Abstract
A method is described to enhance the sensitivity of an immunochromatographic assay for clenbuterol (CLE) by making use of dually-labeled gold nanoparticles (GNPs), background fluorescence blocking, and immunomagnetic separation. The GNPs were labeled with biotinylated antibody and streptavidin, respectively, and dually labeled GNPs were obtained via the biotin-streptavidin interaction to amplify the detection signal. The fluorescent signal was blocked by dually labeled GNPs and decreased as the dually labeled GNPs aggregation increases on nitrocellulose membrane, which derived from fluorescent polyvinylchloride card. However, fluorescence (measured at excitation/emission wavelengths of 518/580 nm) recovers when CLE reacts with dually labeled GNPs. Immunomagnetic separation was first applied for sample pretreatment. This can offset the matrix effect and improves the sensitivity and accuracy of the assay. Under the optimal conditions, the limits of detection of CLE visually were 0.25 μg·L−1. In addition, clenbuterol can be quantified in swine urine with a 0.03 μg·L−1 detection limit. This is 60-fold lower than current immunochromatography. Response is linear in the 0.06–0.59 μg·L−1 concentration range, and the recoveries from spiked swine urine range from 81 to 115%.”

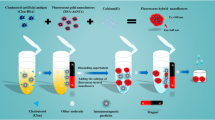
Schematic presentation of the strategies for improving sensitivity of immunochromatographic assay. It includes immunomagnetic separations, dually-labeled gold nanoparticles and background fluorescence blocking. The assay was applied to detect clenbuterol (CLE) in swine urine with an excellent performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lateral flow immunochromatography (LFIC) is a powerful tool for point-of-care testing in many different areas, as it owns the unique advantages of rapidity, simplicity, stability and portability[31]. In food safety monitoring, immunochromatography has been widely applied to rapid screen many analytes, such as toxins [6], veterinary drugs [17], hormones [14], pesticides [29], heavy metal ions [27], among others. Colloidal gold nanoparticles (GNPs), as traditional nanomaterials, is widely used in lateral flow immunoassay, but there are many limitation and challenge for its’ extensive application, such as relatively low sensitivity and susceptible to matrix interference. [2]. Hence, many great efforts have been made to improve the sensitivity of immunochromatography in the past several years, for instance signal amplification system, novel nanomaterials, and immunomagnetic separation [20, 21, 25, 26, 30, 32].
Increasing marker size is a common approach for amplifying the signal to improve the sensitivity of immunochromatography [5, 7]. Biotin−streptavidin system can greatly contribute to signal amplification and increase the sensitivity effectively [20]. GNPs labeled biotin and GNPs labeled streptavidin can be generated to accumulate and amplify the marker size, due to the good reaction specificity and high affinity between biotin and streptavidin. In addition, conventional immunochromatography used to detect small molecules needs the targets in sample reach a certain concentration to cause the visualized signal on test line disappearing completely, in that case, it limits the sensitivity of the immunochromatography [9]. However, a new format of immunochromatography based on positive correlation between analyte concentration and signal intensity would avoid the limitation and acquire higher sensitivity [4]. Background fluorescence blocking technology is an approach to make the signal intensity positively correlate with analyte. The fluorescent signal can be captured when little target competed with antigen on tset zone to react with gold probe, because the block for fluorescence becomes weaker through reducing GNPs of T zone. Besides, the fluorescein on the polyvinyl chloride card does not contact directly with GNPs on the nitrocellulose membranes, which enhances the stability of fluorescence signal on the immunochromatographic test strip (ICTS) [24]. Because indirect contact can reduce interference caused by solvents, pH, salt ions and other adverse factors with fluorescent signals. Furthermore, matrix effect of samples is a common and ubiquitous issue to influence the reaction between antigen and antibody, because the components in samples are various and complicated. The sensitivity of ICTS might be reduced by the matrix effect. Immunomagnetic separation (IMS), a technology to separate and concentrate the analytes in samples directly and efficiently, enables to decrease the matrix effect and increase sensitivity of ICTS [3, 12, 13].
Therefore, three strategies including dual-labeled gold nanoparticles, background fluorescence blocking, and immunomagnetic separation have been employed to improve the sensitivity of immunochromatography. And the novel ICTS system is demonstrated by using clenbuterol (CLE) as a model target. CLE, one of the most common beta-adrenergic agonists, is therapeutically used of the treatment of bronchial asthma or bronchitis [16]. A small amount of CLE can reduce body fat, promote muscle growth and metabolism [10]. However, long-term or excessive consumption of animal foods containing CLE is prone to produce dizziness, palpitations, fatigue and other toxic side effects, and even lead to death [1, 28]. Thus, many countries have explicitly banned the use of CLE in livestock breeding [19]. Urine is the most common biological fluid as sample to monitor illicit drug residues and used to detect residues of CLE generally [8, 15]. In this paper, a novel ICTS system with signal amplifying and immunomagnetic separation has been successfully developed to monitor CLE in swine urine.
Materials and methods
Chemicals and equipment
Bovine serum albumin (BSA) and streptavidin (SA) were purchased from Sigma-Aldrich (St. Louis, MO, USA www.sigmaaldrich.com). SA-magnetic beads were acquired from Roche Diagnostics GmbH (Mannheim, Germany www.roche.com). BSA-CLE (4.46 mg/mL) and biotinylated anti-CLE antibody (mAb-biotin, 2.32 mg/mL) were prepared in our laboratory. Goat anti-mouse IgG (17.13 mg/mL) was purchased from Arista Biologicals (Allentown, PA, USA www.aristabiologicals.com). CLE, salbutamol, cimaterol, terbutaline, ractopamine, vidorol, propranolol, labellol, atenolol, oxadolol, zilpaterol, adrenaline and phenethylamine A were provided by WDWK Bio Co., Ltd. (Beijing, China www.wdwkbio.com). All solvents and other chemicals were of analytical reagent grade.
Nitrocellulose (NC) membranes were purchased from Millipore (Bedford, MA www.merckmillipore.com). Absorbent pad and sample pad were obtained from Kinbio Tech Co., Ltd. (Shanghai, China www.goldbio.cn). Polyvinyl chloride (PVC) cards with fluorescein were from Xinpu Bio Co., Ltd. (Shanghai, China). Ultrapure water was purified with Milli-Q system from Millipore Corp. (Bedford, MA, USA www.merckmillipore.com). Ultrasonic equipment was supplied by Zhixin Instrument Co., Ltd. (Shanghai, China www.zhisun.com). XYZ dispensing platform (HM3035) and programmable strip cutter ZQ4000 were obtained from Kinbio Tech Co., Ltd. (Shanghai, China www.goldbio.cn). Fluorescence Quantitative Reader was offered by WDWK Bio Co., Ltd. (Beijing, China www.wdwkbio.com).
Conjugation of mAb-biotin and streptavidin (SA) to gold nanoparticles (GNPs)
Colloidal gold particles with 30 nm average diameter were prepared according to the protocol described in [11]. The GNP-mAb-Biotin conjugates were prepared as the previously described [18] with a minor modification. Initially, 1 mL of GNPs solution was adjusted to pH 8.0 with 0.1 M potassium carbonate. Next, 100 μL antibody dilution (containing 0.5% BSA) was added and incubated at room temperature for 10 min. Then, 100 μL of 10% BSA (w/v) was added into the mixture for 10 min to block the excess sites. Eventually, the above mixed solution was centrifuged for 10 min (10,000×g, 4 °C) and resuspended with 200 μL of phosphate buffered saline (PBS, 0.01 M, pH 7.4) containing tween-20, PEG 20000 and sucrose. The resuspended solution was sonicated for 3 min and stored at 4 °C.
The GNPs-SA conjugates were prepared according to the method described in reference [11] with a minor modification. 200 μL of SA (1 mg/mL) was added to 1 mL of GNPs solution under stirring. Then, 200 μL of 1.0 M sodium bicarbonate buffer was added immediately. After 10 min at room temperature, 200 μL of 2% PEG6000 was added to stabilize the colloid. And then the mixture was centrifuged at 4 °C for 30 min (10,000×g). Eventually, the GNPs-SA conjugates were resuspended with 200 μL of PBS buffer (0.1 M) containing 0.02% PEG6000 and mixed with the GNPs-mAb-Biotin conjugates. The complexes of gold nanoparticles, properties seen in [23],were stored at 4 °C until use.
Preparation of immunomagnetic beads
In a typically experiment, SA-magnetic beads and mAb-Biotin were incubated in PBS buffer (0.01 M, pH 7.4) at room temperature. After 30 min, the immunomagnetic beads were separated by magnet and resuspended with PBS buffer after washing three times.
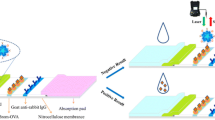
Assembly of the immunochromatography test strip (ICTS)
The sample pad was pretreated with phosphate buffer (PB, 0.02 M, pH 7.4) containing 0.5% BSA (w/v) and 0.5% PVP (w/v) and dried at 60 °C for 2 h. The test and control lines on NC membranes were coated with BSA-CLE dilution (0.22 mg/mL) and goat anti-mouse IgG solution (0.17 mg/mL), respectively. The prepared NC membranes were dried at 45 °C for 2 h. The ICTS was assembled by attaching the sample pad, NC membrane, absorbent pad to the PVC card. Finally, the assembled ICTS was cut into 4.7 mm-wide strips and stored at room temperature.
Sample pretreatment
All swine urine samples were obtained from the local pig farms and with effective pretreatment. One-milliliter of swine urine sample was incubated with 400 μL of immunomagnetic beads for 10 min. The immunomagnetic beads and target composites were separated and resuspended with PB (0.02 M, pH 7.4) after washing twice with ultrapure water. The resuspended solution was treated at 100 °C for 3 min to release the targets captured on the immunomagnetic beads, after returning to room temperature, the sample extraction was obtained after magnetic separation.
Detection of CLE with the immunochromatographic test strip (ICTS)
Gold probe and sample extraction (150 μL) were mixed in the microwell and incubated for 3 min. Then, 120 μL of mixture was dropped onto the sample pad of the ICTS. After 10 min, the results of ICTS can be obtained to qualitatively detect CLE visually. The fluorescent signal of test line, which derived from a layer of fluorescein (excitation/emission wavelengths of 518/580 nm) coated on the polyvinyl chloride card, appeared and enhanced with the increasing of CLE concentration. Fluorescence Quantitative Reader was used to quantitatively determinate CLE, which was according to the alteration of Ft/F0 (F0 and Ft were the fluorescence intensity of the background and test line, respectively). A series of CLE concentrations (0, 0.015, 0.031, 0.062, 0.12, 0.25, 0.5, 1.0 μg·L−1) were spiked, and the sample extractions were tested by the ICTS. A standard curve was established by plotting the Ft/F0 against the logarithmic concentrations of CLE for quantitative detection.
Results and discussion
As shown in Fig. 1, three strategies were employed to improve the sensitivity of immunochromatography for CLE detection, including: (i) dual-labeled GNPs, a system of amplifying the signal via biotin-SA interaction on GNPs-mAb-Biotin and GNPs-SA conjugates; (ii) fluorometric immunochromatography based on background fluorescence blocking, an approach to improve the stability and sensitivity; and (iii) immunomagnetic separatio, a system to reduce the matrix effect of samples.
Optimization of the immunochromatographic test strip (ICTS)
In order to guarantee the sensitivity of the ICTS, the amount of mAb for labeling, the volume and ratio of GNPs-mAb-Biotin and GNPs-SA for detection, and the time for reaction have been optimized. Every parameter was measured in triplicate. As shown in supplementary materials, the following experimental conditions were found to give best results: (a) amount of mAb: (100 μL); (b) amount of GNPs-mAb: (3 μL); (c) ratio of the GNPs-mAb-Biotin and GNPs–SA conjugates: 3:3; and (d) best incubation time: 3 min.
Strategies for improving the sensitivity of ICTS
Under the optimal conditions, the samples containing different concentrations of CLE (0, 0.0075; 0.015, 0.031, 0.062, 0.12, 0.25, 0.5, 1.0, 2.0 μg·L−1) were added to the sample pad of ICTS for detection. After 10 min, the results were judged visually. Figure 2a indicates that the cut-off value of the traditional ICTS with GNPs-mAb as detection probes is at 2.0 μg·L−1. Dual-labeled GNPs obtained by the interaction between GNPs-mAb-Biotin and GNPs-SA conjugates can be used to amplify the detection signal on the test line. As shown in Fig. 2b, the signal intensity of the test and control lines are darker than that in Fig. 2a, and the cut-off value of the double-labeled GNPs based ICTS for CLE detection is 1.0 μg·L−1, which is twice lower than that of the traditional ICTS.
a Appearance of traditional ICTS with GNPs-mAb as detection probes; b Appearance of ICTS with dual-labeled GNPs as probes; c Appearance of ICTS based on gold probes blocking fluorescence under UV lamp; d Standard curve plotted by the Ft/F0 against the logarithmic concentrations of CLE with 0, 0.015, 0.031, 0.062, 0.12, 0.25, 0.5, 1.0 μg·L−1 for quantitative detection
Background fluorescence on the PVC card is blocked by the GNPs aggregated on the test and control lines. Under the detection mode, the amount of GNPs decreased with the increasing CLE concentration, on the contrary, background fluorescence blocking makes the obtained fluorescence signal is proportion to the concentration of CLE, which enables to detect small molecule targets with “turn-on” mode. Hence, fluorescent signals can be captured visually when the CLE concentration reaches a certain level. As shown in Fig. 2c, the fluorescence signal of the test line recovered with the increasing of CLE concentration. The detection limit of the ICTS based on background fluorescence blocking can be determined as 0.25 μg·L−1 visually under UV lamp, which is eight times lower than that of the traditional ICTS. Because the fluorescence signal enhances remarkably with the gold probe accumulation decreases suddenly when the CLE is at 0.25 μg/L. Additionally, for quantitative detection, the fluorescence ratio (Ft/F0) was recorded by the Fluorescence Quantitative Reader, which exhibits a positive correlation with the amount of CLE (Fig. 2d). And it shows a good linear range from 0.13 to 3.10 μg·L−1 with a reliable correlation of coefficient (R2 = 0.988), the linear equation is Y = 1.21–0.66/(1 + (X/0.64)^0.88) (where Y is the Ft/F0, X is CLE concentration). The limit of detection (LOD) was estimated to be 0.09 μg·L−1 from IC10 of the standard curve. [22]
Swine urine contains some complex compounds which would influence the reaction between antigen and antibody on the ICTS, resulting in reducing the sensitivity of the method (Fig.3). To reduce the matrix effect, immunomagnetic separation technology has been used to separate and concentrate CLE directly and efficiently in swine urine samples. A series concentration of CLE with 0, 0.0075; 0.015, 0.031, 0.062, 0.12, 0.25, 0.5, 1.0, 2.0 μg·L−1 was spiked in PB buffer or swine urine (analyte extraction by immunomagnetic separation), and then the above mixtures were tested with the ICTS. Consequently, the two established standard curves were presented in Fig.3. It shows that the standard curve (black line) of pretreated swine urine is approximately the same as that of PB buffer (blue line), which demonstrates that the matrix effect of the swine urine has been markedly reduced with immunomagnetic separation technology. In PB buffer, the calibration curve provided a liner range between 0.056 and 0.775 μg·L−1 with a good correlation of 0.996. And the detection limit was calculated as 0.03 μg·L−1 from IC10. In pretreated swine urine samples, the standard curve shows a good linear range between 0.06–0.59 μg·L−1 for quantitative detection of CLE with the LOD at 0.03 μg·L−1 (the linear equation is Y = 0.94–0.45/(1 + (X/0.19)^1.19, where Y is the Ft/F0, X is CLE concentration), which is consisted with the calibration curve of PB buffer. Moreover, it is three times lower than that of un-pretreated swine urine samples and the sensitivity has been improved by about 60 times compared with the traditional GNPs based ICTS. Those results proved that the novel ICTS system offers a superior sensitivity for detecting CLE.
Evaluation of the immunochromatographic test strip
To evaluated the specificity of the ICTS for Clenbuterol, twelve adrenoceptor agonists (salbutamol, cimaterol, terbutaline, ractopamine, vidorol, propranolol, labellol, atenolol, oxadolol, zilpaterol, adrenaline and phenethylamine A) were spiked into swine urine with the concentrations of 0, 1.5, 3.1, 6.2, 12.5, 25, 50, 100 μg·L−1, respectively. After pretreated with immunomagnetic separation technology, the extractions were tested with the novel ICTS system. The result was shown in Table 1, it indicates that the cross reactivities of salbutamol, cimaterol and terbutaline exhibits are 0.59%, 3.5% and 11.1%, respectively, the other nine adrenoceptor agonists exhibits negligible cross reaction with the ICTS.
The accuracy of the novel ICTS system was evaluated by analyzing the swine urine samples spiked with 0.1, 0.15, 0.3 μg·L−1 of CLE. Table 2 shows that the average recoveries for CLE detection ranges from 81% to 115% with relative standard deviation less than 6%, which is acceptable for rapid and quantitative screening CLE in swine urine samples.
Three blank swine urine samples were fortified with different concentrations of CLE (0.2, 0.25, 0.3 μg·L−1), and detected by ICTS and ELISA kit. The results of the two methods showed good correlation (Table 3). And seven negative swine urine were not detected as false positive by ICTS and ELISA kit. Besides, other methods for rapid screening CLE have been summarized and compared with ICTS in Table 1S, those findings demonstrated that the novel ICTS system is comparable or even better than the other methods. Hence, the fluorometric immunochromatography based on gold probe block fluorescence is a practical and sensitive method for detecting CLE and other small molecules. Moreover, immunomagnetic separations for urine and other sample (meat, milk, feed) can reduce matrix effects and provide better sensitivity and stability for immunochromatography.
Conclusion
The strategies for improving sensitivity of immunochromatographic assay are based on dual-labeled, background fluorescence and immunomagnetic separation. The fluorometric immunochromatography based on dual-labeled gold nanoparticles blocking provided a signal amplification and novel“turn-on” mode for CLE detection, and the immunomagnetic separations for swine urine reduced matrix effects. Ultimately, the ICTS system based on the strategies was established to detect CLE in swine urine samples with a satisfactory performance, and the sensitivity of ICTS system increased 60-fold than traditional gold immunochromatography. It demonstrated that the novel ICTS system provides an approach for sensitive and quantitative detection in food safety and has the potential to a wide variety of other analytes.
References
Barbosa J, Cruz C, Martins J, Manuel Silva J, Neves C, Alves C, Ramos F, Noronha da Silveira MI (2005) Food poisoning by clenbuterol in Portugal. Food Addit Contam 22:563–566. https://doi.org/10.1080/02652030500135102
Cai Y, Kang K, Liu Y, Wang Y, He X (2018) Development of a lateral flow immunoassay of C-reactive protein detection based on red fluorescent nanoparticles. Anal Biochem 556:129–135. https://doi.org/10.1016/J.AB.2018.06.017
Chen J, Park B, Eady M (2017) Simultaneous detection and serotyping of salmonellae by Immunomagnetic separation and label-free surface-enhanced Raman spectroscopy. Food Anal Methods 10:3181–3193. https://doi.org/10.1007/s12161-017-0870-x
Chen X, Xu Y, Yu J, Li J, Zhou X, Wu C, Ji Q, Ren Y, Wang L, Huang Z, Zhuang H, Piao L, Head R, Wang Y, Lou J (2014) Antigen detection based on background fluorescence quenching immunochromatographic assay. Anal Chim Acta 841:44–50. https://doi.org/10.1016/j.aca.2014.07.025
Cho IH, Bhunia A, Irudayaraj J (2015) Rapid pathogen detection by lateral-flow immunochromatographic assay with gold nanoparticle-assisted enzyme signal amplification. Int J Food Microbiol 206:60–66. https://doi.org/10.1016/j.ijfoodmicro.2015.04.032
Dong S, Liu Y, Zhang X, Xu C, Liu X, Zhang C (2018) Development of an immunochromatographic assay for the specific detection of bacillus thuringiensis (Bt) Cry1Ab toxin. Anal Biochem 567:1–7. https://doi.org/10.1016/j.ab.2018.08.014
Dou L, Zhao B, Bu T, Zhang W, Huang Q, Yan L, Huang L, Wang Y, Wang J, Zhang D (2018) Highly sensitive detection of a small molecule by a paired labels recognition system based lateral flow assay. Anal Bioanal Chem 410:3161–3170. https://doi.org/10.1007/s00216-018-1003-0
Feng F, Zheng J, Qin P, Han T, Zhao D (2017) A novel quartz crystal microbalance sensor array based on molecular imprinted polymers for simultaneous detection of clenbuterol and its metabolites. Talanta 167:94–102. https://doi.org/10.1016/J.TALANTA.2017.02.001
Fu Q, Liang J, Lan C, Zhou K, Shi C, Tang Y (2014) Development of a novel dual-functional lateral-flow sensor for on-site detection of small molecule analytes. Sensors Actuators B Chem 203:683–689. https://doi.org/10.1016/j.snb.2014.06.043
Garssen GJ, Geesink GH, Hoving-Bolink AH, Verplanke JC (1995) Effects of dietary clenbuterol and salbutamol on meat quality in veal calves. Meat Sci 40:337–350. https://doi.org/10.1016/0309-1740(94)00034-5
Hermanson GT (2013) Bioconjugate Techniques: Third Edition
Huang Z, Cui X, Xie Q-Y, Liu DF, Lai WH (2016) Short communication: a novel method using immunomagnetic separation with a fluorescent nanobeads lateral flow assay for the rapid detection of low-concentration Escherichia coli O157:H7 in raw milk. J Dairy Sci 99:9581–9585. https://doi.org/10.3168/jds.2016-11780
Liu D, Huang Y, Wang S, Liu K, Chen M, Xiong Y, Yang W, Lai W (2015) A modified lateral flow immunoassay for the detection of trace aflatoxin M1 based on immunomagnetic nanobeads with different antibody concentrations. Food Control 51:218–224. https://doi.org/10.1016/j.foodcont.2014.11.036
Liu J, Fan Y, Kong Z, Wang Y, Luo J, Xu S, Jin H, Cai X (2018) Smartphone-based rapid quantitative detection of luteinizing hormone using gold immunochromatographic strip. Sensors Actuators, B Chem 259:1073–1081. https://doi.org/10.1016/j.snb.2017.12.161
Liu R, Liu L, Song S, et al (2017) Development of an immunochromatographic strip for the rapid detection of 10 β-agonists based on an ultrasensitive monoclonal antibody
Parr MK, Opfermann G, Schäänzer W (2009) Analytical methods for the detection of clenbuterol. Bioanalysis
Pei X, Wang Q, Li X, Xie J, Xie S, Peng T, Wang C, Sun Y, Jiang H (2016) Provision of ultrasensitive quantitative gold Immunochromatography for rapid monitoring of olaquindox in animal feed and water samples. Food Anal Methods 9:1919–1927. https://doi.org/10.1007/s12161-015-0360-y
PENG T, ZHANG FS, YANG WC et al (2014) Lateral-flow assay for rapid quantitative detection of Clorprenaline residue in swine urine. J Food Prot 77:1824–1829. https://doi.org/10.4315/0362-028X.JFP-14-103
Prezelj A, Obreza A, Pecar S (2003) Abuse of Clenbuterol and its detection. Curr Med Chem 10:281–290. https://doi.org/10.2174/0929867033368330
Shan S, Lai W, Xiong Y, Wei H, Xu H (2015) Novel strategies to enhance lateral flow immunoassay sensitivity for detecting foodborne pathogens. J Agric Food Chem 63:745–753. https://doi.org/10.1021/jf5046415
Shellaiah M, Simon T, Venkatesan P, Sun KW, Ko FH, Wu SP (2018) Nanodiamonds conjugated to gold nanoparticles for colorimetric detection of clenbuterol and chromium(III) in urine. Microchim Acta 185. https://doi.org/10.1007/s00604-017-2611-7
Sun Y, Xie J, Peng T, Wang J, Xie S, Yao K, Wang C, Sun S, Xia X, Jiang H (2017) A new method based on time-resolved Fluoroimmunoassay for the detection of streptomycin in Milk. Food Anal Methods 10:2262–2269. https://doi.org/10.1007/s12161-017-0797-2
Taranova NA, Urusov AE, Sadykhov EG, Zherdev AV, Dzantiev BB (2017) Bifunctional gold nanoparticles as an agglomeration-enhancing tool for highly sensitive lateral flow tests: a case study with procalcitonin. Microchim Acta 184:4189–4195. https://doi.org/10.1007/s00604-017-2355-4
Wang J, Cao F, He S, Xia Y, Liu X, Jiang W, Yu Y, Zhang H, Chen W (2018a) FRET on lateral flow test strip to enhance sensitivity for detecting cancer biomarker. Talanta. 176:444–449. https://doi.org/10.1016/j.talanta.2017.07.096
Wang J, Zhang L, Huang Y, Dandapat A, Dai L, Zhang G, Lu X, Zhang J, Lai W, Chen T (2017) Hollow au-ag nanoparticles labeled Immunochromatography strip for highly sensitive detection of Clenbuterol. Sci Rep 7. https://doi.org/10.1038/srep41419
Wang R, Zhang W, Wang P, Su X (2018b) A paper-based competitive lateral flow immunoassay for multi β-agonist residues by using a single monoclonal antibody labelled with red fluorescent nanoparticles. Microchim Acta 185:191. https://doi.org/10.1007/s00604-018-2730-9
Xiao M, Fu Q, Shen H, Chen Y, Xiao W, Yan D, Tang X, Zhong Z, Tang Y (2018) A turn-on competitive immunochromatographic strips integrated with quantum dots and gold nano-stars for cadmium ion detection. Talanta. 178:644–649. https://doi.org/10.1016/j.talanta.2017.10.002
Yan H, Xu D, Meng H, et al (2014) Food poisoning by clenbuterol in China. Qual Assur Saf Crop Foods
Zhang Q, Qu Q, Chen S, Liu X, Li P (2017) A double-label time-resolved fluorescent strip for rapidly quantitative detection of carbofuran residues in agro-products. Food Chem 231:295–300. https://doi.org/10.1016/j.foodchem.2017.02.016
Zhang Y, Li M, Cui Y, Hong X, du D (2018) Using of tyramine signal amplification to improve the sensitivity of ELISA for aflatoxin B1 in edible oil samples. Food Anal Methods 11:2553–2560. https://doi.org/10.1007/s12161-018-1235-9
Zhao B, Huang Q, Dou L, Bu T, Chen K, Yang Q, Yan L, Wang J, Zhang D (2018) Prussian blue nanoparticles based lateral flow assay for high sensitive determination of clenbuterol. Sensors Actuators B Chem 275:223–229. https://doi.org/10.1016/J.SNB.2018.08.029
Zhou J, Zhu K, Xu F, Wang W, Jiang H, Wang Z, Ding S (2014) Development of a microsphere-based fluorescence immunochromatographic assay for monitoring lincomycin in milk, honey, beef, and swine urine. J Agric Food Chem 62:12061–12066. https://doi.org/10.1021/jf5029416
Funding
This study was supported financially by grants from the Ministry of Science and Technology of People’s Republic of China (2017YFF0211003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuyang Zeng and Demei Liang are co-author.
Electronic supplementary material
ESM 1
(DOCX 179 kb)
Rights and permissions
About this article
Cite this article
Zeng, Y., Liang, D., Zheng, P. et al. Immunochromatographic fluorometric determination of clenbuterol with enhanced sensitivity. Microchim Acta 186, 225 (2019). https://doi.org/10.1007/s00604-019-3326-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3326-8