Abstract
A capillary immunochromatographic assay (CICA) using colloidal gold-labeled monoclonal antibodies was developed for semi-quantitative detection of clenbuterol (CLE) in this paper. This novel system fabricated in a competitive format was supported by glass capillary because of its easily modified, low cost, favorable optical properties. Here, two different-sized gold nanoparticles (13 and 26 nm) were prepared and corresponding assay conditions were optimized. By comparing the results between the two different-sized AuNPs, the 26-nm-AuNPs manifested higher sensitivities which the detection limit was estimated to be 0.35 ng/ml and the cut-off level with the naked eye of 2.5 ng/ml was observed. Using the optimized parameters, the intra-assay and inter-assay coefficients of variation (CVs) were ≤10.2 % and the recovery reached 70.8–115.5 % in pig urine, pork, beef, pork sausage and luncheon meat. The performance, including sample preparation, could be accomplished within 24 min. These results suggest that CICA holds promising use for sensitive, simple and low-cost on-site detection of CLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clenbuterol (CLE), a representative ß2-adrenoreceptor agonist, could enhance the growth rate of animals and reduce fat deposition (Mitchell and Dunnavan 1998; Mersmann 1998; Song et al. 2013). However, CLE can be accumulated in animal tissues and remain for a long time, which causes severe adverse effects on human health such as cardiovascular and central nervous diseases (Parr et al. 2009; Martinez-Navarro 1990). Hence, it was prohibited to use in food-producing animals in many countries including China, the USA, and European Union (Song et al. 2013; China’s Ministry et al. 2007; EU Council et al. 1996).
Various methods have been developed for the detection of CLE residues including chromatography, immunoassays, electrochemical analysis et al. (Bo et al. 2013; Wang et al. 2013). Chromatography-based techniques are specific, accurate and sensitive enough but require complicated sample pretreatment and time-consuming procedures (Morales-Trejo et al. 2013; Nicoli et al. 2013; Zhao et al. 2010; Ramos et al. 2003), which makes them mainly used as confirmatory analysis. Immunoassays are widely reported as fast screening methods (Song et al. 2013; Ren et al. 2009; Bucknall et al. 1993; Zhang et al. 2006; Lai et al. 2007; Gao et al. 2014), and the lateral-flow immunoassay (LFIA) is especially preferred for on-site qualitative detection for its significant advantages such as simplicity, rapidity (less than 20 min), and cost-effectiveness in comparison to other techniques.
However, now the application of LFIA for CLE is still mainly limited to simple samples such as urine (Song et al. 2013; Zhang et al. 2006; Lai et al. 2007). For most of complex food stuffs, the matrix interference seems a challenge for the nano-gold immunochromatographic assays. Wu et al. creatively coupled LFIA with magnetic nanobeads to detect CLE in meat with an excellent sensitivity, but quite complex pretreatments of samples were still required, such as disassociation and nitrogen blowing, which reduced the whole simplicity of the technique (Wu et al. 2014). LFIA itself has been verified some disadvantages including towing phenomena, diffusion phenomena, and significant deviation owing to complicated and heterogeneous structure of nitrocellulose membrane (NCM) (Du et al. 2014). Washing step is not supported by NCM, which may also reduce its selectivity, reproducibility and repeatability to some extent (Posthuma-Trumpie et al. 2009; O’Farrell et al. 2009). To solve the problem, there is an increasing attention on the development of new supporting materials to replace NCM, such as paper (Nery et al. 2013), polyethylene (Fernández-Sánchez et al. 2005), polyethersulfone (Kalogianni et al. 2007) and cotton thread (Zhou et al. 2012; Mao et al. 2015; Reches et al. 2010). Also, a glass capillary-based nano-gold immunochromatographic assay (CICA) was firstly proposed (Du et al. 2014). Due to some unique advantages of the glass capillary such as uniform smooth surface, transparent texture, chemical inertness, and tolerance to ionic strength and organic solvent, the developed CICA was expected to demonstrate better precision, simplicity and accuracy in complex biological matrix, and this has been preliminarily verified by the analysis of parvalbumin and furazolidone in fishery products (Du et al. 2014; Du 2015). Based on these works, here the CICA for CLE was improved to provide a new and more efficient technique for the routine fast screening of such hazard in food samples. To fulfill the strict requirements of on-site analysis in farms, supermarkets or factories, the size of gold nanoparticles and corresponding CICA procedures were carefully optimized to increase the sensitivity, and the tolerance of the technique to complex matrix was evaluated with much simpler pretreatments for pork, beef and other food stuffs.
Materials and Methods
Reagents and Instruments
Anti-CLE mouse monoclonal antibody and CLE–BSA conjugates were obtained from Fopan Biotechnology Inc. (Shenzhen, China). Goat anti-mouse IgG were purchased from Immuno Reagents, Inc. (North Carolina, US). CLE, Ractopamine, Salbutamol, Formoterol, were provided by Ditengmin Biotechnology Co. (Wuxi, China). Isoproterenol and Epinephrine were obtained from National institutes for Food and Drug Control (Beijing, China) 3-glycidyloxypropyltrimethoxysilane (98 %, GPTMS) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Hydrogen tetrachloroaurate (III) (HAuCl4), trisodium citrate, toluene, acetone, triethylamine (TEA), sucrose were purchased from National Chemical Pharmaceutical Co. (Shanghai, China). Bovine serum albumin (BSA), tris (hydroxymethylamino) methane (Tris) and Tween-20 were purchased from Solarbio (Beijing, China). Unless otherwise stated, all other regents were of analytical reagent grade or better. Aqueous solutions were prepared using 18.2 MΩ purified by Unique-R20 from Research Scientific Instruments Co. (Beijing, China). The urine samples were collected from local farms in Qingdao, and pork, beef and other food samples were collected from local supermarkets in Qingdao. All these biological samples were confirmed to be free of CLE by LC-MS-MS (Jin et al. 2007).
The glass capillary (d = 1.1 mm) was purchased from Instrument factory of West China University of Medical Sciences (Chengdu, China). The UV–vis spectra were recorded with a UV-1101 from Techcomp (Shanghai, China). The AuNPs were characterized by transmission electron microscope (TEM, JEOL JEM-1200EX, Japan). All centrifugations were accomplished with Z36HK (HERMLE Labortechnik GmbH, Germany). The final results used for semi-quantitative analysis were collected by HP Scanjet G4050 scanner from Seiko Epson Nagano (HP, Japan). Vortex mixer employed throughout experimentation was MS1 Minshaker (IKA, Germany).
Preparation and Characterization of AuNPs and AuNPs–mAb
Two different-sized gold nanoparticles (AuNPs) were prepared by the sodium citrate method as previously reported (Liu et al. 2006). In brief, 100 ml 1 mM HAuCl4 solution was loaded into a three-neck flask and heated to boiling adequately. By quickly adding a suitable amount (10 ml for 13-nm-AuNPs and 6.5 ml for 26-nm-AuNPs, respectively) of 38.8 mM sodium citrate, the color of the solution would change from pale yellow to red in 1 min. The system was boiled for another 15 min and the solution was naturally cooled down to room temperature. After being filtered through 0.45 μm water membrane, the mixture was stored at 4 °C in the dark for further use. The prepared AuNPs were characterized by UV–vis spectroscopy and TEM in Medical College of Qingdao University.
The optimal pH and concentration of anti-CLE monoclonal antibody to AuNPs were selected according to Slot (1985). The anti-CLE monoclonal antibody labeled with AuNPs (AuNPs–mAb) was prepared according to the method of Li with some modification (Li et al. 2013a). In summary, the AuNP solution was adjusted to the optimal pH with K2CO3 (0.1 M). While gently stirring, 1 ml anti-CLE mAb at the optimum concentration was added to 10 ml colloidal gold solution by dropping and the mixture was incubated for 40 min at room temperature. Subsequently, the AuNPs–mAb conjugates were blocked by adding bovine serum albumin (10 %) and polyethylene glycol (PEG)-20000 (1 %) to the final concentration of 1.0 % (w/v) and 10 % (w/v), respectively, incubating for 40 min. Next, the mixtures were centrifuged at 4 °C, 2500g for 15 min and the supernatant was centrifuged at 4 °C, 8500–10000g for 50 min. Then, the precipitates were washed by repeated centrifugation with 1 % BSA in 0.01 M Tris–HCl. Finally, the labeled anti-CLE mAb was re-suspended in 1 ml Tris–HCl (0.01 M, pH 8.2, containing 5 % sucrose, 1 % BSA, 0.1 % Tween-20, and 0.02 % NaN3), and stored at 4 °C for further use.
Construction of CICA Apparatus
The glass capillary was pretreated according to previous method with some modification (Du et al. 2014). Briefly, glass capillaries were sonicated in “piranha solution” (H2SO4:30 % H2O2 = 3:1, <80 °C) for 15 min and washed with excess ultrapure water to remove the residual acid. Then the capillaries were dried at 105 °C for 60 min. Subsequently, the capillaries were sonicated in KOH (1 M), ultrapure water, HCl (1 M), ultrapure water and acetone in order, at each step for 15 min. Next, the capillaries were dried at 105 °C for 60 min. After cooling to room temperature, the capillaries were immersed in modifying solution (toluene containing 5 % GPTMS, 0.5 % triethylamine) for 12 h at 37 °C. Finally, the capillaries were subsequently rinsed in toluene and acetone for 5 min and can be used after fully air-drying.
CLE–BSA conjugates were immobilized on the one end of the capillary as test zone, while goat anti-mouse IgG on the other end of the capillary as control zone. Specific operation as follows: 2 μl CLE–BSA conjugates were injected into the capillary by a pipettor incubated at 37 °C for 40 min. Then, the capillary was washed by phosphate-buffered saline with Tween-20 (PBST, 1 g/l Tween-20 in 0.01 M PBS, pH 7.4) for 5 min. 2 μl anti-mouse IgG were injected into the other end of the capillary incubated at 37 °C for 40 min, and then, washed again. Finally, the modified capillary was blocked with 2 % BSA in 0.01 M PBS, incubated at 37 °C for 40 min, and washed again. The fabricated capillary was stored at 4 °C before use.
To optimize the concentration of CLE–BSA conjugates and goat anti-mouse IgG, the AuNPs–mAb conjugates were diluted with the same volume of PBS (0.01 M, pH 7.4), and the reaction time was 10 min. For further optimization of the dilution ratio of AuNPs–mAb, the reaction time was also fixed as 10 min. With the optimal parameters mentioned above, the effect of reaction time on the brightness of CICA was investigated.
CICA Performance
The capillary immunochromatographic assay was carried out by injecting 3 μl of mixture (AuNP-mAb and sample at 1:4, v/v) into test zone. After 7 min of reaction at room temperature, the capillary was placed upside down to make the mixture moving down to control zone, reacting for another 7 min. Then, unbound conjugates were discarded and the capillary was rinsed in PBST three times. After removal of the residual moisture in the capillary, the result was observed. A range of CLE standard solutions were prepared and determined to establish the calibration curve according to the assay procedure. The reaction mixture was AuNPs–mAb and PBS (0.01 M, pH 7.4) with a ratio of 1:4. For qualitative analysis, the cut-off level by naked eyes was defined as the lowest CLE concentration sufficient to make the color in test zone disappeared. For semi-quantitative analysis, the image of the CICA result was obtained by a flatbed scanner. The relative brightness value of test zone was calculated by subtracting background gray value from the test zone signal. Then, the calibration curve was obtained by plotting B x /B0 against the concentration of CLE and was fitted by a four-parameter logistic function (Du et al. 2014). B0 and B x were used to designate the relative brightness of test zone at zero (B0) and a given concentration of analyte (B x ), respectively. The semi-quantitative detect limit (LOD) was defined as the mean determined concentration of blanks plus threefold standard deviation (n = 3).
The specificity of the CICA for CLE was evaluated by ractopamine, salbutamol, formoterol, isoproterenol, and epinephrine at the concentration of 10, 100, 500, and 1000 ng/ml in PBS (0.01 M, pH 7.4) respectively (n = 3). The accuracy and precision of the CICA were estimated by calculating the recoveries and the intra- and inter-assay coefficients of variation (CV) (Li et al. 2013a). The intra-assay CV was analyzed with blank sample (AuNP-mAb and PBS at 1:4, v/v) by six different batches of CICA (n = 6) and the inter-assay CV was calculated by the same batch CICA at three spiked concentrations (n = 6). The recoveries were conducted by CLE spiked urine, pork, beef, pork sausage, and luncheon meat at the concentrations of 0.5, 1.0, 2.0, and 3.0 ng/ml. The quantitative results were calculated by the calibration curve.
Validation with Real Samples
The urine samples were adjusted to neutral by 2 % 5-sulfosalicylic acid dehydrate, followed by centrifuge (2500 g) for 2 min. The supernatants were collected for detection by CICA. For pork, beef, pork sausage, and luncheon meat, the spiked samples were homogenized with extraction buffer (2 % 5-sulfosalicylic acid dehydrate in water: methanol (95:5; v/v)) at a ratio of 1:1 (w/v). Then the mixture was heated in boiling water for 5 min, followed by centrifuge (2500g) for 3 min. The supernatants were collected and adjusted to pH 7.0–7.5 with 2 M NaOH (10–20 μl in per milliliter extract with a slight difference among samples), followed by centrifuge (7200 g) for 2 min. The supernatants were collected for CICA performance as mentioned above.
Results
The Principle of CICA for CLE
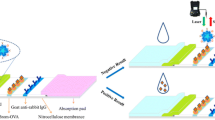
The proposed CICA for CLE is based on the competitive theory similar with that of traditional LFIA (Fig. 1). CLE–BSA conjugates and Goat anti-mouse IgG are immobilized by epoxy groups on the inner wall of both ends of the capillary and represent the test zone and control zone, respectively. As the mixture of AuNPs–mAb and sample incubates on the test zone, CLE residues in the sample compete with CLE–BSA conjugates for the limited number of AuNPs–mAb binding sites, which decreases the accumulation of AuNPs–mAb on the test zone and reduces the intensity of color. Therefore for negative samples, red bands will be formed on both of test zone and control zone, and for positive samples, the intensity of red band on test zone will decrease and even disappear. Within certain ranges, there is a specific functional relationship between the brightness of the test zone and the concentration of analyte in samples (Blažková et al. 2009). Thus, the image of results can be used not only for qualitative analysis but also for semi-quantitative analysis.
Optimization of the CICA Conditions
Respectively, 13 and 26 nm (diameter) gold nanoparticles (AuNPs) were made to test the influences of the particle size on CICA. The absorbance peak in UV–vis (517 and 524 nm, respectively in Fig. 2a) indicated the success of preparation as well as the uniformity of the particles, and based on the transmission electron microscope (TEM), the average diameter was approximately calculated as 13.5 ± 1.0 and 26.3 ± 2.5 nm, respectively in Fig. 2b (Lou et al. 2012).
The pH value and the concentration of antibody used in labeling process were critical for the stability of AuNPs–mAb conjugates. In principle low absorbance values (ABS) at 520 nm usually indicates the appearance of aggregation and poor stability of AuNPs–mAb conjugates. Here, with an increase of the amount of K2CO3 (in the range of 0–50 μl in per milliliter AuNPs: 0–50 μl/ml), the absorbance value (ABS) of AuNPs–mAb conjugates climbed up firstly and then slightly declined. The maximum ABS was corresponding with the optimal addition of K2CO3 which was 30 μl/ml (pH 8.0) for 13-nm-AuNPs and 25 μl/ml (pH 7.5) for 26-nm-AuNPs (Fig. 3a). As well, with the growth of the antibody concentration (in the range of 0–50 μg in per milliliter AuNPs: 0–50 μg/ml), the absorbance value (ABS) of AuNPs–mAb conjugates was significantly increased firstly and then tended to be stable. Thus the antibody concentration was optimized to be 25 μg/ml for 13-nm-AuNPs and 30 μg/ml for 26-nm-AuNPs (Fig. 3b). Based on these results, the conjugation of AuNPs with anti-CLE monoclonal antibody was also verified by the significant red shift in the UV–vis spectrum (Fig. 2a, 12 nm for 13-nm-AuNPs and 10 nm for 26-nm-AuNPs, respectively).
The amount of capture reagents and the dilution ratio of AuNPs–mAb conjugates were believed to significantly affect the color intensity of the test zone and therefore the cut-off level of the assay (Li et al. 2013a). Considering the competitive format of CICA, the concentration of CLE-BSA conjugates and the dilution ratio of AuNPs–mAb conjugates should be as minimal as possible while the color intensity of the test zone is enough to distinguish in blank samples. The concentration of the goat anti-mouse IgG should make color intensity of the control zone close to that of the test zone (Du et al. 2014). According to these criteria, the concentration of CLE-BSA conjugates, goat anti-mouse IgG and the dilution ratio of AuNPs–mAb conjugates were optimized and the results were summarized in Table 1. The relative brightness rose rapidly with reaction time increased from 1 to 7 min, and then flattened out slightly, indicating that 7 min was enough for the color development. Therefore, the total performance can be accomplished within 14 min.
Performance Evaluation of the CICA
The sensitivity of the developed CICA was determined by a range of CLE standard solutions. With 13-nm-AuNPs, the cut-off level by naked eyes was estimated about 5.0 ng/ml and the semi-quantitative detection limit (LOD) was calculated as 1.22 ng/ml, while for 26-nm-AuNPs the value was 2.5 ng/ml and 0.35 ng/ml, respectively (Fig. 4). Based on calibration curves, the linear range was 1.22–6 ng/ml with 13-nm-AuNPs and 0.35–3.5 ng/ml with 26-nm-AuNPs, respectively. The size of AuNPs showed significant influence on the efficiency of CICA, and the performance with 26-nm-AuNPs exhibited 2–3 times higher sensitivity than that with 13-nm-AuNPs.
The specificity of the CICA was examined by running some structural analogs of CLE including ractopamine, salbutamol, formoterol, isoprotereno and epinephrine. The cross-reactivity was estimated less than 2.5 % to salbutamol and less than 0.1 % to ractopamine, formoterol, isoprotereno and epinephrine, which indicated a satisfactory specificity of the technique (Table 2).The precision of CICA was evaluated by the intra- and inter-assay coefficients of variation (CV), and the intra-assay CV was observed within the range of 5.9–9.4 %, while the inter-assay CV was calculated as 8.5 % (n = 6).
The accuracy of CICA was evaluated by urine and food samples fortified with different concentration of CLE. Besides urine samples, the developed technique demonstrated excellent tolerance to the complex food matrix. After simple pretreatments (less than 10 min) without nitrogen blowing and other cleaning procedures (except centrifugation), satisfactory recovery (70.8–115.5 %) and precision (CV ≤ 10.2 %) were observed in pork, beef and even meat products such as pork sausage and luncheon meat (Table 3) For testing blank samples, the red signal on the test zone was still stable after two month storage at 4 °C.
Discussion
The sensitivity of nano-gold immunochromatographic performance is usually poor in comparison to ELISA or other immunoassays, and in many situations it limits the real application of such techniques, especially for the detection of trace hazards. Here the task was solved by a careful selection of AuNPs. The amount of CLE–BSA conjugates with the 26-nm-AuNPs was about 2.5 times lower than that of 13-nm-AuNPs. For a competitive analysis, reduced amount of coating antigens would indicate improved sensitivity. Moreover, with the same dilution ratio, the conjugates with larger AuNPs demonstrated relatively higher brightness, which therefore significantly increased the sensitivity of the CICA with 26-nm-AuNPs. These results consisted well with previous reports for NCM-based immunochromatographic strips (Lou et al. 2012; Safenkova et al. 2012), and the reasons may be attributed to the preferable optical properties and the superior affinity of larger AuNPs–mAb conjugates (Safenkova et al. 2010; Safenkova et al. 2012; Liu et al. 2007).
Different methods for detection of CLB residues were summarized including chromatography, electrophoresis and ELISA (Table 4). The developed CICA demonstrated similar sensitivity with the traditional NCM-based immunochromatographic strips, but just as expected, since no need for complicated sample pretreatment, it was simpler and more time-saving. Also the technique exhibited superior precision and excellent tolerance to food matrix, which could be illustrated by the much lower CV values in urine, pork, beef, and even food stuffs containing multiple raw materials as well additives. To large extent such advantages of the CICA may be attributed to the unique features of glass capillaries, especially its uniform surface smoothness, ionic strength and organic solvent tolerance and easiness for cleaning. In addition, the covalent immobilization of capture regents on glass capillaries may also contribute to increase the reproducibility and stability of CICA through the formation of more homogenous binding sites and the significant decrease in non-specific sites (Du et al. 2014; Tang et al. 2016).
It is also interesting to imagine the possible reuse of the CICA. Unlike NCM and other disposable materials, glass capillaries have the potential to be recycled by suitable dissociation of antibody-antigen complex on the surface (Kandimalla et al. 2004; Venkatesh and Murthy 1996). In fact such a re-generation has been realized in previous analysis of furazolidone (Du 2015). Therefore, one can also expect a CICA for CLE with even higher economical efficiency, which is important to the routine on-site screening in a large number of food samples.
Conclusions
A new immunochromatographic assay fabricated by glass capillary (CICA) was developed for semi-quantitative detection of clenbuterol (CLE). The size of gold nanoparticles was verified to have significant effect on the performance, and the CICA with 26-nm-AuNPs showed satisfactory sensitivity, precision and specificity. With simpler and faster pretreatments in comparison to traditional techniques, such efficiency was also validated with biological samples including urine, pork, beef and pork sausage, and luncheon meat, which allowed us to suggest the CICA as a new and promising technique for on-site screening of CLE.
Reference
Blažková M, Mičková-Holubová B, Rauch P, Fukal L (2009) Immunochromatographic colloidal carbon-based assay for detection of methiocarb in surface water. Biosens Bioelectron 25:753–758
Bo B, Zhu XJ, Miao P, Pei D, Jiang B, Lou Y, Shu YQ, Li GX (2013) An electrochemical biosensor for clenbuterol detection and pharmacokinetics investigation. Talanta 113:36–40
Bucknall SD, MacKenzie AL, Sauer MJ, Everest DJ, Newman R, Jackman R (1993) Determination of clenbuterol in bovine liver by enzyme immunoassay. Anal Chim Acta 275:227–230
China’s Ministry of Agriculture Bulletin No. 235 (2007) The maximum residue limit in animal food.
Du SY (2015) Development of nano-gold capillary immunochromatographic assay for rapid detection of hazards in aquatic products. Ocean university of China, Dissertation
Du SY, Lin H, Sui JX, Wang XD, Cao LM (2014) Nano-gold capillary immunochromatographic assay for parvalbumin. Anal Bioanal Chem 406:6637–6646. doi:10.1007/s00216-014-8093-0
EU Council Directive 96/22/EC (1996) Official Journal of the European Union. Council Directive 96/22/EC of 29 April 1996 concerning the prohibition on the use in stock farming of certain substances having a hormonal or thyrostatic action and of beta-agonists and replacing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC. Off J Eur Union L125:3–9
Fernández-Sánchez C, McNeil CJ, Rawson K, Nilsson O, Leung HY (2005) One-step immunostrip test for the simultaneous detection of free and total prostate specific antigen in serum. J Immunol Methods 307:1–12
Gao HF, Han J, Yang SJ, Wang ZX, Wang L, Fu ZF (2014) Highly sensitive multianalyte immunochromatographic test strip for rapid chemiluminescent detection of ractopamine and salbutamol. Anal Chim Acta 839:91–96
Jin YE, Guo DH, Zhang Y, Wang GQ (2007) Determination of 11β2-agonists in foodstuff of animal origin by liquid chromatography with tandem mass spectrometric detection. J Chinese Mass Spectrometry Soci 28 (4). doi:10.3969/j.issn.1004-2997.2007.04.001
Kalogianni DP, Goura S, Aletras AJ, Christopoulos TK, Chanos MG, Christofidou M, Skoutelis A, Ioannou PC, Panagiotopoulos E (2007) Dry reagent dipstick test combined with 23S rRNA PCR for molecular diagnosis of bacterial infection in arthroplasty. Anal Biochem 361:169–175
Kandimalla VB, Neeta NS, Karanth NG, Thakur MS, Roshini KR, Rani BE et al (2004) Regeneration of ethyl parathion antibodies for repeated use in immunosensor: a study on dissociation of antigens from antibodies. Biosens Bioelectron 20:903–6
Lai WH, Xu Y, Fung DYC, Xiong YH (2007) Development of a lateral-flow assay for rapid screening of the performance-enhancing sympathomimetic drug clenbuterol used in animal production; food safety assessments. Asia Pac J Clin Nutr 16(Suppl 1):106–110
Li CH, Luo W, Xu HY, Zhang Q, Xu H, Aguilar ZP, Lai WH, Wei H, Xiong YH (2013a) Development of an immunochromatographic assay for rapid and quantitative detection of clenbuterol in swine urine. Food Control 34:725–732
Li LB, Du HW, Yu H, Xu L, You TY (2013b) Application of ionic liquid as additive in determination of three-agonists by capillary electrophoresis with amperometric detection. Electrophoresis 34:277–283
Liu JW, Lu Y (2006) Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat Protoc 1:246–252
Liu X, Atwater M, Wang JH, Huo Q (2007) Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloid Surface B 58:3–7
Lou S, Ye JY, Li KQ, Wu AG (2012) A gold nanoparticle-based immunochromatographic assay: the influence of nanoparticulate size. Analyst 137:1174–1181
Mao X, Du TE, Wang YY, Meng LL (2015) Disposable dry-reagent cotton thread-based point-of-care diagnosis devices for protein and nucleic acid test. Biosens Bioelectron 65:390–396
Martinez-Navarro JF (1990) Food poisoning related to consumption of illicit b-agonist in liver. The Lancet 336:1311. doi:10.1016/0140-6736(90)92990-Y
Mersmann HJ (1998) Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J Anim Sci 76:160–172
Mitchell GA, Dunnavan G (1998) Illegal use of beta-adrenergic agonists in the United States. J Anim Sci 76(1):208–211
Morales-Trejo F, León SV, Escobar-Medina A, Gutiérrez-Tolentino R (2013) Application of high-performance liquid chromatography-UV detection to quantification of clenbuterol in bovine liver samples. J Food Drug Anal 21:414–420
Nery EW, Kubota LT (2013) Sensing approaches on paper-based devices: a review. Anal Bioanal Chem 405:7573–7595
Nicoli R, Petrou M, Badoud F, Dvorak J, Saugy M, Baume N (2013) Quantification of clenbuterol at trace level in human urine by ultra-high pressure liquid chromatography–tandem mass spectrometry. J Chromatogr A 1292:142–150
O’Farrell B (2009) Evolution in lateral flow-based immunoassay systems. Lateral Flow Immunoassay pp 8–9. doi 10.1007/978-1-59745-240-3_1
Parr MK, Opfermann G, Schänzer W (2009) Analytical methods for the detection of clenbuterol. Bioanalysis 1(2):437–450. doi:10.4155/bio.09.29
Posthuma-Trumpie G A, Korf J and van Amerongen A (2009) Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem 393:569–582. doi:10.1007/s00216-008-2287-2
Ramos F, Cristino A, Carrola P, Eloy T, Silva JM, Castilho MC, Silveira MIN (2003) Clenbuterol food poisoning diagnosis by gas chromatography–mass spectrometric serum analysis. Anal Chim Acta 483:207–213
Reches M, Mirica KA, Dasgupta R, Dickey MD, Butte MJ, Whitesides GM (2010) Thread as a matrix for biomedical assays. ACS Appl Mater Interfaces 2:1722–1728
Ren XF, Zhang FM, Chen FJ, Yang TB (2009) Development of a sensitive monoclonal antibody-based ELISA for the detection of clenbuterol in animal tissues. Food Agric Immunol 20:333–344
Safenkova IV, Zherdev AV, Dzantiev BB (2010) Correlation between the composition of multivalent antibody conjugates with colloidal gold nanoparticles and their affinity. J Immunol Methods 357:17–25
Safenkova I, Zherdev A, Dzantiev B (2012) Factors influencing the detection limit of the lateral-flow sandwich immunoassay: a case study with potato virus X. Anal Bioanal Chem 403:1595–1605
Slot JW, Geuze HJ (1985) new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol 38:87–93
Song CM, Zhi A, Liu Q, Yang J, Jia G, Shervin J, Tang L, Hu X, Deng RG, Xu CL, Zhang GP (2013) Rapid and sensitive detection of β-agonists using a portable fluorescence biosensor based on fluorescent nanosilica and a lateral flow test strip. Biosens Bioelectron 50:62–65. doi:10.1016/j.bios.2013.06.022
Tang YW, Lan JX, Gao X, Liu XY, Zhang DF, Wei LQ, Gao ZY, Li JR (2016) Determination of clenbuterol in pork and potable water samples by molecularly imprinted polymer through the use of covalent imprinting method. Food Chem 190:952–959
Venkatesh N, Murthy GS (1996) Dissociation of monoclonal antibody-antigen complexes: implication for ELISA procedures. J Immunol Method 199:167–174
Wang H, Zhang Y, Li H, Du B, Ma HM, Wu D, Wei Q (2013) A silver–palladium alloy nanoparticle-based electrochemical biosensor for simultaneous detection of ractopamine, clenbuterol and salbutamol. Biosens Bioelectron 49:14–19
Wu KS, Guo L, Xu W, Xu HY, Aguilarc ZP, Xu GM et al (2014) Sulfonated polystyrene magnetic nanobeads coupled with immunochromatographic strip for clenbuterol determination in pork muscle. Talanta 129:431–437
Xu JY, Li Y, Guo JJ, Shen F, Luo YL, Sun CY (2014) Fluorescent detection of clenbuterol using fluorophore functionalized gold nanoparticles based on fluorescence resonance energy transfer. Food Control 46:67–74
Zhang GP, Wang XN, Yang JF, Yang YY, Xing GX, Li QM et al (2006) Development of an immunochromatographic lateral flow test strip for detection of β-adrenergic agonist Clenbuterol residues. J Immunol Methods 312:27–33
Zhou G, Mao X, Juncker D (2012) Immunochromatographic assay on thread. Anal Chem 84:7736–7743
Zhao L, Zhao J, Huangfu WG, Wu YL (2010) Simultaneous determination of melamine and clenbuterol in animal feeds by GC–MS. Chromatographia 72:365–368
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Independent Innovation and Achievements Transformation Project of Shandong Province (2014ZZCX02703).
Conflict of Interest
Xueli Qu declares that she has no conflict of interest; Hong Lin declares that he has no conflict of interest; Shuyuan Du declares that she has no conflict of interest; Jianxin Sui declares that he has no conflict of interest; Xinlei Zhang declares that she has no conflict of interest; Limin Cao declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Qu, X., Lin, H., Du, S. et al. Development of a Nano-Gold Capillary Immunochromatographic Assay for Rapid and Semi-Quantitative Detection of Clenbuterol Residues. Food Anal. Methods 9, 2531–2540 (2016). https://doi.org/10.1007/s12161-016-0442-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0442-5