Abstract
A nanosorbent for dispersive solid-phase extraction was fabricated by functionalizing multiwalled carbon nanotubes with sulfosalicylic acid after oxidation and thiolation followed by decoration with Fe3O4 nanoparticles. Incorporation of hydrophilic carboxylic and sulfonic acid groups introduces coordinating ability and accessibility for cadmium and lead, resulting in high sorption capacity of 217.39 and 454.54 mg g−1, respectively. Decoration by magnetic nanoparticles enhances its dispersibility and facilitates the separation of solid phase without tedious centrifugation or filtration processes, which is the exclusive objective of dispersive solid-phase extraction. The functionalized sorbent was characterized by FT-IR, SEM and TEM. The uniform and monolayer sorption behavior of the sorbent was proved by an evident fit of the equilibrium data to Langmuir isotherm model. The analytical method developed after optimizing the experimental variables such as solution pH, sorption time, amount of sorbent, desorption condition for preconcentration and separation enables the use of an economically viable less sensitive AAS for trace determination due to the improved detection limit of 0.13 and 1.21 µg L−1 for cadmium and lead, respectively. Commonly occurring concomitant ions in the real samples was not found to interfere in the trace determination of analyte. The good precision was assessed by the determined average day-to-day coefficient of variation of 3.02% for cadmium and 2.29% for lead. The accuracy and applicability of the present method for sequential cadmium and lead determination are substantiated by the analysis of Standard Reference Material and environmental water samples from electroplating industries, river water and tap water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current trend in solid-phase extraction (SPE) is the use of nascent (Kocot et al. 2013), oxidized (Sitko et al. 2013) or modified carbon nanotubes (CNTs) (Sahmetlioglu et al. 2014). An important objective in this field is the design of protocols that will achieve the correct functionalization of the CNTs. This will improve the analytical parameters and physicochemical stability of CNTs for their better use in various environmental matrices. The present-day environmental jeopardy is mainly due to the diversified uses of metals in varied forms. The US Agency for Toxic Substances and Disease Registry (ATSDR) 2013 Substance Priority List ranks cadmium as 7th and lead as 2nd priority substances based on a combination of their frequency, toxicity and potential for human exposure at National Priorities List sites (ATSDR, 2013). Both cadmium and lead are ubiquitous heavy metal pollutants which are toxic, persist in the environment and bioaccumulate in food chains (Cheng et al. 2005; Saleh 2016; Islam et al. 2015a). Individually or synergistically, these metals pose a serious threat to human health on every encounter and remain as toxicants (Watson 1999; Sigel et al. 2011). The maximum permissible levels of the toxic metals have been set at the lower limits by both national and international regulations. This makes their accurate quantification the foremost step to check their contamination level, to monitor the efficiency of the action plan taken for pollution control and to assess its exposure and effects on biota (Islam et al. 2012, 2013a, 2014a). Since the discovery of CNTs they have been widely explored for SPE (Saleh 2015; Pyrzynska 2010; Liang et al. 2004; Zawisza et al. 2012; Taghizadeh et al. 2014) because of their high surface area-to-volume ratio, physiochemical stability and mechanical strength. In addition, the manipulable surface chemistry of CNTs offers both chemical or physical functionalization by introducing simple organic groups, biomolecules, polymers, or bacteria in order to optimize the specificity of the approach or improve subsurface mobility for sorption of target analyte even at trace levels (Cui et al. 2011; Vicentini et al. 2014). Sorption studies using carbon-based nanomaterials report rapid equilibrium rates, high sorption capacity, effectiveness over a broad pH range, and consistency with sorption isotherms (Cheng et al. 2005; Danmaliki and Saleh 2017; Saleh and Danmaliki 2016, 2017a; Ding et al. 2016). The tunable properties of CNTs in combination with adaptability of magnetic solids inspire innovative solutions to persistent environmental challenges. Since the introduction of magnetic separation method in 1999 by Safarikova et al. (Safarikova and Safarik 1999) the research in magnetic solids is continuously increasing (Islam et al. 2016a). The use of magnetic extraction in analytical method has proved to be highly efficient owing to the enhancement of dispersibility of solid phase which facilitates its easy separation from the aqueous solution by applying an external magnetic field. Hence, dispersive solid-phase extraction (DSPE) overcomes the demerits of conventional solid-phase extraction like packing of sorbent into the column, tedious centrifugation and filtration processes where loss of nanosorbent was observed. (Chen et al. 2011). The versatility of magnetic materials in analytical chemistry proposes their use for very different types of analyte from a simple inorganic ion to complex biomolecules. The present research is an effort dedicated to the challenge in the improvement of SPE-CNT coupled atomic spectrometric determination methods for metal ion (Latorre et al. 2012).
Considering the above, it is for the first time a nanosorbent is fabricated by functionalizing the nascent multiwalled CNTs (MWCNTs) with 5-sulfosalicylic acid (SSA) reagent followed by decoration with magnetic nanoparticles for DSPE. The presence of carboxyl and highly hydrophilic sulfonic group in the chosen SSA reagent introduces the metal chelating sites and accessibility to the metal ions resulting in higher sorption capacity compared to the recent researches on SPE-CNT (Table 1). The detection limits reported here by the adjunction of the proposed DSPE with a cheap and reliable technique like flame atomic absorption spectrometry (FAAS) are comparable to those obtained by sophisticated analytical techniques as well as FAAS with MWCNTs or other sorbents (Table 1). The proposed methodology lies in the green analytical chemistry rules and is environmentally innocuous as it restricts the release of MWCNTs and there is no use of any toxic solvent for elution purpose (Pena-Pereira et al. 2010). The adaption of proposed optimized and validated DSPE coupled with sequential FAAS resulted in accurate and precise determination of Cd and Pb in SRM NIES 8 and real environmental water samples.
The whole research had been carried out during the year 2014–2016 in Analytical Research Laboratory, Department of Chemistry, Aligarh Muslim University, Aligarh, India.
Materials and methods
Materials
The chemicals used throughout this study were of analytical reagent grade (Merck, Mumbai, India). Stock solutions of 1000 mg L−1 of Cd and Pb each were procured (Merck, Darmstadt, Germany). These solutions were then further diluted with triple-distilled water (TDW) to obtain working solutions. The pH of all the solutions was adjusted by suitable buffer solutions (Orion 2 star model pH meter, Thermo Scientific, MA, USA): HCl/glycine (pH 1.2–3.6), CH3COOH/CH3COONa (pH 4.0–6.0), Na2HPO4/C6H8O7 (pH 7.0–7.8) and NH4Cl/NH3 (pH 8–10). Long MWCNTs with mentioned D × L 110–170 nm × 5–9 µm of > 90% carbon basis and SSA reagent were purchased (Sigma-Aldrich, MO, USA, and Merck, Mumbai, India, respectively). The environmental SRM of vehicle exhaust particulates (NIES 8) was obtained from the National Institute of Environmental Studies (Ibaraki, Japan).
Fabrication of MS-CNTs
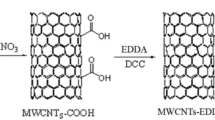
One gram of MWCNTs was purified with 200 mL of 10% HCl to eliminate the impurities and possible residues (Li et al. 2011). Purified MWCNTs were treated with a mixture of oxidant (15 mL), H2SO4:HNO3 (12:3 mL), for 10 h in a water bath at 55 °C. This introduces –COOH groups not only at original defect sites, but also at newly created defect sites along the walls during oxidation, improving dispersibility (Zhang et al. 2003). The oxidized MWCNTs (ox-MWCNTs) were filtered and washed repeatedly with TDW to neutral pH. This resulted in open-ended tubes, functionalized with carboxylic acid groups (Savio et al. 2011). For further functionalization, ox-MWCNTs were subjected to thiolation, a chemical defect reaction in which ox-MWCNTs were charged with 30 mL of thionyl chloride (SOCl2) and 2 mL of dimethylformamide (DMF) with stirring at 70 °C for 24 h, filtered and washed with tetrahydrofuran (THF) (Li et al. 2007). The prepared MWCNT-COCl was refluxed with an aqueous solution of SSA (5 g) for 10 h in the presence of triethylamine (5 mL) as catalyst to capture the HCl produced during synthesis. The novel SSA-fabricated nanotubes were then magnetized by gradually adding a 250 mL aqueous solution of mixed FeCl3·6H2O (1.86 g) and FeSO4·7H2O (0.96 g) into SSA-fabricated nanotubes (1.0 g in 200 mL) at 80 °C, and then 5 mL of 30% ammonia solution was added quickly for the coprecipitation of Fe3O4 nanoparticles on nanotubes. The temperature was raised to 85 °C, and 5 mL of 25% ammonia solution was added to adjust the pH to 10. After rapid stirring of 45 min, the resultant nanotubes were cooled to room temperature and separated from the solution with the aid of external permanent magnet followed by washing with TDW. The finally obtained magnetic MWCNTs fabricated with SSA were oven-dried at 60 °C for 12 h and abbreviated as MS-CNTs (Fig. 1).
Characterization of MS-CNTs
Fourier transform infrared spectra (FT-IR) was (PerkinElmer spectrum two spectrometer, MA, USA) obtained using KBr disk method in the range between 500 and 4000 cm−1 with a resolution of 2.0 cm−1, and the interferograms were recorded by accumulating 32 scans. Scanning electron microscopy (SEM) along with energy-dispersive X-ray analysis (EDS) spectra (Jeol JSM-6510LV, Tokyo, Japan) after-coated with a gold overlayer to avoid charging during electron irradiation was used to record the micrographs for the surface morphology and micro-compositional analysis of the sorbent (Haruna et al. 2016; Al-Shalalfeh et al. 2016). Transmission electron microscope (TEM) images were recorded to examine the diameter of nanotubes and Fe nanoparticles (Jeol JEM-2100 microscope, MA, USA) at a maximum accelerating voltage of 200 kV.
Pretreatment of samples and SRMs
To evaluate the proposed DSPE method, three types of environmental water samples were used for evaluation: tap water, river water and electroplating waste water (EPW). To calculate the presence of potential pollutants in different areas tap water samples were collected from two locations. Three municipality taps (A) in the residential area near the industries (Aligarh City, UP, India) and tap (B) in our laboratory. Tap water (1 L) was collected after allowing to flow for 10 min three times in the interval of 1 week and then pooled to make a composite tap water sample. Similarly, EPW samples were collected at two different spots (A and B) dependent on varying distance from the main industrial area (Aligarh, UP, India) (Islam et al. 2015b). The difference in distance is an inverse measure of the vulnerability of the nearby residents to the potential hazards by analyzed carcinogens. Spot A was located in the vicinity of the main source, and spot B was the enclosed water pond, in the residential area 10 km away from spot A. River water sample was also investigated for the same river in two different cities. The river water was collected from the Ganga in Narora (A) and Kanpur (B) cities in UP, India. These river waters are exposed mainly to the local residents. The sampled water, immediately after collection, was filtered through Millipore cellulose membrane filter (0.45 µm pore size), acidified to pH 2 with nitric acid and stored in a precleaned polyethylene bottles. 100 mL volume of each type of samples was further subjected to the developed protocol. The digestion of environmental SRM NIES 8 was carried out as reported in an earlier work (Islam et al. 2010a, b). The solution was evaporated to near dryness, dissolved in minimum volume of 2% HCl, filtered and made up to a 500 mL volume in a calibrated flask. From this an aliquot (100 mL) of prepared SRM solution was taken for the determination of metal ions. The pH was optimized accordingly, and all the samples and SRM were subjected to the given protocol. The concentration of desorbed Cd(II) and Pb(II) in the eluent was determined by FAAS.
Dispersive SPE/FAAS method for sequential determination of Cd and Pb
A thermostatted mechanical shaker (NSW-133, New Delhi, India) at 120 strokes min−1 and 30 ± 0.2 °C was used to get the simultaneous equilibration states for both targeted Cd and Pb. Batch static procedure was followed to optimize the studied experimental variables. An optimized amount of MS-CNTs was stirred for an optimized set of time and pH conditions with 100 mL of model solution containing both Cd and Pb for the simultaneous sorption. After equilibration the sorbent was easily separated from the solution by utilizing an external permanent magnet (Fig. 2), in fraction of minute without any loss of MS-CNTs. The sorbed analyte ions were then simultaneously eluted with 5 mL of eluate and sequentially determined in an integrated mode on an automatic flame control atomic absorption spectrometer (GBC 932 + , Dandenong, Australia) with deuterium background correction and slit width of 0.5 nm each at the wavelength of 228.8 nm and 217.0 nm, respectively, on air-acetylene flame. Prior to next sorption/desorption cycle the sorbent was flushed to neutrality by TDW to ensure similar pH conditions.
Results and discussion
Characterization of MS-CNTs
In the FT-IR spectra (ESI Fig. 1) the characteristic peak appearing at 3440 cm−1 corresponds to structural O–H stretching vibrations (Saleh et al. 2017b). The peaks at 1265 and 1600 cm−1 are associated with C–O and phenolic C=O stretching vibrations, respectively (Saleh et al. 2017c). The band at 1448 cm−1 is assigned to the stretching of C=C bonds (Drago 1965). The appearance of medium-intensity peak at 757 cm−1 corresponds to C–S stretching vibrations of bonded sulfonic groups. In addition, the peaks at 2922 and 3024 cm−1 are assigned for aliphatic C–H and aromatic C–H stretching vibrations, respectively (Drago 1965). This confirmed the functionalization of SSA onto MWCNTs. The FT-IR spectra of Fe2O3-modified CNTs show a peak at 696 cm−1 which represents the presence of iron oxides in the prepared composite. Such peak can be assigned to Fe–O bond (Saleh et al. 2017b). The SEM and TEM micrographs of MS-CNTs (Fig. 3) indicate the decoration of nanotubes with magnetic nanoparticles in the range of 8–15 nm (inset Fig. 3b with direct magnification of 50,000x). The SEM image shows the long-sized tubular structure of CNTs with identical diameter. In TEM image the deposition of Fe particles which roughened the surface is clearly visible and indicates the successful preparation of magnetite CNTs. The EDS spectra shown in Fig. 4a confirm the successful oxidation of MS-CNTs. The presence of both S and Fe (Fig. 4b) along with the O atoms was noted in the micro-compositional analysis of MS-CNTs which are not observed in oxidized CNTs suggesting the fabrication of MS-CNTs. The add-on of analyte atoms Cd and Pb peaks in EDS of MS-CNTs after the sorption equilibrium confirms the uptake of analytes (Fig. 4c).
Optimization of DSPE method
Effect of solution pH on simultaneous Cd and Pb sorption
The sorption mechanism is mainly attributable to chemical interactions between metal ions and the surface functional groups rendering the sorption capacity sensitive to changes in pH. The effect of pH on the extraction recovery of analyte ions was examined by subjecting the Cd and Pb sorption (each 0.20 mM, volume 100 mL) to pH changes in the range of 2–10. The resulting effect of pH on analyte sorption is illustrated in Fig. 5. The low sorption in acidic region can be attributed, in part, to competition between protons and analyte ions on the same sites. The increase in sorption on decrease in protonation may be due to mono-, di- and trianionic SSA species (H2SSA−, HSSA2− and SSA3−) (Smith et al. 2007) involving chelation through the potential donor sites of the SSA molecule (the sulfonic acid, the carboxylic acid and the phenolic groups), added with the presence of more oxygen acceptor atoms from the remaining carboxylic functionality introduced on MWCNTs. The charge of MS-CNTs surface becomes more negative with the increase in pH, which causes electrostatic interactions and results in higher sorption of analyte ions. In conclusion, for the maximum uptake without the doubt of hydroxide formation pH 8 ± 0.1 was optimized in all further experiments. The higher uptake for Pb than for Cd is in agreement with the general trend attributed to higher electronegativity and first stability constant of the associated metal hydroxide (Dastgheib and Rockstraw 2002). The high hydrophobicity and lack of functionality make the nascent MWCNTs weak sorbents for both Cd and Pb (Fig. 5). The proposed material after the fabrication of MWCNTs was advantageous regarding these two drawbacks. The nascent MWCNTs generally contain a fraction of catalytic metal residues, which may release into the aqueous phase and account for the very low sorption of metals shown in Fig. 5 for the case of nascent MWCNTs. The oxidized and then SSA functionalized MWCNTs also tend to show sorption capacities for both Cd and Pb much lower than the final product MS-CNTs as shown in Fig. 5. Thus, the effective approach adopted here brings in dual functionality and improves the dispersibility of MS-CNTs in aqueous solutions, resulting in drastically enhanced Cd and Pb uptake.
Effect of sorption time
The sorption kinetics that described the metal ion uptake rate and governed the contact time of the sorption reaction is one of the important characteristics. The sorption kinetics were studied in the time range of 10–80 min. ESI graphics 2 depicts that approximately 30% Cd and 20% Pb were sorbed in initial 10 min. 30 min was required for 50% saturation (t1/2) of the total uptake capacity, while the two reached the equilibrium and get saturated in 40 min and 50 min, respectively. A further increase in the contact time does not show any increase in the sorption of either of the analyte ions. The time of 50 min was sufficient for the complete accessibility of the active sites by the analyte ions and available surface offered by MS-CNTs for the analytes to reach 100% saturation. Hence, the optimized equilibration time of 50 min was used in all subsequent experiments.
Amount of sorbent, desorption conditions and stability
The effect of the MS-CNTs amount on the DSPE efficiency following the univariate approach was tested in the range from 20 to 100 mg of MS-CNTs for 100 mg L−1 of both metals. In practice, 50 mg of MS-CNTs was sufficient enough to provide more sorption sites and gain high enrichment efficiency to ensure the quantitative extraction of trace target analytes from the analyzed real samples. The aim of this study is to sequentially determine the two stated toxic metals. This makes it an essential requisite to select the same eluent for both the targeted metal ions. Different acidic and basic solutions with variations in volumes (1–10 mL) and concentrations (0.1–2.0 M) were used. The solutions of HCl and HNO3 resulted in quantitative recovery of analyte ions but also the leaching of decorated Fe3O4 particles, resulting in loss of magnetism and restricting the sorbent’s recycling. This was not preferred. To avoid this basic solutions of NH3 and NaOH were studied. It was found that > 98% recovery of both Cd and Pb was possible with 5 mL of 2 N NH3. The repeated availability of MS-CNTs through sorption/desorption cycles is quite critical for its application in the DSPE of Cd and Pb sorption in real samples. Herein, the stability of MS-CNTs was investigated following the above-stated batch procedure. The followed up sorption/desorption depicts that there is no significant decrease in the simultaneous sorption capacity of both analyte ions even after 12 cycles. This established that MS-CNTs could be regenerated up to 12 times without any noticeable loss in its properties. The sorbent not only possesses higher sorption capability, but also shows better desorption property. This in combination with the fact that only a small amount of 50 mg sorbent is required per cycle will reduce the overall costing for the fabricated sorbent.
Sorption isotherms
The sorption behavior of the developed DSPE for Cd and Pb was evaluated by the individual analysis of Langmuir and Freundlich isotherms. The concentration was increased in the range of 450–600 and 830–920 mg L−1 for Cd and Pb, respectively. The initial concentration of sorbate is the driving force to overcome the mass transfer resistance between the two phases. The experimental data were treated with the linearized form of both isotherm equations (Islam et al. 2010a, 2013b). The Langmuir plot (Fig. 6) for both the analytes resulted in a correlation coefficient value of > 0.99 in comparison with lower r2 values obtained by Freundlich plot (ESI Fig. 3, 4). The experimentally obtained sorption capacities for Cd and Pb are 208.86 and 435.72 mg g−1 of MS-CNTs, in good agreement with the capacity values of 217.39 and 454.54 mg g−1 as determined by Langmuir model, respectively. It confirms the Langmuir fit to the present data, which assumes the monolayer sorption.
Effect of interferents
To investigate the effects of potential interferents at the levels at or above which they may occur in the extraction recovery of Cd and Pb synthetic Cd and Pb solutions (100 µg L−1 each) were prepared along with the interferents and the recovery was monitored. The presence of alkali, alkaline earth metals and certain anions and few transition metal cations was considered as interference if the tested interferents caused a variation greater than ± 5% in the quantitative recovery of either of the analytes. From Table 2 it was observed that at the tested conditions none of the interferents caused a noticeable deterioration of both Cd and Pb signals as the % recovery for both the analytes is > 96%. This establishes the efficiency and ultimate utility of the proposed DSPE protocol in the trace determination of Cd and Pb in the complex environmental matrices.
Analytical figures of merit and method validation
At optimal DSPE conditions and instrumental parameters the calibration curves with regression equations of A = 0.306CCd + 0.001 (R2 = 0.999) and A = 0.033CPb + 0.027 (R2 = 0.993) for Cd and Pb were found linear. The limit of detection (LOD) for Cd and Pb evaluated as 3S/m (where S and m are the standard deviation of the blank and the slope of the calibration graph, respectively) corresponding to a preconcentration factor of 20 (Islam et al. 2016b) was found to be 0.13 and 1.27 µg L−1, respectively. It is evident from Table 1 that the LOD data are superior than those of the other nanosorbent coupled FAAS method (Sitko et al. 2013; Ramandi et al. 2014; Ramandi and Shemirani 2015; Alvand and Shemirani 2014) and comparable with other sophisticated analytical techniques (Kocot et al. 2013; Zawisza et al. 2012; Cui et al. 2011). Limit of quantification (LOQ) calculated as 10S/m corresponding to a preconcentration factor of 20 was found to be 0.43 and 4.03 µg L−1 for Cd and Pb, respectively. The good precision for the proposed DSPE was assessed by running 3 intra-batch replicates of 100 μg L−1 Cd and Pb each for three consecutive days. The sequentially determined average day-to-day coefficient of variation was 3.02% for Cd and 2.29% for Pb. The absence of systematic and constant errors in the proposed DSPE was confirmed by the analysis of SRM and recovery experiments in spiked environmental samples (Islam et al. 2015c, 2016c, Islam and Kumar 2016), respectively. An accurately weighed amount of SRM NIES 8 was analyzed by the proposed DSPE method. Considering the presence of many diverse concomitant ions in the analyzed SRM regarding its composition it is critical to highlight that none of the potential interferents deviated the quantitative recovery of either Cd or Pb. The relative standard deviation was assessed by the sequentially determined average day-to-day coefficient of variation of 3.02% for Cd and 2.29% for Pb. In case of Cd tcal (1.81) < ttab (4.303), while for Pb tcal (1.21) < ttab (4.303), calculated by using Student’s t test at 95% confidence limit (n = 3). The absence of statistical significance for mean observed Cd (1.06 μg g−1) and Pb (217.30 μg g−1) values from the certified values of 1.1 and 219 μg g−1 in NIES 8, respectively, clearly indicates that the developed method has no systematic errors. To evaluate the matrix effects and affirm the absence of constant errors, the real samples were spiked with varying amount of both analytes and were analyzed by the same optimized method (Table 3). The recovery of spiked Cd and Pb was observed to be > 96%, which would have been impossible without the adjunction of the proposed DSPE coupled sequential FAAS determination.
Application to real samples
The practical utility of the developed DSPE protocol was examined by the simultaneous extraction followed by sequential determination of both Cd and Pb in real environmental water samples with 95% confidence level. The obtained results are listed in Table 3. The concentration of both pollutants in the EPW samples was found to decrease on moving away from the main industrial source. The municipality tap water showed pollutant content higher than the river water. However, the river water of Ganga, Kanpur, showed higher content of both analytes than the water sample of Ganga, Narora. The least is reported in the laboratory tap water. This trend concludes that even down the drain the potential metal ions pollution is present, sorbed by the ground water, risking the interacting biota and ecosystem. The detected trace concentration of Cd and Pb cannot be directly determined by FAAS owing to its inherent low detection limit and matrix effect.
Conclusion
The objective behind the presented state-of-the-art material MS-CNTs is to develop a DSPE method with improved analytical figures of merit for both the proactive and retroactive applications monitoring the level of environmental contamination by highly toxic Cd and Pb. Herein, the pretreatment followed by oxidation and functionalization of MWCNTs greatly reduced the large amount of residual metallic impurities from the metal catalysts used in their synthesis which might have a negative influence on the applications of MWCNTs. The functionalization by a cheap reagent like SSA followed up by magnetic decoration resulted in the usage of merely 50 mg MS-CNTs for sorption along with the increased regeneration ability of up to 12 cycles. This delimits the applications of CNTs regarding its comparatively higher operational cost and exhibited its advantages over methods such as membrane filtration, centrifugation and precipitation by centrifugation. The analytical merits of the developed DSPE method are highlighted by reliability, reproducibility and linearity, evaluated by analysis of SRM, recovery experiments (recovery > 96%), and a good sequentially determined average day-to-day linear coefficient of variation (< 5%) for both Cd and Pb. The combined advantages of functionalization and magnetization are commendable in terms of high sorption capacity (217.39 mg Cd and 454.54 mg Pb g−1 sorbent), low detection limits (0.13 and 1.27 µg L−1 for Cd and Pb, respectively), reduced analysis time and environmental innocuousness. Owing to this high sorption capacity the fabricated material may be further explored for recovery and removal of toxic analytes. Herein, potential application was exhibited by the analysis of EPW, river and tap waters for their trace Cd and Pb contents. This sequential determination of Cd and Pb by FAAS in these environmental samples usually containing many possible interfering constituents would not have been possible without such DSPE conjunction.
References
Alvand M, Shemirani F (2014) Preconcentration of trace cadmium ion using magnetic graphene nanoparticles as an efficient adsorbent. Microchimica Acta 181(1–2):181–188
Al-Shalalfeh MM, Saleh TA, Al-Saadi AA (2016) Silver colloid and film substrates in surface-enhanced Raman scattering for 2-thiouracil detection. RSC Adv 6(79):75282–75292
Cheng X, Kan AT, Tomson MB (2005) Uptake and sequestration of Naphthalene and 1,2-Dichlorobenzene by C60. J Nanopart Res 7(4–5):555–567
Chen L, Wang T, Tong J (2011) Application of derivatized magnetic materials to the separation and the preconcentration of pollutants in water samples. TrAC Trends Anal Chem 30(7):1095–1108
Cui Y, Liu S, Hu Z-J, Liu X-H, Gao H-W (2011) Solid-phase extraction of lead(II) ions using multiwalled carbon nanotubes grafted with tris(2-aminoethyl)amine. Microchimica Acta 174(1–2):107–113
Danmaliki GI, Saleh TA (2017) Effects of bimetallic Fe−Ce nanoparticles on the desulfurization of thiophenes using activated carbon. Chem Eng J 307(1):914–927
Dastgheib SA, Rockstraw DA (2002) A model for the adsorption of single metal ion solutes in aqueous solution on to activated carbon produced from pecan shells. Carbon 40(11):1843–1852
Ding Z, Hu X, Wan Y, Wang S, Gao B (2016) Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. Journal of Ind Eng Chem 33:239–245
Drago RS (1965) Physical Methods in Inorganic Chemistry. Litton Education Publishing Inc, New York
Haruna K, Saleh TA, Hossain MK, Al-Saadi AA (2016) Hydroxylamine reduced silver colloid for naphthalene and phenanthrene detection using surface-enhanced Raman spectroscopy. Chem Eng J 304:141–148
Islam A, Kumar S (2016) Glycidylmethacrylate based resin functionalized with graphene oxide for column preconcentration and trace determination of Cd(II) and Ni(II) in environmental and food samples. RSC Adv 6:77629–77635
Islam A, Laskar MA, Ahmad A (2010a) Characterization of a novel chelating resin of enhanced hydrophilicity and its analytical utility for preconcentration of trace metal ions. Talanta 81(4–5):1772–1780
Islam A, Laskar MA, Ahmad A (2010b) Characterization and application of 1-(2-pyridylazo)-2-naphthol functionalized amberlite XAD-4 for preconcentration of trace metal ions in real matrices. J Chem Eng Data 55(12):5553–5561
Islam A, Ahmad A, Laskar MA (2012) Characterization of a chelating resin functionalized via azo spacer and its analytical applicability for the determination of trace metal ions in real matrices. J Appl Polym Sci 123(6):3448–3458
Islam A, Laskar MA, Ahmad A (2013a) Preconcentration of metal ions through chelation on a synthesized resin containing O, O donor atoms for quantitative analysis of environmental and biological samples. Environ Monit Assess 185(3):2691–2704
Islam A, Ahmad H, Zaidi N, Yadav S (2013b) Selective separation of aluminum from biological and environmental samples using glyoxalbis(2-hydroxyanil) functionalized amberlite XAD-16 resin: kinetics and equilibrium studies. Ind Eng Chem Res 52(14):5213–5220
Islam A, Zaidi N, Ahmad H, Yadav S (2014a) Synthesis, characterization, and systematic studies of a novel aluminum selective chelating resin. Environ Monit Assess 186(9):5843–5853
Islam A, Ahmad H, Zaidi N, Kumar S (2014b) Graphene oxide sheets immobilized polystyrene for column preconcentration and sensitive determination of lead by flame atomic absorption spectrometry. ACS Appl Mater Inter 6(15):13257–13265
Islam A, Zaidi N, Ahmad H, Kumar S (2015a) Efficacy of dihydroxy-mercaptopyrimidine functionalized polymeric resin for the trace determination of Cd by SPE coupled flame atomic absorption spectrometry. RSC Adv 5:46662–46671
Islam A, Zaidi N, Ahmad H, Kumar S (2015b) Amine-functionalized mesoporous polymer as potential sorbent for nickel preconcentration from electroplating wastewater. Environ Sci Pollut Res 22(10):7716–7725
Islam A, Ahmad A, Laskar MA (2015c) Flame atomic absorption spectrometric determination of trace metal ions in environmental and biological samples after preconcentration on a newly developed amberlite XAD-16 chelating resin containing p-aminobenzene sulfonic acid. J AOAC Int 98(1):165–175
Islam A, Ahmad H, Zaidi N, Kumar S (2016a) Graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim Acta 183(1):289–296
Islam A, Ahmad H, Zaidi N, Kumar S (2016b) Copper selective self-sorting polymeric resin with mixed-mode functionality for column preconcentration and atomic absorption spectrometric determination. RSC Adv 6:5590–5598
Islam A, Kumar S, Zaidi N, Ahmad H (2016c) SPE coupled to AAS trace determination of Cd(II) and Zn(II) in food samples using amine functionalized GMA-MMA-EGDMA terpolymer: isotherm and kinetic studies. Food Chem 213:775–783
Kocot K, Zawisza B, Margu E, Queralt I, Hidalgo M, Sitko R (2013) Dispersive micro solid-phase extraction using multiwalled carbon nanotubes combined with portable total-reflection X-ray fluorescence spectrometry for the determination of trace amounts of Pb and Cd in water samples. J Anal At Spectrom 28(5):736–742
Latorre CH, Mendez JA, Garcia JB, Martin SG, Pena-Crecente RM (2012) Carbon nanotubes as solid-phase extraction sorbents prior to atomic spectrometric determination of metal species: a review. Anal Chim Acta 749:16–35
Li J, He W, Yang L, Sun X, Hua Q (2007) Preparation of multi-walled carbon nanotubes grafted with synthetic poly(l-lysine) through surface-initiated ring-opening polymerization. Polymer 48(15):4352–4360
Li R, Chang X, Li Z, Zang Z, Hu Z, Li D, Tu Z (2011) Multiwalled carbon nanotubes modified with 2-aminobenzothiazole modified for uniquely selective solid-phase extraction and determination of Pb(II) ion in water samples. Microchim Acta 172(3–4):269–276
Liang P, Liu Y, Guo L, Zeng J, Lu H (2004) Multiwalled carbon nanotubes as solid-phase extraction adsorbent for the preconcentration of trace metal ions and their determination by inductively coupled plasma atomic emission spectrometry. J Anal At Spectrom 19:1489–1492
Pena-Pereira F, Lavilla I, Bendicho C (2010) Liquid-phase microextraction approaches combined with atomic detection: a critical review. Anal Chim Acta 669(1–2):1–16
Pyrzynska K (2010) Carbon nanostructures for separation, preconcentration and speciation of metal ions. TrAC Trends Anal Chem 29(7):718–727
Ramandi NF, Shemirani F (2015) Selective ionic liquid ferrofluid based dispersive-solid phase extraction for simultaneous preconcentration/separation of lead and cadmium in milk and biological samples. Talanta 131:404–411
Ramandi NF, Shemirani F, Farahani MD (2014) Dispersive solid phase extraction of lead(II) using a silica nanoparticle-based ionic liquid ferrofluid. Microchim Acta 181(15–16):1833–1841
Safarikova M, Safarik I (1999) Magnetic solid phase extraction. J Magn Magn Mater 194(1–3):108–112
Sahmetlioglu E, Yilmaz E, Aktas E, Soylak M (2014) Polypyrrole/multi-walled carbon nanotube composite for the solid phase extraction of lead(II) in water samples. Talanta 119:447–451
Saleh TA (2015) Mercury sorption by silica/carbon nanotubes and silica/activated carbon: a comparison study. J Water Supply Res Technol-AQUA 64(8):892–903
Saleh TA (2016) Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb(II): from surface properties to sorption mechanism. Desalin Water Treat 57(23):10730–10744
Saleh TA, Danmaliki GI (2016) Adsorptive desulfurization of dibenzothiophene from fuels by rubber tyres-derived carbons: kinetics and isotherms evaluation. Process Saf Environ Prot 102:9–19
Saleh TA, Sarı A, Tuzen M (2017a) Effective adsorption of antimony(III) from aqueous solutions by polyamide graphene composite as a novel adsorbent. Chem Eng J 307:230–238
Saleh TA, Tuzen M, Sarı A (2017b) Magnetic activated carbon loaded with tungsten oxide nanoparticles for aluminum removal from waters. J Environ Chem Eng 5(3):2853–2860
Saleh TA, Tuzen NM, Sarı A (2017c) Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem Eng Res Des 117:218–227
Savio M, Parodi B, Martinez LD, Smichowski P, Gil RA (2011) On-line solid phase extraction of Ni and Pb using carbon nanotubes and modified carbon nanotubes coupled to ETAAS. Talanta 85(1):245–251
Sigel A, Sigel H, Sigel RKO (2011) Metal Ions in Life Sciences. R Soc Chem, Cambridge
Sitko R, Gliwinska B, Zawisza B, Feist B (2013) Ultrasound-assisted solid-phase extraction using multiwalled carbon nanotubes for determination of cadmium by flame atomic absorption spectrometry. J Anal At Spectrom 28(3):405–410
Smith G, Wermuth UD, Young DJ, White JM (2007) Polymeric structures in the metal complexes of 5-sulfosalicylic acid: the rubidium(I), caesium(I) and lead(II) analogues. Polyhedron 26(14):3645–3652
Taghizadeh M, Akbar A, Asgharinezhad Samkhaniany N, Tadjarodi A, Abbaszadeh A, Pooladi M (2014) Solid phase extraction of heavy metal ions based on a novel functionalized magnetic multi-walled carbon nanotube composite with the aid of experimental design methodology. Microchim Acta 181(5–6):597–605
Vicentini FC, Silva TA, Pellatieri A, Janegitz BC, Fatibello-Filho O, Censi R (2014) Pb(II) determination in natural water using a carbon nanotubes paste electrode modified with crosslinked chitosan. Microchem J 116:191–196
Watson JS (1999) Separation methods for waste and environmental applications. Marcel Dekker, New York
U.S. Agency for Toxic Substances and Disease Registry Website. http://www.atsdr.cdc.gov/spl/
Zawisza B, Skorek R, Stankiewicz G, Sitko R (2012) Carbon nanotubes as a solid sorbent for the preconcentration of Cr, Mn, Fe Co, Ni, Cu, Zn and Pb prior to wavelength-dispersive X-ray fluorescence spectrometry. Talanta 99:918–923
Zhang J, Zou H, Qing Q, Yang Y, Li Q, Liu Z, Guo X, Du X (2003) Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J Phys Chem B 107(16):3712–3718
Zheng H, Jia B, Zhu Z, Tang Z, Hu S (2014) Determination of trace amounts of Pb, Cd, Ni and Co by wavelength-dispersive x-ray fluorescence spectrometry after preconcentration with dithizone functionalized graphene. Anal Methods 6(21):8569–8576
Acknowledgement
The authors are grateful to the University Grant Commission India for providing research fellowship to Noushi Zaidi (UGC-SRF D-658/MA) and Hilal Ahmad (UGC-BSR D-752/MR). The authors acknowledge the support provided by UGC-SAP program and DST (FIST & PURSE), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Q. Aguilar-Virgen
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Islam, A., Zaidi, N., Ahmad, H. et al. Functionalized carbon nanotubes for dispersive solid-phase extraction and atomic absorption spectroscopic determination of toxic metals ions. Int. J. Environ. Sci. Technol. 16, 707–718 (2019). https://doi.org/10.1007/s13762-018-1700-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1700-4