Abstract
Streptomycin (STR), used extensively in the treatment of bovine mastitis, may cause damage such as ototoxicity, allergic reaction, and increasing bacterial resistance to consumers on account of remnant in milk. A time-resolved fluoroimmunoassay (TRFIA) was developed to quantify STR for the first time to ensure food safety. Using secondary antibody labeled with europium (Eu3+) chelate as a tracer, the proposed TRFIA showed that the linear working range was 0.32–5.0 ng/mL under the optimal conditions. Milk samples were deproteinized by trichloroacetic acid and the limit of detection of STR in milk was 1.8 μg/kg. The recoveries of milk samples fortified with 4.0, 20, and 40 μg/kg of STR ranged from 86.2 to 96.3% with relative standard deviations less than 11%. Results of TRFIA for the authentic samples were coincided with those of UHPLC-MS/MS analyses. This study confirmed that the established TRFIA was sensitive as well as reliable and could be an alternative method to monitor STR residue in milk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptomycin (STR), a member of aminoglycoside antibiotics, is efficient to treat gram-negative infectious diseases of food animals. Because of its effectiveness and low cost, STR has been widely used in cattle as mastitis curative (Granja et al. 2009; Haasnoot et al. 2003; Mishra et al. 2015). Excessive use or inobservance of withdrawal time may result in the residue of STR in milk and present potential hazard to consumers in terms of ototoxicity, allergic reaction, inhibiting growth of marrow, and the development of bacterial resistance (de Oliveira et al. 2009; Wan et al. 2006). To protect public health, maximum residue limit (MRL) for STR in milk has been set at 200 μg/kg in China, European Commission, and Codex Alimentarius Commission (MOA 2002; Regulation 2010; Commission 2014). As a great deal of attention has been paid to the risk of antibiotic residual all around the world, the restriction of antibiotic in animal-derived food will be more rigid (Organization 2015; European Commission 2011; NHFPC 2016).
To detect the residue level of STR, a variety of analytical methods have been developed, including instrumental methods such as HPLC (Furusawa 2001; Vinas et al. 2007) and HPLC-MS (Aksu et al. 2004; Arsand et al. 2016; van Bruijnsvoort et al. 2004) and immunoassay methods such as ELISA (Aksu et al. 2004; Samsonova et al. 2005; Unusan 2009) and immunochromatographic assay (ICA) (Byzova et al. 2011; Wu et al. 2010). On the one hand, immunoassay methods have attracted more and more attention by virtue of their simplicity, rapidness, and high throughput when compared with instrumental methods. On the other hand, the commonly used immunoassay methods for STR detection are susceptible to matrix interference and suffer from limited sensitivity (Taghdisi et al. 2016). Time-resolved fluoroimmunoassay (TRFIA) is an ultrasensitive and highly selective immunoassay method using long fluorescence life time lanthanide chelates as the labels. It can reduce background fluorescence by extending the measurement time and detecting the specific fluorescence of lanthanide chelates after the decay of short-life background fluorescence (Peippo et al. 2005; Shi et al. 2015). TRFIA has been successfully employed to test several drugs residue in animal-derived food (Le et al. 2013; Shen et al. 2007; Wei et al. 2013; Zhou et al. 2015).
In this study, we firstly established and optimized a TRFIA for detection of STR. The method was proven to be appropriate for screening STR in milk and had been confirmed by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS), which would satisfy the tendency of more strict management of antibiotics.
Materials and Methods
Chemicals and Reagents
STR, gentamycin, spectinomycin, kanamycin, tobramycin, amikacin, and dihydrostreptomycin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Goat anti-mouse antibody, anti-STR monoclonal antibodies (anti-STR McAb), and STR-ovalbumin (STR-OVA) were obtained from our laboratory. The 96-well microtiter plates were obtained from Nunc International (Roskilde, Denmark). Sephadex G-50 was from Pharmacia (Uppsala, Sweden). N′-[p-isothiocyanato-benzyl]-diethylene-triamine-N 1,N 2,N 3,N 4-tetraacetate-Eu3+ (DTTA-Eu3+) and enhancement solution were purchased from Jiangyuan Industrial Technology and Trade Corporation (Jiangsu, China). All other chemicals and solvents were of analytical grade or better and were obtained from Beijing Chemical Reagent Co., Ltd.
Preparation of Labeled Eu3+-Secondary Antibody Conjugate
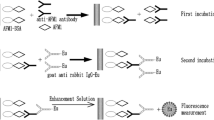
The procedure of labeling goat anti-mouse secondary antibody with Eu3+ chelate (DTTA-Eu3+) was performed according to the previous literatures with some modifications (Liang et al. 2015; Lin et al. 2013). In brief, secondary antibody was dialyzed for 3 days with labeling buffer to remove interfering compounds such as Tris and NaN3. Then, 1 mL purified antibody diluted to a final concentration of 5 mg/mL was mixed with 1 mg Eu3+ chelate, and the mixture was stirred gently at 4 °C overnight. The labeled Eu3+-secondary antibody conjugate was separated from unreacted chelate using a Sephadex G-50 column with Tris–HCl as the elution buffer. The gel filtration eluates were collected 1 mL/tube, and UV abortion at 280 nm was monitored by a NanoDrop 2000c UV–vis spectrophotometer (Thermo Fisher, USA) and fluorescence intensity (cps) at 615 nm by a multifunctional microplate reader SpectraMax M5 (Molecular Devices Co., Ltd. USA). The eluates of the first separation peak with higher UV abortion value and fluorescence intensity were pooled and stored at −20 °C. The principle of conjugation reaction between Eu3+ chelate and secondary antibody is shown in Fig. 1.
Indirect Competitive TRFIA Protocol for STR Detection
The wells of polystyrene microtiter plates were coated with 100 μL STR-OVA (0.33 μg/mL) in 50 mM carbonate buffer (pH 9.6) overnight at 4 °C. The plates were washed three times with TBST and blocked at 37 °C for 1 h. After another washing step, either 50 μL of the standards (0, 0.16, 0.32, 0.625, 1.25, 2.5, 5, 10 ng/mL diluted by PBS) or 50 μL of the samples was added followed by dispensing 50 μL anti-STR McAb into each well. Nonspecific binding was determined by adding 100 μL PBS without antibody. The plates were incubated at 37 °C for 30 min and rinsed three times. A volume of 100 μL labeled Eu3+-secondary antibody (1.6 μg/mL) was applied to the wells for 30 min before another washing procedure. One hundred-microliter enhancement solution was added to dissociate Eu3+ from Eu3+-secondary antibody conjugate and formed high fluorescent micelle with Eu3+ wrapped in agents (TOPO, 2-NTA). The fluorescence intensity of Eu3+ was measured by using a SpectraMax M5 setting excitation wavelength at 340 nm, an emission wavelength at 615 nm, and a delay time at 400 μs (Fig. 2). Blocking reagent (1–5% BSA, 1–5% skim milk, 2% casein, and 2% PEG20000), salt concentration (0, 0.05, 0.1, 0.2, and 0.4 M), pH (5.0, 6.0, 7.0, 8.0, and 9.0) of assay buffer, and diluent of Eu3+-secondary antibody were optimized. All the optimization experiments were measured in triplicate. Based on the optimal conditions, the standard curve was constructed and served to determine the concentration of STR. The sensitivity of TRFIA defined as IC50 and linear working range were obtained from a four-parameter logistic equation of the sigmoidal curve.
Sample Preparation
All raw milk samples were obtained from the local cattle farms. Except the test of confirmation analysis, the samples applied in the experiment were confirmed as free of STR by UHPLC-MS/MS. Different pretreatments–dilution methods and trichloroacetic acid (TCA) method were taken to overcome the matrix interference. The dilution method was as follows: milk samples were centrifuged at 4000g for 10 min to remove the fat, and the upper layer was employed for TRFIA analysis after being diluted different times by PBS (no dilution, 20- and 40-fold). In the TCA method, equivalent 1% TCA (w/v) was added in the milk followed by vortex and centrifugation. The supernatant was diluted by PBS different times (no dilution, 2- and 4-fold) and subsequently employed for analysis. The spiked samples were fortified with STR to gain the final concentration at 4.0, 20, and 40 μg/kg. The preparation procedure of samples for UHPLC-MS/MS analysis was performed according to Gremilogianni et al. (2010). All the samples and the standards were evaluated in triplicate.
UHPLC-MS/MS Conditions
Confirmation analysis was conducted using a Waters Acquity UHPLC system combined with a Waters Xevo TQ-S triple-quadrupole mass spectrometer. A volume of 10-μL solution was injected onto an Acquity UHPLC HSS T3 column (2.1 mm × 100 mm; particle size, 1.7 μm) to separate. The mobile phase consisted of methanol (mobile phase A) and water (mobile phase B) at a volume ratio of 35:65 at a flow rate of 0.300 mL/min. The gradient elution program was as follows: 0–2 min, 35% A and 65% B; 2–3 min, 100% A; and 3–4 min, 35% A and 65% B. Analytes were quantified by multiple reaction monitoring mode using positive-ion electrospray interface (ESI+). The transitions of precursor and product ions for STR were m/z 582 → 263 and 582 → 246. Interface conditions were as follows: nebulizer gas, N2 (45 psi); cone gas, N2 (150 L/h, 350 °C); desolvation gas, N2 (1000 L/h, 400 °C); and capillary voltage, 2.8 kV. The corresponding cone voltage and collision energy optimized for maximum detection sensitivity were 85 V and 42/32 eV for product/precursor ion.
Results and Discussion
Evaluation of Eu3+-Secondary Antibody Conjugate
Based on the principle of gel filtration, the substance of which molecule was too big to penetrate into the pores of the gel would appear early. Thus, the first peak monitored for UV abortion value and fluorescence intensity contained Eu3+-secondary antibody conjugates; moreover, the second peak contained the unreacted Eu3+ chelate. The eluates of the first peak were pooled and evaluated according to the previous report (Zhang et al. 2010). The labeling yield of Eu3+ to secondary antibody was calculated to be 12.9. There was no loss of Eu3+-secondary antibody conjugate immunoreactivity observed in 8 months, demonstrating good stability of the conjugate during long-term storage.
Optimization of TRFIA Conditions
As is known to all, a large number of factors in immunoassay would affect assay sensitivity (Sheng et al. 2013). To attain a better performance, the TRFIA conditions including blocking reagent, assay buffer, and diluent of Eu3+-secondary antibody were optimized. The fluorescence intensity of control and spiked samples and the inhibition ratio were utilized to select the optimal parameters. The inhibition ratio was calculated by the following formula: Inhibition ratio = 1 − F/F 0 (F and F 0 were defined as the fluorescence intensity of control and spiked samples, respectively), and the concentration of STR in spiked samples was 5 ng/mL.
Blocking reagent could reduce the unspecific binding of the secondary antibody onto the plate and thereupon improve the sensitivity of immunoassay (Deng et al. 2003). As shown in Table 1, among the blocking reagents, the maximum value of F 0 was acquired by BSA; nevertheless, the unspecific binding value was also increased. In consideration of inhibition ratio and F 0, skim milk at a concentration of 2% was selected as the optimal blocking reagent.
The salt concentration and pH value of the assay buffer could influence the equilibrium constant of antigen-antibody reaction (Reverberi and Reverberi 2007). The inhibition ratio was improved as the concentration of NaCl in assay buffer increased to 0.05 M; meanwhile, higher concentration of NaCl might result in the diminution of fluorescence intensity (Fig. 3a). Therefore, 0.05 M NaCl was added in the assay buffer to get a satisfying compromise between the inhibition ratio and F 0. Using a too low/high pH may suppress the combination of antibody and antigen. The inhibition ratio as well as F 0 was lower in the conditions of acidic; the maximum values could be obtained when the pH was 8.0 (Fig. 3b).
The optimized results of TRFIA conditions (n = 3). a Influence of the concentration of NaCl in the assay buffer on the TRFIA performance. b Influence of pH of the assay buffer on the TRFIA performance. c Influence of the concentration of NaCl in the Eu3+-secondary antibody diluent on the TRFIA performance. d Influence of the content of Tween-20 in the Eu3+-secondary antibody diluent on the TRFIA performance
In order to improve the conjugate activity, the concentrations of NaCl and Tween-20 in Eu3+-secondary antibody dilution were optimized. The tendency of conjugate fluorescence intensity influenced by salt concentration as seen in Fig. 3c is similar to Fig. 3a. Fluorescence intensity gradually decreased after salt concentration reaching an optimum at 0.08 M. It may be explained that polar environment generated by different salt concentrations could impact the antibody activity. As presented in Fig. 3d, suitable surfactant would make TRFIA performance better and 0.5% Tween-20 in Eu3+-secondary antibody dilution was chosen as the optimum condition.
Evaluation of the TRFIA
TRFIA Standard Curve for STR Detection
The standard curve was obtained from five separate assays under the optimal assay conditions, by plotting F/F 0 value versus logarithm concentration (Fig. 4). The IC50 of the method for STR was 1.4 ng/mL, and the within-assay coefficient of variation (CV) was below 5.8%, indicating high sensitivity and reproducibility. The linear working range was from 0.32 to 5.0 ng/mL with satisfactory correlation coefficients (R 2) more than 0.999. The limit of detection (LOD) defined as IC10 was 0.19 ng/mL. According to the previous reports, the LOD values of ELISA (Gaudin et al. 2013; Wang et al. 2013) and ICA (Byzova et al. 2011; Wu et al. 2010) were 0.24 and 20 ng/mL, respectively; the lower LOD of TRFIA manifested the better performance than the previous assays. There was no significant change in the signal value of TRFIA in 24 h; however, the accuracy of ELISA was decreased sharply with time delaying, which indicated that the stability of TRFIA was superior than that of ELISA.
Specificity
The specificity of antibody was estimated in cross-reactivity (CR) with other aminoglycoside antibiotics using TRFIA, including gentamycin, spectinomycin, kanamycin, tobramycin, amikacin, and dihydrostreptomycin. As illustrated in Table 2, the anti-STR McAb had negligible CR (<0.1%) with other aminoglycosides except dihydrostreptomycin (118.8%), because dihydrostreptomycin held the similar structure with STR. The antibody proved to be very specific for STR and dihydrostreptomycin.
Matrix Effect
Matrix effect might bring down the sensitivity of immunoassay on account of interfering the combination of antibody with analyte (Jiang et al. 2013; Kolosova et al. 2006). If the two calibration curves generated in PBS and sample matrix were superimposed approximately, the matrix effect was overcome and analytes in samples could be determined using a calibration curve in PBS (Sheng et al. 2013). Different pretreatments were applied to eliminate the matrix effect of milk. Results of the sample preparation experiments are depicted in Fig. 5. Compared with the TCA method, dilution alone did not work well in reducing the matrix interference. Therefore, the milk samples were disposed via diluting 4-fold with PBS after using TCA to deproteinize for quantification of STR. In milk samples, the LOD defined as the concentration corresponding to the mean value of the 20 buffer blank values plus three times the standard deviation (SD) was 1.8 μg/kg, which was far below the MRL 200 μg/kg.
Standard curves of TRFIA for STR by different pretreatments to remove the matrix effect of milk. a Dilution method: standard curve in PBS (squares); standard curve in milk (circles); standard curve in milk diluted 20-fold by PBS (triangles); standard curve in milk diluted 40-fold by PBS (inverted triangles). b TCA method: standard curve in PBS (squares); standard curve in milk treated with TCA (circles); standard curve in milk treated with TCA following 2-fold dilution (triangles); standard curve in milk treated with TCA following 4-fold dilution (inverted triangles)
Accuracy and Precision
To evaluate the accuracy and precision of the developed TRFIA, the recoveries of STR from milk samples were studied. All samples were fortified with STR at three levels (4.0, 20, and 40 μg/kg). The average recoveries of STR in milk (Table 3) ranged from 86.2 to 96.3%, and the RSDs were 1.7 to 10.6%. The recovery results were in the allowed range of 80–110% for all concentration levels (European Commission. 2002), showing good performance of the TRFIA method.
Method Validation
To validate the developed method, TRFIA and UHPLC-MS/MS were used to analyze the concentration of STR in 20 milk samples. STR was detected in 12 samples ranging from 2.02 to 14.1 μg/kg by TRFIA, while in 13 samples ranging from 1.84 to 13.37 μg/kg by UHPLC-MS/MS. As shown in Fig. 6, a good correlation was obtained between the TRFIA and UHPLC-MS/MS results. These results suggested that the TRFIA could be applied to measure real samples as a reliable method.
Conclusion
In this study, an indirect competitive TRFIA for STR in milk was established with favorable accuracy, precision, and sensitivity. Compared with ELISA using enzyme as a tracer, TRFIA with lanthanide chelate as the label was more stable and sensitive. Moreover, compared with UHPLC-MS/MS, TRFIA shortened the measure duration and simplified the procedure, generating coincident results in real sample analysis. Thereby, this proposed TRFIA could be a suitable method for screening of STR in milk.
References
Aksu H, Cetin O, Arun O, Ergun O (2004) Determination of tetracyclin and streptomycin residues by means of ELISA in pasteurized and UHT-sterilized milk. Med Weter 60:1171–1173
Arsand JB, Jank L, Martins MT, Hoff RB, Barreto F, Pizzolato TM, Sirtori C (2016) Determination of aminoglycoside residues in milk and muscle based on a simple and fast extraction procedure followed by liquid chromatography coupled to tandem mass spectrometry and time of flight mass spectrometry. Talanta 154:38–45. doi:10.1016/j.talanta.2016.03.045
Byzova NA, Zvereva EA, Zherdev AV, Eremin SA, Sveshnikov PG, Dzantiev BB (2011) Pretreatment-free immunochromatographic assay for the detection of streptomycin and its application to the control of milk and dairy products. Anal Chim Acta 701:209–217. doi:10.1016/j.aca.2011.06.001
Commission CA (2014) Maximum residue limits for veterinary drugs in foods. Updated as at the 35th session of the Codex Alimentarius Commission (July 2012) CAC/MRL:2–2012
de Oliveira RC, Paschoal JAR, Sismotto M, Airoldi FPD, Reyes FGR (2009) Development and validation of an LC-APCI-MS-MS analytical method for the determination of streptomycin and dihydrostreptomycin residues in milk. J Chromatogr Sci 47:756–761
Deng A et al (2003) Residue analysis of the pharmaceutical diclofenac in different water types using ELISA and GC-MS. Environ Sci Technol 37:3422–3429
European Commission (2002) Commission decision (2002)/657/EC of 12 August (2002) implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Comm L 221:8–28
European Commission (2011) Action plan against the rising threats from antimicrobial resistance. http://ec.europa.eu/dgs/health consumer/docs/communication amr 2011 748 en.pdf. Accessed 16 January 2012
Furusawa N (2001) Determining the procedure for routine residue monitoring of sulfamethazine in edible animal tissues. Biomed Chromatogr 15:235–239. doi:10.1002/bmc.48
Gaudin V, Hedou C, Verdon E (2013) Validation of two ELISA kits for the screening of tylosin and streptomycin in honey according to the European decision 2002/657/EC. Food Addit Contam A 30:93–109. doi:10.1080/19440049.2012.722696
Granja R, Nino AMM, Zucchetti RAM, Nino REM, Patel R, Salerno AG (2009) Determination of streptomycin residues in honey by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 637:64–67. doi:10.1016/j.aca.2009.01.006
Gremilogianni AM, Megoulas NC, Koupparis MA (2010) Hydrophilic interaction vs ion pair liquid chromatography for the determination of streptomycin and dihydrostreptomycin residues in milk based on mass spectrometric detection. J Chromatogr A 1217:6646–6651. doi:10.1016/j.chroma.2010.04.059
Haasnoot W, Bienenmann-Ploum M, Kohen F (2003) Biosensor immunoassay for the detection of eight sulfonamides in chicken serum. Anal Chim Acta 483:171–180. doi:10.1016/s0003-2670(02)01545-3
Jiang WX, Wang ZH, Nolke G, Zhang J, Niu LL, Shen JZ (2013) Simultaneous determination of aflatoxin B-1 and aflatoxin M-1 in food matrices by enzyme-linked immunosorbent assay. Food Anal Methods 6:767–774. doi:10.1007/s12161-012-9484-5
Kolosova AY, Shim WB, Yang ZY, Eremin SA, Chung DH (2006) Direct competitive ELISA based on a monoclonal antibody for detection of aflatoxin B-1. Stabilization of ELISA kit components and application to grain samples. Analytical Anal Bioanal Chem 384:286–294
Le T, Yan PF, Liu J, Wei S (2013) Simultaneous detection of sulfamethazine and sulfaquinoxaline using a dual-label time-resolved fluorescence immunoassay. Food Addit Contam A 30:1264–1269. doi:10.1080/19440049.2013.801084
Liang QN, Chen PQ, Liu TC, Zhou JW, Chen JJ, Wu YS (2015) Development of a time-resolved fluoroimmunoassay for Epstein-Barr virus viral capsid antigen IgA antibody in human serum. J Virol Methods 222:16–21. doi:10.1016/j.jviromet.2015.03.024
Lin GF, Liu TC, Zou LP, Hou JY, Wu YS (2013) Development of a dual-label time-resolved fluoroimmunoassay for the detection of α-fetoprotein and hepatitis B virus surface antigen. Luminescence 28:401–406
Mishra GK, Sharma A, Bhand S (2015) Ultrasensitive detection of streptomycin using flow injection analysis-electrochemical quartz crystal nanobalance (FIA-EQCN) biosensor. Biosens Bioelectron 67:532–539. doi:10.1016/j.bios.2014.09.033
MOA (2002) No. 235 Bulletin of the Ministry of Agriculture of the People’s Republic of China. Available at http://www.moa.gov.cn/zwllm/tzgg/gg/200302/t20030226_59300.htm Accessed 28.09.12
NHFPC (2016) National action plan on antimicrobial resistance 2016 to 2020. National Health and Family Planning Commission of China, available at http://www.gov.cn/xinwen/2016-08/25/content_5102348.htm
Organization WH (2015) Global action plan on antimicrobial resistance
Peippo P, Lovgren T, Tuomola M (2005) Rapid screening of narasin residues in poultry plasma by time-resolved fluoroimmunoassay. Anal Chim Acta 529:27–31. doi:10.1016/j.aca.2004.07.013
Regulation C (2010) No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union L 15:1–72
Reverberi R, Reverberi L (2007) Factors affecting the antigen-antibody reaction. Blood Transfus 5:227
Samsonova JV, Bashkurov ML, Ivanova NL, Rubtsova MY, Egorov AM (2005) ELISA of streptomycin in buffer and milk: effect of reagents’ structure and analysis format on assay performance. Food Agr Immunol 16:47–57. doi:10.1080/09540100400029944
Shen JZ, Zhang Z, Yao Y, Shi WM, Liu YB, Zhang SX (2007) Time-resolved fluoroimmunoassay for ractopamine in swine tissue. Anal Bioanal Chem 387:1561–1564. doi:10.1007/s00216-006-1063-4
Sheng W, Yang L, Wang JP, Zhang Y, Wang S (2013) Development of an enzyme-linked immunosorbent assay for the detection of gentamycin residues in animal-derived foods. LWT-Food Sci Technol 50:204–209. doi:10.1016/j.lwt.2012.05.028
Shi HY, Sheng EZ, Feng L, Zhou LL, Hua XD, Wang MH (2015) Simultaneous detection of imidacloprid and parathion by the dual-labeled time-resolved fluoroimmunoassay. Environ Sci Pollut R 22:14882–14890. doi:10.1007/s11356-015-4697-y
Taghdisi SM, Danesh NM, Nameghi MA, Ramezani M, Abnous K (2016) A label-free fluorescent aptasensor for selective and sensitive detection of streptomycin in milk and blood serum. Food Chem 203:145–149. doi:10.1016/j.foodchem.2016.02.017
Unusan N (2009) Occurrence of chloramphenicol, streptomycin and tetracycline residues in ultra-heat-treatment milk marketed in Turkey. Int Food Sci Nutr 60:359–364. doi:10.1080/09637480701664555
van Bruijnsvoort M, Ottink SJM, Jonker KM, de Boer E (2004) Determination of streptomycin and dihydrostreptomycin in milk and honey by liquid chromatography with tandem mass spectrometry. J Chromatogr A 1058:137–142. doi:10.1016/j.chroma.2004.07.101
Vinas P, Balsalobre N, Hernandez-Cordoba M (2007) Liquid chromatography on an amide stationary phase with post-column derivatization and fluorimetric detection for the determination of streptomycin and dihydrostreptomycin in foods. Talanta 72:808–812. doi:10.1016/j.talanta.2006.12.006
Wan GH, Cui H, Zheng HS, Pang YQ, Liu LJ, Yu XF (2006) Flow-injection determination of streptomycin residues in milk using the luminol-periodate-Mn2+ chemiluminescence system. Luminescence 21:36–42. doi:10.1002/bio.880
Wang JP, Zhang HY, Sheng W, Liu W, Zheng LL, Zhang XZ, Wang S (2013) Determination of streptomycin residues in animal-derived foods by a reliable and accurate enzyme-linked immunosorbent assay. Anal Methods 5:4430–4435. doi:10.1039/c3ay40131f
Wei S, Le T, Chen Y, Xu J, He HQ, Niu XD, Luo JH (2013) Time-resolved fluoroimmunoassay for quantitative determination of tylosin and tilmicosin in edible animal tissues. Chin Sci Bull 58:1838–1842. doi:10.1007/s11434-013-5749-7
Wu JX, Zhang SE, Zhou XP (2010) Monoclonal antibody-based ELISA and colloidal gold-based immunochromatographic assay for streptomycin residue detection in milk and swine urine. J Zhejiang Univ Sci 11:52–60. doi:10.1631/jzus.B0900215
Zhang J, Guo J-Z, Xiao H-L, Zhu L, Liu H-Y, Zhang Y, Huang B (2010) Simultaneous detection of different serum pepsinogens and its primary application. World J Gastroenterol 16:3072–3077. doi:10.1007/s11356-015-4697-y
Zhou B, Zhang J, Fan J, Zhu L, Zhang Y, Jin J, Huang BA (2015) A new sensitive method for the detection of chloramphenicol in food using time-resolved fluoroimmunoassay. European Eur Food Res Technol 240:619–625. doi:10.1007/s00217-014-2363-0
Acknowledgements
This study was supported financially by grants from the Ministry of Science and Technology of China (2015BAK36B03). The authors would like to thank colleagues at the Department of Veterinary Pharmacology & Toxicology, College of Veterinary Medicine of China Agricultural University, for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported financially by grants from the Ministry of Science and Technology of China (2015BAK36B03).
Conflict of Interest
Yuanze Sun declares that she has no conflict of interest. Jie Xie declares that she has no conflict of interest. Tao Peng declares that he has no conflict of interest. Jianyi Wang declares that he has no conflict of interest. Sanlei Xie declares that he has no conflict of interest. Kai Yao declares that he has no conflict of interest. Cheng Wang declares that he has no conflict of interest. Shujuan Sun declares that she has no conflict of interest. Xi Xia declares that he has no conflict of interest. Haiyang Jiang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Sun, Y., Xie, J., Peng, T. et al. A New Method Based on Time-Resolved Fluoroimmunoassay for the Detection of Streptomycin in Milk. Food Anal. Methods 10, 2262–2269 (2017). https://doi.org/10.1007/s12161-017-0797-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0797-2