors for biochemical assays. Theranostics 6(7):969–985. https://doi.org/10.7150/thno.14856
Fu S, Wang S, Zhang X, Qi A, Liu Z, Yu X, Chen C, Li L (2017) Structural effect of Fe3O4 nanoparticles on peroxidase-like activity for cancer therapy. Colloids Surf B Biointerfaces 154:239–245. https://doi.org/10.1016/j.colsurfb.2017.03.038
Zhu MJ, Liu WP, Liu HX, Liao YH, Wei JT, Zhou XM, Xing D (2015) Construction of Fe3O4/vancomycin/PEG magnetic nanocarrier for highly efficient pathogen enrichment and gene sensing. ACS Appl Mater Interfaces 7(23):12873–12881. https://doi.org/10.1021/acsami.5b02374
Ye R, Zhu C, Song Y, Song J, Fu S, Lu Q, Yang X, Zhu MJ, Du D, Li H, Lin Y (2016) One-pot bioinspired synthesis of all-inclusive protein-protein nanoflowers for point-of-care bioassay: detection of E. coli O157:H7 from milk. Nanoscale 8(45):18980–18986. https://doi.org/10.1039/c6nr06870g
Cui J, Jia S (2017) Organic–inorganic hybrid nanoflowers: a novel host platform for immobilizing biomolecules. Coord Chem Rev 352:249–263. https://doi.org/10.1016/j.ccr.2017.09.008
Guo RY, Wang SY, Huang FC, Chen Q, Li YB, Liao M, Lin JH (2019) Rapid detection of Salmonella Typhimurium using magnetic nanoparticle immunoseparation, nanocluster signal amplification and smartphone image analysis. Sensors and Actuators B-Chemical 284:134–139. https://doi.org/10.1016/j.snb.2018.12.110
Bu S, Wang K, Ju C, Wang C, Li Z, Hao Z, Shen M, Wan J (2019) Point-of-care assay to detect foodborne pathogenic bacteria using a low-cost disposable medical infusion extension line as readout and MnO2 nanoflowers. Food Control 98:399–404. https://doi.org/10.1016/j.foodcont.2018.11.053
Wang KY, Bu SJ, Ju CJ, Li CT, Li ZY, Han Y, Ma CY, Wang CY, Hao Z, Liu WS, Wan JY (2018) Hemin-incorporated nanoflowers as enzyme mimics for colorimetric detection of foodborne pathogenic bacteria. Bioorg Med Chem Lett 28(23–24):3802–3807. https://doi.org/10.1016/j.bmcl.2018.07.017
Fu MH, Xing JF, Ge ZQ (2019) Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol a. Sci Total Environ 651:2857–2865. https://doi.org/10.1016/j.scitotenv.2018.10.145
Ye R, Zhu C, Song Y, Lu Q, Ge X, Yang X, Zhu MJ, Du D, Li H, Lin Y (2016) Bioinspired synthesis of all-in-one organic-inorganic hybrid nanoflowers combined with a handheld pH meter for on-site detection of food pathogen. Small 12(23):3094–3100. https://doi.org/10.1002/smll.201600273
Yang H, Li H, Jiang X (2008) Detection of foodborne pathogens using bioconjugated nanomaterials. Microfluid Nanofluid 5(5):571–583. https://doi.org/10.1007/s10404-008-0302-8
Zhang JJ, Xing H, Lu Y (2018) Translating molecular detections into a simple temperature test using a target-responsive smart thermometer. Chem Sci 9(16):3906–3910. https://doi.org/10.1039/c7sc05325h
Fu G, Sanjay ST, Zhou W, Brekken RA, Kirken RA, Li X (2018) Exploration of nanoparticle-mediated photothermal effect of TMB-H2O2 colorimetric system and its application in a visual quantitative photothermal immunoassay. Anal Chem 90(9):5930–5937. https://doi.org/10.1021/acs.analchem.8b00842
Qin TY, Liu B, Zhu KN, Luo ZJ, Huang YY, Pan CJ, Wang L (2018) Organic fluorescent thermometers: highlights from 2013 to 2017. Trac-Trends in Analytical Chemistry 102:259–271. https://doi.org/10.1016/j.trac.2018.03.003
Zhu JL, Wen MQ, Wen W, Du D, Zhang XH, Wang S, Lin YH (2018) Recent progress in biosensors based on organic-inorganic hybrid nanoflowers. Biosens Bioelectron 120:175–187. https://doi.org/10.1016/j.bios.2018.08.058
Zhu Z, Guan ZC, Liu D, Jia SS, Li JX, Lei ZC, Lin SC, Ji TH, Tian ZQ, Yang CYJ (2015) Translating molecular recognition into a pressure signal to enable rapid, sensitive, and portable biomedical analysis. Angewandte Chemie-International Edition 54(36):10448–10453. https://doi.org/10.1002/anie.201503963
Wang KY, Bu SJ, Ju CJ, Han Y, Ma CY, Liu WS, Li ZY, Li CT, Wan JY (2019) Disposable syringe-based visual immunotest for pathogenic bacteria based on the catalase mimicking activity of platinum nanoparticle-concanavalin A hybrid nanoflowers. Microchim Acta 186(2):57. https://doi.org/10.1007/s00604-018-3133-7
Ge J, Lei J, Zare RN (2012) Protein-inorganic hybrid nanoflowers. Nat Nanotechnol 7(7):428–432. https://doi.org/10.1038/nnano.2012.80
He Y, Ren Y, Guo B, Yang Y, Ji Y, Zhang D, Wang J, Wang Y, Wang H (2020) Development of a specific nanobody and its application in rapid and selective determination of Salmonella enteritidis in milk. Food Chem 310:125942. https://doi.org/10.1016/j.foodchem.2019.125942
Duan N, Shen M, Qi S, Wang W, Wu S, Wang Z (2020) A SERS aptasensor for simultaneous multiple pathogens detection using gold decorated PDMS substrate. Spectrochim Acta A Mol Biomol Spectrosc 230:118103. https://doi.org/10.1016/j.saa.2020.118103
Cai G, Zheng L, Liao M, Li Y, Wang M, Liu N, Lin J (2019) A microfluidic immunosensor for visual detection of foodborne bacteria using immunomagnetic separation, enzymatic catalysis and distance indication. Microchim Acta 186(12):757. https://doi.org/10.1007/s00604-019-3883-x
Bhandari D, Chen FC, Bridgman RC (2019) Detection of Salmonella typhimurium in romaine lettuce using a surface plasmon resonance biosensor. Biosensors (Basel) 9(3). https://doi.org/10.3390/bios9030094
Xiong J, Wang WX, Fu ZF (2017) Fluorimetric sandwich affinity assay for Staphylococcus aureus based on dual-peptide recognition on magnetic nanoparticles. Microchim Acta 184(10):4197–4202. https://doi.org/10.1007/s00604-017-2396-8
Zhu FJ, Zhao GY, Dou WC (2018) Electrochemical sandwich immunoassay for Escherichia coli O157:H7 based on the use of magnetic nanoparticles and graphene functionalized with electrocatalytically active Au@Pt core/shell nanoparticles. Microchim Acta 185(10):455. https://doi.org/10.1007/s00604-018-2984-2
Li T, Yu LJ, Li MT, Li W (2006) A new approach to the standard addition method for the analysis of F, Al and K content in green tea. Microchim Acta 153(1–2):109–114. https://doi.org/10.1007/s00604-005-0454-0
Zhan Z, Li H, Liu J, Xie G, Xiao F, Wu X, Aguilar ZP, Xu H (2020) A competitive enzyme linked aptasensor with rolling circle amplification (ELARCA) assay for colorimetric detection of Listeria monocytogenes. Food Control 107(0956–7135):106806. https://doi.org/10.1016/j.foodcont.2019.106806
Mannoor MS, Zhang S, Link AJ, McAlpine MC (2010) Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc Natl Acad Sci U S A 107(45):19207–19212. https://doi.org/10.1073/pnas.1008768107
Xie S, Ye J, Yuan Y, Chai Y, Yuan R (2015) A multifunctional hemin@metal-organic framework and its application to construct an electrochemical aptasensor for thrombin detection. Nanoscale 7(43):18232–18238. https://doi.org/10.1039/c5nr04532k
Pang B, Zhao C, Li L, Song X, Xu K, Wang J, Liu Y, Fu K, Bao H, Song D, Meng X, Qu X, Zhang Z, Li J (2018) Development of a low-cost paper-based ELISA method for rapid Escherichia coli O157:H7 detection. Anal Biochem 542:58–62. https://doi.org/10.1016/j.ab.2017.11.010
Funding
This work was financially supported by the National Key Research and Development Program of China (no. 2016YFD0501001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 481 kb)
Rights and permissions
About this article
Cite this article
Bu, S., Wang, K., Wang, C. et al. Immunoassay for foodborne pathogenic bacteria using magnetic composites Ab@Fe3O4, signal composites Ap@PtNp, and thermometer readings. Microchim Acta 187, 679 (2020). https://doi.org/10.1007/s00604-020-04657-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04657-1