Abstract
An ultrasound-assisted method is described for dispersive solid phase extraction of trace levels of triazole fungicides. A sorbent was prepared from an N-heterocyclic carbene copper complex that was supported on ionic liquid-modified graphene oxide. The sorbent was characterized by scanning electron microscopy, transmission electron microscopy, Raman and FT-IR spectroscopy, energy-dispersive X-ray spectroscopy and elemental mapping. The capability of sorption and extraction is mainly based on complexation with Cu (I) ions. The variables affecting extraction were optimized. Following desorption with ethanol, the fungicides were quantified by corona discharge ion mobility spectrometry. Under optimized conditions (solution pH value: 7.0; amount of sorbent: 10 mg; extraction time: 3 min; desorption agent: ethanol), the technique provides good linearity (>0.994), repeatability (RSD < 4.1%), low limits of detection (0.18 ng.mL−1), excellent preconcentration factors (468–476) and high recoveries from spiked environmental water samples (92–94%). The sorbent can be reused over five cycles without significant loss of its activity.

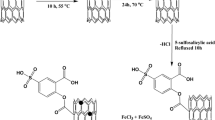
Schematic presentation of design and synthesis of the N-heterocyclic carbene copper complex supported on ionic liquid-modified graphene oxide as a sorbent for triazole fungicides and its application in ultrasound-assisted dispersive solid phase extraction with ion mobility spectrometric detection
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triazole fungicides (TFs) are systemic pesticides that are broadly used to prevent fungal sickness in agriculture. [1, 2]. Because of their moderate lipophilicity, long-term stability and long-term photochemical and chemical half-lives, they can enter and accumulate in waters such as river, wastewater, and lake. Researches have shown that these substances have a substantial toxicity [3, 4]. Furthermore, they endanger human health by troublemaking the endocrine function of the body [5]. Also, TFs are hardly degraded and are considered as persistent organic contaminants [6]. European Union Directive, to maintain human health and nontarget organism, has controlled the limits for individual pesticide and for the sum of all pesticides in drinking water are 0.1 μg.L−1 and 0.5 μg.L−1, respectively [7]. Based on the information given, designing a simple, fast, very sensitive and trustworthy technique to analyze trace levels of TFs in the environment is one of the prime necessary. Various techniques of chromatography such as gas chromatography (GC) [8] and high-performance liquid chromatography (HPLC) [9] have been applied to quantification TFs in environmental media and food samples. Notwithstanding their efficiency, selectivity, and sensitivity most of these methods are not suitable for direct analysis of complex samples because they are vulnerable to matrix interference from the real samples [10, 11]. Sample preparation methods that which enrich and pre-concentrate the target analytes in the real samples are very significant [12]. Given the toxicity of TFs, designing of a sample preparation method having appearances such as fast, reliability, sensitivity, cost-effectiveness and acceptability for its quantification in complexes matrices is necessary. These sample preparation methods can be divided into adsorbent-based extraction such as solid-phase microextraction (SPME) [13], solid-phase extraction (SPE) [8], bar adsorptive microextraction (BAME) [12] and in-disk solid-phase extraction (ID-SPE) [14], and solvent-based extraction such as liquid-liquid extraction (LLE) [9], Liquid phase microextraction (LPME) [15], liquid-liquid microextraction (LLME) [16] and cloud point extraction (CPE) [17]. With the comparison of adsorbent-based and solvent based extraction methods, adsorbent-based extraction methods are worthier and less risky because of less toxic solvent, and samples are needed. The ultrasound wave is widely applied in solid phase extraction. Ultrasonic-assisted solid phase extraction makes extraction of target analytes happen in a shorter time due to the ultrasonic power makes it possible to get absorbent vacancies by dispersion of sorbents into the sample solution [18].
In this approach, based on previous studies, the selective interaction between triazole and Cu (I) has been considered [19, 20]. According to previous studies, GO@IL@NHC-Cu sorbent for microextraction of triazole fungicides was successfully designed and synthesized. Then it applied in ultrasonic assisted dispersive solid phase sample preparation method for the extraction and preconcentration of TFs from water samples. Consequently, some main factors affecting the adsorption efficiency of TFs were optimized in sequence and the optimal conditions were established. In other words, this is the first report on the absorption, extraction, and determination of TFs in environmental water samples using a GO@IL@NHC-Cu based sorbent. The separation of TFs by ultrasonic assisted dispersive solid phase extraction was carried out with the complexion of triazole cycle of analytes with Cu (I) ‘s fixed in sorbent and hydrophobicity adsorbing of TFs GO@IL@NHC-Cu sorbent. It should be noted that the attendance of semi polar Cu (I) and semi nonpolar (graphene) sites facilitates the extraction of nonpolar compounds. This is due to the presence of Cu (I) that may increase the dispersity of the sorbent.
Ion mobilty spectrometry (IMS) is industrialized as an influential and well-known instrumental technique [21]. Combining IMS with a variety of various sample preparation methods would provide a high fast, low cost and sensitive determination. CD-IMS is followed with various sample preparation methods such as electromembrane extraction (EME) [22], hollow fiber liquid-liquid-liquid microextraction (HF-LLLME) [23], dispersive solid phase extraction (DSPE) [24], and MIP-SPE [25]. In the present report, we combine an IMS analysis method with CD ionization source after UA-DSPE system for the concurrent extraction and quantification of three OPPs which are broadly used containing tebuconazole, propiconazole and difenoconazole in environmental water samples. This method can provide short analysis time, high preconcentration factors, simplicity, low detection limit, portability and comparatively low cost.
Experimental
Reagent and materials
The information about reagent and materials is presented in the electronic supplementary materials.
Instrumentation
Ion mobility spectrometer (model 1000) that used this work was fabricated at Isfahan University of Technology. IR spectra were recorded on a Bomem MB-Series FT-IR spectrophotometer. Raman spectras of GO, GO-IL, and GO@IL@NHC-Cu nanosobent were recorded on a Bruker SENTERR (2009) with an excitation beam wavelength at 785 nm. Scanning electron microscopy and EDX mapping characterizations of GO@IL@NHC-Cu nanosobent was performed using an electron microscopy Hitachi SU3500. Transmission electron microscopy characterization of GO@IL@NHC-Cu nanosobent was performed using a transmission electron microscope Philips CM-30 with an accelerating voltage of 150 kV. X-ray photoelectron spectroscopy (XPS) analysis of GO@IL@NHC-Cu nanosobent was performed using a Thermo Scientific, ESCALAB 250Xi with Mg X-ray resource.
Calculation of extraction recovery and enrichment factor
The extraction recovery (ER %) of the UA-DSPE procedure was calculated according to the following equation:
where ns,initial and na,final are the number of moles of analyte originally present in the sample and the number of moles of analyte finally collected in the eluted solution, respectively. Va is the volume of the eluted solution, Vs is the volume of sample solution, Ca,final is the final concentration of analyte in the eluted solution, and Cs,initial is the initial the analyte concentration in the sample solution. The preconcentration factor (PF) of UA-DSPE procedure was calculated according to the following equation:
Ion mobility spectrometry
All information about the ion mobility spectrometry is described in the electronic supplementary materials.
Synthesis of the sorbent
The preparation of GO@IL@NHC-Cu (the sorbent) follows a method described earlier [26] and is described in some detail in the Electronic Supplementary Material section, along with its spectral characterization.
Ultrasound assisted dispersive solid phase extraction (UA-DSPE) procedure
50 mL of a solution containing sample or standards of TFs at pH of 7.0 was poured into a conical-bottom centrifuge tube with cap. Then the 15 mg of sorbent was added in fortified/non-fortified samples. Ultrasound power was used to immerse the GO@IL@NHC-Cu nanosorbents in the studied solutions. Ultrasound waves can increase the mass transfer and interaction of target molecules with the graphene sites of the modified GO@IL@NHC-Cu nanosorbents. After sonication for 2.0 min, the created black solution was centrifuged at 10000 rpm for 3 min. By centrifuging of the black solution, the fine particles of sorbent that dispersed in the sample solution were accumulated in the lowest point of the conical test tube. After that, the aqueous phase was totally divided by a syringe. Then, in the desorption step, 100 μL of ethanol was added to the sediment phase in the centrifuge tube. After vortexing, black solution was centrifuged at 10000 rpm for 2 min. The resulted ethanol solution was analyzed by CD-IMS.
Results and discussion
Characterization
The Fourier-transform infrared spectroscopy (FT-IR), Raman spectroscopy, and scanning electron microscopy (SEM) information about the Characterization of sorbent is described in the electronic supplementary materials.
The transmission electron microscopy (TEM) image of GO@IL@NHC-Cu sorbent possesses similarly wrinkled sheet morphology (see Fig. 1). The sorbent also was characterized by X-ray photoelectron spectroscopy as decribed in ref. [26].
Optimization of extraction conditions
The following parameters were optimized: (a) Sample pH value; (b) amount of sorbent; (c) extraction time; (d) centrifuge time; (e) desorption agent; (f) reusability of the sorbent. Respective data and Figures are given in the Electronic Supporting Material. In short, the following experimental conditions were found to give best results: (a) sample pH value: 7; (b) amount of sorbent: 10 mg; (c) extraction time: 3 min (d) centrifuge time: 3 min (e) desorption agent: ethanol (f) reusability of the sorbent: five cycles.
Method validation
To evaluate the real application of the presented method, the optimized extraction parameters were approved to appraise its quantitative efficiency. Table 1 shows the summarized results. Extraction was repeated five times and the extraction solution was considered with a CD-IMS system. Repeatability of the UA-DSPE-IMS quantities was obtained as the relative standard deviation (RSD%) in the range of 3.3 to 4.6. External calibration plots were obtained and good linearity in two various ranges with correlation coefficients between 0.994 up to 0.998 was achieved. Preconcentration factor (PF) and extraction recovery (ER %) using Eqs. 1 and 2, “Calculation of extraction recovery and enrichment factor Section” was calculated.
Preconcentration factors (PFs) of 469–477 that corresponded to extraction recoveries ranging from 92 up to 94% were obtained. Limits of quantification (LOQ) was in 0.6 ng.mL−1. Limits of detections (LODs), calculated according to the signal-to-noise ratio of 3, and were found to be in the 0.18 ng.mL−1.
Analysis of real samples
To evaluate the effects of the matrix, the procedure as described in the “UA-DSPE procedure section” UA-DSPE procedure was applied on the lake water, river water, wastewater and agricultural wastewater samples without any pre-treatment. Table 1 shows the analytical figure of merits for monitoring of TFs in various real samples.
To monitor trace amounts of triazole fungicides (TFs) in real matrices, UA-DSPE was used as a sample preparation method for TFs in optimized condition. Table 2 shows the TFs relative recoveries in distilled water, lake water, river water, waste water, and agricultural wastewater samples. Fig. 2 show the spectra of the lake water, river water, waste water and agricultural wastewater samples after the UA-DSPE under optimal conditions.
Spectra of the nonspiked and spiked in waste water, lake water, river water and agricultural wastewater samples with 10 ng.mL−1 of TFs after solvent assisted dispersive solid phase extraction under optimum conditions (solution pH value: 7.0; amount of sorbent: 10 mg; extraction time: 3 min; desorption agent: ethanol)
Table 3 shows the analytical performance of the proposed method compared with the formerly reported methods. Given that the analysis time in CD-IMS has meaningfully short, IMS analysis time less than 20 ms, compared with other detection techniques. Consequently, UA-DSPE-CD-IMS can provide high fast and sensitive results for the preconcentration and quantification of these TFS in lake water, river water, wastewater and agricultural wastewater samples.
Conclusion
According to previous studies, GO@IL@NHC-Cu sorbent for microextraction of triazole fungicides was successfully designed and synthesized. UA-DSPE method using a novel sorbent GO@IL@NHC-Cu was successfully implemented for the extraction and sorption of TFs from various real samples (river water, lake water and waste water) prior to the CD-IMS quantification. The presented sample preparation method is a new extraction method for separation and trace detection of TFs in environmental water samples. The material was designed for high dispersion of the sorbent in environmental water samples and high adsorption capacity for fast and efficient extraction of target molecules in the media. Therefore, the new coupling of the sorbent by ultrasonic assisted dispersive solid phase extraction method was developed for fast extraction and reliable trace determination of TFs by instrumental techniques. GO@IL@NHC-Cu was successfully characterized by SEM, FT-IR, EDS and elemental mapping analysis. The sorbent exhibiting an excellent sorption of TFs via complexation with Cu (I). Moreover, the method was shown to provide several distinct advantages such as high extraction efficiency, short analysis time, simplicity, portability and relatively low cost and easy elution of the analytes. Furthermore, the results indicated that the applied GO@IL@NHC-Cu sorbent can efficiently adsorb and extract TFs from several real samples. Finally, it is highly anticipated that the proposed method has the great analytical potential to be implemented as a routine means for monitoring TFs in a wide variety of real samples.
References
Kahle M, Buerge IJ, Hauser A et al (2008) Azole fungicides: occurrence and fate in wastewater and surface waters. Environ Sci Technol 42:7193–7200. https://doi.org/10.1021/es8009309
Buerge IJ, Poiger T, Müller MD, Buser H-R (2006) Influence of pH on the stereoselective degradation of the fungicides epoxiconazole and cyproconazole in soils. Environ Sci Technol 40:5443–5450
Lv X, Pan L, Wang J et al (2017) Effects of triazole fungicides on androgenic disruption and CYP3A4 enzyme activity. Environ Pollut 222:504–512. https://doi.org/10.1016/J.ENVPOL.2016.11.051
Zhu B, Liu L, Gong Y-X et al (2014) Triazole-induced toxicity in developing rare minnow (Gobiocypris rarus) embryos. Environ Sci Pollut Res 21:13625–13635. https://doi.org/10.1007/s11356-014-3317-6
Abolghasemi MM, Hassani S, Bamorowat M (2016) Efficient solid-phase microextraction of triazole pesticides from natural water samples using a Nafion-loaded trimethylsilane-modified mesoporous silica coating of type SBA-15. Microchim Acta 183:889–895. https://doi.org/10.1007/s00604-015-1724-0
Oller I, Malato S, Sánchez-Pérez JA et al (2007) Detoxification of wastewater containing five common pesticides by solar AOPs–biological coupled system. Catal Today 129:69–78. https://doi.org/10.1016/J.CATTOD.2007.06.055
Risica S, Grande S, Fisica L (2000) ISTITUTO SUPERIORE DI SANITÀ council directive 98 / 83 / EC on the quality of water intended for human consumption : calculation of derived activity concentrations Rapporti ISTISAN. 1–49
Charlton AJA, Jones A (2007) Determination of imidazole and triazole fungicide residues in honeybees using gas chromatography–mass spectrometry. J Chromatogr A 1141:117–122. https://doi.org/10.1016/J.CHROMA.2006.11.107
Jeannot R, Sabik H, Sauvard E, Genin E (2000) Application of liquid chromatography with mass spectrometry combined with photodiode array detection and tandem mass spectrometry for monitoring pesticides in surface waters. J Chromatogr A 879:51–71. https://doi.org/10.1016/S0021-9673(00)00098-4
Su H, Lin Y, Wang Z et al (2016) Magnetic metal–organic framework–titanium dioxide nanocomposite as adsorbent in the magnetic solid-phase extraction of fungicides from environmental water samples. J Chromatogr A 1466:21–28. https://doi.org/10.1016/J.CHROMA.2016.08.066
Miao Q, Wang J, Nie J et al (2016) Magnetic dispersive solid-phase extraction based on a novel adsorbent for the detection of triazole pesticide residues in honey by HPLC-MS/MS. Anal Methods 8:5296–5303. https://doi.org/10.1039/C6AY00376A
Almeida C, Nogueira JMF (2012) Comparison of the selectivity of different sorbent phases for bar adsorptive microextraction—application to trace level analysis of fungicides in real matrices. J Chromatogr A 1265:7–16. https://doi.org/10.1016/J.CHROMA.2012.09.047
Abolghasemi MM, Habibiyan R, Jaymand M, Piryaei M (2018) A star-shaped polythiophene dendrimer coating for solid-phase microextraction of triazole agrochemicals. Microchim Acta 185:179. https://doi.org/10.1007/s00604-017-2639-8
Vieira AC, Santos MG, Figueiredo EC (2017) Solid-phase extraction of triazole fungicides from water samples using disks impregnated with carbon nanotubes followed by GC-MS analysis. Int J Environ Anal Chem 97:29–41. https://doi.org/10.1080/03067319.2016.1272679
Farajzadeh MA, Sorouraddin SM, Mogaddam MRA (2014) Liquid phase microextraction of pesticides: a review on current methods. Microchim Acta 181:829–851. https://doi.org/10.1007/s00604-013-1157-6
Nie J, Chen F, Song Z et al (2016) Large volume of water samples introduced in dispersive liquid–liquid microextraction for the determination of 15 triazole fungicides by gas chromatography-tandem mass spectrometry. Anal Bioanal Chem 408:7461–7471. https://doi.org/10.1007/s00216-016-9835-y
Tang T, Qian K, Shi T et al (2010) Determination of triazole fungicides in environmental water samples by high performance liquid chromatography with cloud point extraction using polyethylene glycol 600 monooleate. Anal Chim Acta 680:26–31. https://doi.org/10.1016/J.ACA.2010.09.034
Kakavandi MG, Behbahani M, Omidi F, Hesam G (2017) Application of ultrasonic assisted-dispersive solid phase extraction based on ion-imprinted polymer nanoparticles for Preconcentration and trace determination of Lead ions in food and water samples. Food Anal Methods 10:2454–2466. https://doi.org/10.1007/s12161-016-0788-8
Zhao J, Lai S, Ruan L, Cheng J (2013) Structure , bioactivity and implications for environmental remediation of complexes comprising the fungicide hexaconazole bound to copper. https://doi.org/10.1002/ps.3536
Qiu R, Luo H (2015) Copper(I)–triazole dimer formation and rate acceleration in in-source click reaction. RSC Adv 5:96213–96221. https://doi.org/10.1039/C5RA19855K
Sorribes-Soriano A, de la Guardia M, Esteve-Turrillas FA, Armenta S (2018) Trace analysis by ion mobility spectrometry: from conventional to smart sample preconcentration methods. A review. Anal Chim Acta 1026:37–50. https://doi.org/10.1016/J.ACA.2018.03.059
Aladaghlo Z, Fakhari AR, Hasheminasab KS (2016) Application of electromembrane extraction followed by corona discharge ion mobility spectrometry analysis as a fast and sensitive technique for determination of tricyclic antidepressants in urine samples. Microchem J 129:41–48. https://doi.org/10.1016/J.MICROC.2016.05.013
Mirmahdieh S, Khayamian T, Saraji M (2012) Analysis of dextromethorphan and pseudoephedrine in human plasma and urine samples using hollow fiber-based liquid–liquid–liquid microextraction and corona discharge ion mobility spectrometry. Microchim Acta 176:471–478. https://doi.org/10.1007/s00604-011-0743-8
Mohammadnejad M, Gudarzi Z, Geranmayeh S, Mahdavi V (2018) HKUST-1 metal-organic framework for dispersive solid phase extraction of 2-methyl-4-chlorophenoxyacetic acid (MCPA) prior to its determination by ion mobility spectrometry. Microchim Acta 185:495. https://doi.org/10.1007/s00604-018-3014-0
Jafari MT, Rezaei B, Zaker B (2009) Ion mobility spectrometry as a detector for molecular imprinted polymer separation and metronidazole determination in pharmaceutical and human serum samples. Anal Chem 81:3585–3591. https://doi.org/10.1021/ac802557t
Dabiri M, Alavioon SI, Movahed SK (2018) N-Heterocyclic carbene–copper complex supported on ionic liquid-modified graphene oxide: versatile catalyst for synthesis of (i) 1,2,3-triazole and (ii) propargylamine derivatives. J Iran Chem Soc 15:2463–2474. https://doi.org/10.1007/s13738-018-1435-7
Wei Q, Song Z, Nie J et al (2016) Tablet-effervescence-assisted dissolved carbon flotation for the extraction of four triazole fungicides in water by gas chromatography with mass spectrometry. J Sep Sci 39:4603–4609. https://doi.org/10.1002/jssc.201600619
Farajzadeh MA, Djozan D, Mogaddam MRA, Bamorowat M (2011) Extraction and preconcentration technique for triazole pesticides from cow milk using dispersive liquid-liquid microextraction followed by GC-FID and GC-MS determinations. J Sep Sci 34:1309–1316. https://doi.org/10.1002/jssc.201000928
Wang H, Yang X, Hu L et al (2016) Detection of triazole pesticides in environmental water and juice samples using dispersive liquid–liquid microextraction with solidified sedimentary ionic liquids. New J Chem 40:4696–4704. https://doi.org/10.1039/C5NJ03376D
Bolaños PP, Romero-González R, Frenich AG, Vidal JLM (2008) Application of hollow fibre liquid phase microextraction for the multiresidue determination of pesticides in alcoholic beverages by ultra-high pressure liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A 1208:16–24. https://doi.org/10.1016/J.CHROMA.2008.08.059
Acknowledgements
Financial support from the Research Affairs of Shahid Beheshti University is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 12788 kb)
Rights and permissions
About this article
Cite this article
Aladaghlo, Z., Fakhari, A.R., Alavioon, S.I. et al. Ultrasound assisted dispersive solid phase extraction of triazole fungicides by using an N-heterocyclic carbene copper complex supported on ionic liquid-modified graphene oxide as a sorbent. Microchim Acta 186, 209 (2019). https://doi.org/10.1007/s00604-019-3276-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3276-1