Abstract
In this paper, we describe ultrasonic assisted-dispersive solid phase extraction based on ion-imprinted polymer (UA-DSPE-IIP) nanoparticles for the selective extraction of lead ions. Ultrasound is a good and robust method to facilitate the extraction of the target ions in the sorption step and elution of the target ions in the desorption step. The ion-imprinted polymer nanoparticles used in the UA-DSPE-IIP were prepared by precipitation polymerization technique. The ion-imprinted polymer nanoparticles was synthesized using 2-vinylpyridine as a functional monomer, ethylene glycol dimethacrylate as the cross-linker, 2,2′- azobisisobutyronitrile as the initiator, 1,3,4-thiadiazole-2,5-dithiol as the ligand, methanol/dimethyl sulfoxide as the solvent, and lead as the template ion, through precipitation polymerization technique. The IIP nanoparticles were characterized by Fourier transformed infra-red spectroscopy (FTIR), thermogravimetric and differential thermal analysis (TGA/DTA), and scanning electron microscopy (SEM). Box-Behnken design (BBD) was used for optimization of sorption and desorption steps in UA-DSPE-IIP. In the sorption step: pH of solution, IIP amount (mg), sonication time (min) for sorption and in the desorption step: concentration of eluent (mol L−1), volume of eluent (mL), and sonication time (s) for desorption was investigated and optimized by the Box-Behnken design. The optimum conditions for the method were pH of solution: 7.5, sonication time for sorption 7.5 min, IIP amount 24 mg, type and concentration of eluent HCl 1.4 mol L−1, volume of eluent 2.1 mL, and sonication time for desorption 135 s. Under the optimized conditions, the limit of detection and relative standard deviation for the detection of lead ions by UA-DSPE-IIP was found to be 0.7 μg L−1 and <4%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination is an important topic due to its various effects on human health and the environment, and furthermore, exposure to certain heavy metals at trace amounts can entail irrecoverable and severe effects (Jamali et al. 2010, Khan et al. 2008). In addition, the presence of heavy metals in water has been a major concern for many years due to their toxicity which endangers the aquatic life, and yet, the worldwide tendency to decrease the permitted level of contamination in drinking water is a big challenge for the environmental researchers (Jia et al. 2009). The sources of heavy metal pollution are mainly the industries such as metal cleaning, mining activities, metal finishing, and etc. (Divrikli et al. 2007, Elci et al. 2003).
The toxicity of lead which widely exists in the environment is worth mentioning. This metal can accumulate in the vital organs of human and animals, and its cumulative poisoning effects can cause serious hematological damages such as brain damage, anemia, and kidney malfunctioning. Lead concentration in natural waters is typically between 2 to 10 ng mL−1 for which the upper limit recommended by World Health Organization (WHO) is less than 10 ng mL−1 (Mayer and Wilson 1998). As it was previously stated, lead is one of the most ubiquitous elements in the environment and has become recognized as a major health risk to the environment. Lead can release into the biosphere particularly as a fuel additive in large quantities as a result of widespread industrial applications (Hu et al. 2002). The primary sources of lead in humans and other animals include food, beverages, water, soil, and paint (Kuban et al. 2005). Therefore, the development of analytical methods that are rapid with low detection limits is necessary.
Analysis of trace heavy metals is difficult because of their very low concentrations in samples and high complexity of sample matrices; therefore, preliminary preconcentration and matrix removal steps are highly needed to ensure the accuracy and precision of the analytical results (Yang et al. 2009). Various preconcentration techniques such as solvent extraction, coprecipitation, cloud point extraction, ion-exchange, solid phase extraction, solvent-assisted dispersive solid phase extraction, and electroanalytical techniques (Behbahani et al. 2013a, 2015b, Aladaghlo et al. 2016, Divrikli et al. 2007, Elci et al. 2003, Giokas et al. 2001, Hu et al. 2002, Kuban et al. 2005) have been developed for different environmental samples. Among these techniques, SPE procedures in the offline or online mode are superior to other procedures for their high preconcentration factor, simplicity, consumption of small volumes of organic solvent, and low-cost sample preparation (Yilmaz and Soylak 2016, Ghaedi et al. 2016, Omidi et al. 2015, Behbahani et al. 2015a).
Although SPE has superior position over other sample preparation methods, it suffers from the selectivity of conventional sorbents for extraction and preconcentration of target analytes. In order to improve the selectivity in SPE methods, the use of ion-imprinted polymers (IIPs) has been proposed especially for the clean-up of potentially interfering compounds. IIPs are highly selective sorbent because the ion imprinting process is based on the preparation of a highly cross-linked polymer around a complex (target ions with appropriate ligand) in the presence of a suitable monomer. Therefore, imprinted polymer shows a tendency for selective preconcentration of the template ion over other components in a sample (Behbahani et al. 2015c, Ghorbani-kalhor et al. 2015, Behbahani et al. 2015d).
During recent years, new polymerization techniques have been presented dealing with the obtainment of imprinted sorbents in order to improve the analytical characteristic of IIPs. Among the methods for synthesis of ion-imprinted polymers, precipitation polymerization is a suitable method to obtain spherical particles with the desired performances. In the precipitation polymerization method, polymerization begins in the presence of a larger amount of porogen than that typically used in the bulk polymerization method. In the more diluted reaction system, the growing polymer chains are unable to engross the entire volume of the vessel leading to a dispersion of microgel particles in the solvent. In addition, it has been presented that the selectivity, sorption capacity, and homogeneity of binding sites related to polymers prepared by precipitation polymerization are clearly progressed compared to those present in imprinted polymers obtained by bulk polymerization [Tamayo et al. 2003, Cacho et al. 2004].
Recently, ultrasound wave is widely used in solid phase extraction. Ultrasonic-assisted solid phase extraction is applied for rapid extraction of analytes at lower time according to the high available surface area and empty site of sorbent by dispersion of sorbents into solution via ultrasonic power (Asfaram et al. 2017a, b, Dashamiri et al. 2017, Bagheri et al. 2017, Asfaram et al. 2017c, Ansari et al. 2016).
Optimization of the factors by experimental design methodology leads to cost-effective and acceptable results with least number of experimental runs. With experimental design methodology, the interaction and relationship between variables can be evaluated (Ebrahimzadeh et al. 2013). Box–Behnken design (BBD) is an efficient option (three-level factor quadratic design) at which the experimental points are located on the midpoints of the edges of a cube and at the center (central points). The special arrangement of the BBD levels allows the number of design points to increase at the same rate as the number of polynomial coefficients. The spherical nature of the BBD combined with the fact that the design is rotatable or nearly rotatable suggests that ample center runs should be used (Stalikas et al. 2009). An advantage of BBD is that it does not contain combinations for which all factors are simultaneously at their highest or lowest levels. Therefore, these designs are useful in avoiding experiments that would be performed under extreme conditions, for which unsatisfactory results might occur (Ebrahimzadeh et al. 2013).
In the present study, ultrasonic assisted-dispersive solid phase extraction based on ion-imprinted polymer nanoparticles (UA-DSPE-IIP) was used as a new sample preparation method for selective extraction and fast preconcentration of lead ions. The proposed method followed by flame atomic absorption spectroscopy (FAAS) was applied for trace monitoring of lead ions in several water samples. The ion-imprinted polymer nanoparticles were synthesized by precipitation polymerization of 2-vinilpyridine (4-VP), ethylene glycol dimethacrylate (EGDMA), 2,2′-azobisisobutyronitrile (AIBN), lead (II) as the template ion, and 1,3,4-thiadiazole-2,5-dithiol as the lead-binding ligand. 1,3,4-Thiadiazole-2,5-dithiol is sulfur containing organic compound. It is a good ligand and forms complexes with lead ions. To evaluate and optimize the influence of factors on sorption and desorption steps, the Box–Behnken design (BBD) was used. To examine the applicability of the method, UA-DSPE-IIP was applied for preconcentration and trace monitoring of lead ions in different food and water samples.
Experimental
Reagents and Materials
High pure 1,3,4-thiadiazole-2,5-dithiol and 2-vinyl pyridine were purchased from Sigma Aldrich. Ethylene glycol dimethacrylate (EGDMA) was obtained from Fluka (Buchs, Switzerland). 2, 2′-azobisisobutyronitrile (AIBN) was purchased from Acros Organics (New Jersey, USA). NaOH, HCl, HNO3, acetic acid, dimethyl sulfoxide, and methanol were purchased from Merck (Darmstadt, Germany). The other applied reagents for the method were of analytical grade and obtained from Merck (Darmstadt, Germany). Ultrapure water was prepared using a Milli-Q system from Millipore (Bedford, MA, USA). Ore polymetallic gold Zidarovo-PMZrZ as a certified reference material obtained from Bulgaria was used to validate the proposed sample preparation method.
Preparation of Standard Solutions
The nitrate salts of Pb(II), Cd(II), Cu (II), Ni(II), Zn(II), and Co(II) were used to prepare stock solutions (1000 mg L−1) of these ions in 2% (v/v) HNO3. The used standard solutions in the experiments were prepared by appropriate dilution of the stock solution with double distilled water. All of these solutions were stored in ambient temperature. The pH of sample solution was adjusted by drop wise addition of 2 M sodium hydroxide or nitric acid solutions.
Sample Treatment
The real samples that were used to evaluate the applicability of the proposed method for trace detection of lead ions were distilled, tap (Tehran, Iran), sea (Caspian Sea), and river water samples. Ore polymetallic gold Zidarovo-PMZrZ obtained from Bulgaria was applied as the certified reference material for validation of the proposed sample preparation method. The water samples were stored in cleaned polyethylene bottles and were filtered through nylon filters (Millipore) before the analysis. Certified reference material was digested in 6 mL of HCl (37% (v/v)) and 2 mL of HNO3 (65% (v/v)) as the extraction solvent. Digestion was carried out for 5 min at 250 W, 4 min at 400 W, 5 min at 550 W, and then venting for 6 min. The residue from digestion was then diluted with deionized water (Behbahani et al. 2013). Finally, for separation and preconcentration of lead ions in the real samples, the pH of solutions was adjusted at optimum condition (pH of 7.5).
To evaluate the applicability of the method for food analysis, eight selected samples was chosen. These tested food samples in the experiments for lead detection were celery, cucumber, pepper, cabbage, persimmon, kiwi, carrot, and pear. The named samples were collected from the local supermarket (Tehran, Iran). Dried and grounded samples (1.0 g) with 15 mL of pure NHO3 were put into burning cup. The samples were digested in a MARS 5 microwave oven at 200 °C. After the digestion step, the resulted solution was filtrated through Whatman No. 42. After filtration, the obtained clear solution was diluted to 210.0 mL (pH of 7.5) for lead analysis (Behbahani et al. 2015b).
Apparatus
Flame atomic absorption spectrometer (Analyst 200 PerkinElmer, USA) with a deuterium background corrector was applied for monitoring of lead ions. A lead hollow cathode lamp (HCL) was used as the emission light source operated at 10 mA. The wavelength was set at 283-nm resonance line and the spectral band width at 0.5 nm. All measurements were carried out in an air/acetylene flame. A digital pH meter, Metrohm 827 Ion analyzer (Herisau, Switzerland), equipped with a combined glass calomel electrode was used for the pH adjustments at 25 ± 1 °C temperature. Heidolph heater stirrer model MR 3001 (Germany) was used for heating and stirring of the solutions. IR spectra were recorded on a Bruker IFS-66 FT-IR spectrophotometer. Scanning electron microscopy (SEM) was performed by gently distributing the powder sample on the stainless steel stubs, using SEM (KYKY, EM3200) instrument. The thermal properties of samples were determined, using a BAHR-Thermoanalyse GmbH (Germany) with employing, heating, and cooling rates of 10 °C min−1. The samples were weighed as a thin film and carefully packed into a clean aluminum pan (11.5–12.5 mg) and sealed by crimping an aluminum lid on the pan (Shimadzu universal crimper). An Al2O3 empty pan sealed with a cover pan was used as a reference sample. A scanning range of 10 to 800 °C was used for samples at 10 °C min−1 in nitrogen gas.
Synthesis of Lead Imprinted Polymer Nanoparticles
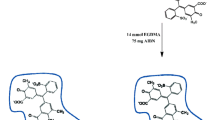
The ion-imprinted polymer nanoparticles used in the UA-DSPE-IIP was prepared by precipitation polymerization technique. To synthesize the ion-imprinted polymer nanoparticles, 4 mmol of 2-VP (functional monomer) and 1 mmol of 1,3,4-thiadiazole-2,5-dithiol (ligand) were dissolved in 40 mL of progen (10 mL methanol and 30 mL DMSO) in a 100-mL glass flask. Afterward, as the second step, 0.5 mmol of Pb(NO3)2 as a template ion was added slowly to a glass flask and the resulted mixture was stirred for 4 h at room temperature. In the third step, 25 mmol of EGDMA and 0.1 mmol of AIBN were added as cross-linker and initiator. The oxygen of the sample solution was removed by bubbling nitrogen through the sample for 10.0 min. Polymerization was performed in an oil bath at 65 °C for 24 h in the presence of nitrogen under magnetic stirring at 400 rpm. The prepared polymer was washed several times with 1:4 (v/v) acetone/water to remove the unreacted materials and then with HCl (1.4 mol L−1) for leaching the imprinted metal ions until the washing solution was free from lead ions (Fig. 1 presents a schematic to present the synthesis process of ion-imprinted polymer nanoparticles). Finally, it was washed with double-distilled water until it reached a neutral pH. The resulting fine powder was dried under vacuum in desiccators before the sorption and desorption studies. In the same way, the non-imprinted polymer (NIP) was also prepared without ions.
The Ultrasonic-Assisted Solid Phase Extraction Procedure
The ultrasonic-assisted solid phase extraction based on ion-imprinted polymer nanoparticles was applied in the batch mode. Ultrasound power was used to immerse the ion-imprinted polymer (24 mg) nanoparticles in a solution containing 2 mg L−1 concentration (pH of 7.5) of lead ions. Ultrasound waves can increase the mass transfer and interaction of target ions with recognition sites on ion-imprinted polymer nanoparticles. After sonication for 7.5 min, the solution was centrifuged and ultrapure water was used for remove of interfering compounds. The ion-imprinted polymer nanoparticles was suspended in 2.1 mL of HCl (1.4 mol L−1) as the eluent and sonicated for 135 s. The lead ions in the elution solvent were measured by FAAS. Figure 2 provides a scheme to illustrate the proposed sample preparation method.
Optimization Strategy
To optimize the optimum conditions for sorption and desorption steps, two Box–Behnken designs were applied separately. The experimental design matrix and data analysis were performed by Statgraphics-Centurion-16.1.11.
Results and Discussion
Characteristics of the Synthesized IIP Nanoparticles
The synthesized ion-imprinted polymer nanoparticles were characterized by IR spectroscopy, scanning electron microscopy, and thermogravimetric and differential thermal analysis. The IR spectra of unleached and leached ion-imprinted polymer (IIP) nanoparticles were recorded by using KBr pellet method. In the IR spectra, the absorptions due to C=O (1731 cm−1) and C–O (1152 cm−1) from EGDMA, SH (1433 cm−1) from 1,3,4-thiadiazole-2,5-dithiol, and C–H bands (2955 cm−1) were observed. The obvious absorption peak of 1,3,4-thiadiazole-2,5-dithiol (SH) on leached and unleached IIP nanoparticles can prove the presence of ligand in the structure of synthesized ion-imprinted polymer nanoparticles. Thermal stability of the leached and unleached polymer nanoparticles was examined by thermogravimetric analysis. Figure 3 shows that thermogravimetric (TG) and differential thermal analysis (DTA) plots for leached and unleached ion-imprinted polymer nanoparticles. As can be seen in the DTA plots, the maximum peak in lead-IIP was observed at higher temperature of 344 and 497 °C, while this event for the leached IIP happened in the lower temperature of 336 and 491 °C. The observation proves the higher thermal stability of the unleached relative to leached polymer, which is due to the presence of lead ions in the unleached polymer and also its strong complexation with 1,3,4-thiadiazole-2,5-dithiol in the polymeric matrix. As it is shown in TG plot for unleached IIP nanoparticles, weight loss for lead IIP was about 84.4%, and this amount of reduction in weight is due to the presence of lead ions in polymer network. The weight decrease up to 100% for leached imprinted polymer is due to the absence of lead ions in polymer. These obtained data from TGA indicate that the formation of IIP nanoparticles and elution of lead ions from the polymer was carried out successfully.
The morphology of the synthesized IIP nanoparticles was evaluated by scanning electron microscopy. As can be seen in the SEM image (Fig. 4), the size of synthesized IIP nanoparticles was in the range of 29–45 nm.
Elemental analysis (EA) was used for further characterization of leached IIPs and the percentage of elements by EA was found to be carbon (59.87%), hydrogen (7.42%) and nitrogen (1.542%), sulfur (0.91%).
Optimization of Sorption Step
Three factors such as solution pH, sorbent amount, and sonication time on the retention efficiency of lead ions by UA-DSPE-IIP was evaluated and optimized by Box–Behnken design. According to the few numbers of parameters in UA-DSPE-IIP, the Box–Behnken design was directly applied for the optimization of the factors without screening. The Box–Behnken design is an independent quadratic design in that it does not contain an embedded factorial or fractional factorial design. In this design, the treatment combinations are at the midpoints of edges of the process space and at the center. These designs are rotatable (or near rotatable) and require three levels of each factor. The levels of factors selected according to the primary experiments based on one variable at the time are presented in Table 1. This design is appropriate for exploration of quadratic response surface and construction of a second-order polynomial model which can be expressed as the following equation:
where Y is the response variable (area); b o is an intercept; b i , b ii , and b ij are constant regression coefficients of the model; and X i , X j ( i = 1,4; j = 1,4; and i ≠ j) represent the coded level of an independent variable. The number of runs (N) for the experiment was N = 2 k (k − 1) + C 0, where k is the number of factors and C 0 is the number of center points. In this study, k and C o were set at 3 and 3, respectively, which meant 15 experiments had to be done. The experimental data showed a good accordance with the second-order polynomial equations. The data for R-square and adjusted R-square was 99.5 and 98.8%, respectively. These data illustrate a good correlation between experimental and theoretical results.
The effects of factors on the retention of target ions by the proposed method are shown as the Pareto chart in Fig. 5. The bars, extending beyond the line, correspond to effects that are statistically significant at the 95% confidence level. Increase or decrease of retention by the UA-DSPE-IIP is shown with the positive or negative mark (corresponding to a gray or blue color), respectively. According to the obtained data by Box–Behnken design, three selected factors were the most significant and positive variables on the retention of lead ions by ion-imprinted polymer nanoparticles under ultrasonic condition. In this section, retention of the target ions by the sorbent was selected as the response. The retention of Pb(II) on the IIP nanoparticles increases as the pH increases (the pH of the sample solution was adjusted by drop-wise addition of 2 M sodium hydroxide or nitric acid solutions). The lower retention of target ions in the acidic conditions is due to the protonation of active sites of the polymer. With increases of the solution’s pH, the protonation of ligand decreases and the condition for complex formation and retention of lead ions by ion-imprinted polymer nanoparticles becomes more favorable. Therefore, the pH of 7.5 was chosen as the best pH in the next experiments. The effect of sonication time and sorbent amount on the retention of target ions by the proposed sample preparation method was significant and positive factor. According to the obtained data, the sonication time of 7.5 min and IIP amount of 24 mg were selected as the optimum sonication time and IIP amount. The effect of interaction between independent factors on UA-DSPE-IIP retention was demonstrated as three-dimensional surface and contour plots. These plots were represented as a function of two factors while other factors were kept at center level (Fig. 6).
Pareto charts of the main effects in the Box–Behnken design for retention step. AA, BB, and CC are the quadratic effects of the pH, sonication time, and IIP amount, respectively. AB, AC, and BC are the interaction effects between pH and sonication time, pH and IIP amount, and sonication time and IIP amount, respectively
The maximum retention of lead ions by the IIP nanoparticles was achieved in short contact time. These observations strongly prove the high contribution of ultrasound power in mass transfer of target ions. High available surface area and vacant sites of the IIP nanoparticles which can be assisted by ultrasonic power via the enhancement in diffusion coefficient can accelerate the extraction of lead ions by IIP nanoparticles.
Based on the obtained results from the optimization experiments, the following data were chosen as the optimum conditions: solution’s pH, 7.5; IIP amount, 24 mg; and sonication time, 7.5 min.
Selection of Desorption Solvent
Complete desorption of lead ions from recognition sites of ion-imprinted polymer is an important step to guarantee the absence of memory effect. To evaluate the effect of the eluent’s type, the dried IIP nanoparticles (24 mg) that extract the target ions under the optimized condition for the sorption step was immersed in 3 mL of several solvents such as HCl, HNO3, and CH3COOH for 3 min under ultrasonic condition. Based on the results in Table 2, the efficient desorption of lead ions from IIP nanoparticles was achieved in HCl as the best elution solvent.
Optimization of Desorption Step
To optimize the desorption parameters, the Box–Behnken design was used while other factors were kept constant in the previous optimized conditions (sorption step). In this section, the efficiency of desorption was obtained based on the recovery of lead ions. For desorption of the lead ions from IIP nanoparticles, three factors including sonication time, concentration, and volume of selected eluent (HCl) were examined. The number of the runs for the experiment was 15. R-square and adjusted R-square (with values 99.8 and 99.4%, respectively), illustrating a good correlation between experimental and theoretical results. In the ion-imprinted techniques, the complete desorption of target ions from IIP nanoparticles is a very important step to guarantee the absence of memory effect. After choosing a suitable elution solvent for the desorption of lead ions from the recognition sites of the IIP nanoparticles, the removal efficiency of target ions was evaluated by the Box–Behnken design in the three main factors (volume of eluent, concentration of eluent, and sonication time). The effects of factors on the desorption of target ions by the used elution solvent are shown as the Pareto chart in Fig. 7. According to the obtained data by the Box–Behnken design, three selected factors were the most significant and positive variables on the removal of lead ions from interaction sites of ion-imprinted polymer nanoparticles under ultrasonic condition. Figure 8 demonstrates the effect of interaction between independent variables on ultrasonic-assisted dispersive solid phase extraction based on ion-imprinted polymer nanoparticles as three-dimensional surface and contour plots. The obtained plots were represented as a function of two factors while the other factors were kept at center level. According to the obtained data, the efficient removal of lead ions from IIP nanoparticles in the acidic condition was achieved in a short contact time and these observations strongly support the high contribution of ultrasound power in facilitating desorption of target ions from the interaction sites of the IIP nanoparticle. The optimum condition after investigation of overall results was obtained: eluent volume, 2.1 mL; eluent concentration, 1.4 mol L−1; and sonication time, 135 s.
Pareto charts of the main effects in the Box–Behnken design for desorption step. AA, BB, and CC are the quadratic effects of the concentration of eluent, volume of eluent, and sonication time, respectively. AB, AC, and BC are the interaction effects between the concentration of eluent and volume of eluent, the concentration of eluent and sonication time, and volume of eluent and sonication time, respectively
Effect of Sample Volume
In the real samples analysis, the volume of the samples can have an effect on the preconcentration factor by the applied sample preparation method. Therefore, to examine the possible preconcentration factors by the method, the effect of sample volume on lead ion extraction was investigated. For this purpose, 24 mg of the polymer was suspended in different sample volumes (25, 50, 100, 150, 200, 250, 300, and 400 mL) containing 0.01 mg of lead ions. All solutions were extracted under the optimum conditions assisted by ultrasonic power. The results demonstrated that the dilution effect was not significant for the sample volumes up to 250 mL for lead ions (Fig. 9).
Sorption Capacity
The sorption capacity defined as the maximum amount of target ions can be sorbed per gram of the sorbent, and this parameter is used as a factor to evaluate the successful synthesization of the sorbent. To evaluate this factor, 100 ml of a solution containing 1 mg of lead ions was used for the extraction procedure under the optimized conditions and the sorption capacity was calculated (the amount of sorbent for the experiment was 10 mg). In order to evaluate the maximum adsorption capacity, the difference between concentration of the solution before extraction and the concentration of the solution after extraction was calculated. Based on three replicate measurements, the sorption capacities of the ion-imprinted polymer and non-imprinted polymer were calculated to be 81.0 ± 0.5 and 21.5 ± 0.6 mg g−1, respectively.
Selectivity Study
The distribution ratio (mL g−1) of target ions between the IIP particles and aqueous solution was also calculated by the following equation:
where, Ci (mg L−1) and Cf (mg L−1) are concentrations before and after extraction, respectively; V is the volume of initial solution and m is mass of IIP nanoparticles. Selectivity coefficients (K) and relative selectivity coefficients (K′) for lead ions relative to potentially interfering ions in the solution are defined as
where Kd (Pb2+) and Kd (Mn+) are the distribution ratios of lead and potentially interfering ions, respectively.
In order to evaluate the selectivity of the synthesized lead IIP nanoparticles, during several batch experiments, pairs of lead and potentially interfering ions were extracted by 24.0 mg of IIP nanoparticles at a pH of 7.5. The competitive adsorption of lead ions over the selected inorganic ions such as Cu2+, Ni2+, Cd2+, Zn2+, and Co2+ for IIP and NIP nanoparticles from their binary mixture was investigated under optimum conditions and then the distribution ratios (Kd), selectivity coefficients (K), and relative selectivity coefficients (K′) for lead ion relative to foreign ions were calculated using Eqs.(1)–(3), respectively. The results are summarized in Table 3. As seen in Table 3, the competitive adsorption capacity of lead IIP nanoparticles for lead ions is higher than NIP.
By considering the high selectivity coefficients obtained by IIP nanoparticles and a significant difference between the binding of lead ion and competitor ions to the imprinted sorbent versus non-imprinted polymer, clearly, it could be suggested that the prepared IIP can be applied as a selective sorbent for separation of lead ion in the presence of other metal cations in various real samples with different complex matrices.
Statistical and Calibration Parameters
Under the optimized conditions, UA-DSPE-IIP showed a linear calibration curve within the concentration ranging from 3 to 900 μg L−1. The least square equation at above dynamic linear range was as follows:
The limits of detection (LODs), which is defined as CLOD = 3Sb/m, where Sb is the standard deviation of seven replicate blank signals and m is the slope of the linear section of calibration curve after preconcentration; for a sample volume of 210 mL, it was found to be 0.7 μg L−1 for lead ions. The relative standard deviation for five separate batch experiments with 24 mg of sorbent under ultrasound power for determination of 3 μg L−1 of water was 3.1%.
To validate the obtained data by the proposed method (SA-DSPE-IIP-FAAS), certified reference material (ore polymetallic gold Zidarovo-PMZrZ and NIST SRM 1515 apple leaves) was used. As Table 4 shows, a good correlation was obtained between the certified value and the amounts found by the present method. Therefore, the applied sample preparation method can be used as a validate solid-phase for extraction and trace detection of lead in environmental samples.
The figure of merits of the used sample preparation method for extraction and determination of lead ions were compared (Table 5) with other reported methods (Narin et al. 2007, Zhu et al. 2009, Ebrahimzadeh and Behbahani 2013, Liu et al. 2011, Esen et al. 2009). The UA-DSPE-IIP is a fast and selective extraction technique for trace detection of lead ions in complex matrices.
Real Sample Analysis
To evaluate the applicability of the sample preparation method for trace analysis of lead ions in real samples with different matrices containing various amounts of coexisting ions, the method was applied for trace determination of lead ions from different food and water samples (the used samples for the aim was 210 mL). As shown in Table 6, a good correlation was obtained between the added and measured amounts of the analyte.
Conclusion
In this study, the ultrasonic-assisted dispersive solid phase extraction based on ion-imprinted polymer nanoparticles followed by FAAS was used for preconcentration and trace detection of lead ions in environmental water and food samples. Two Box–Behnken designs were separately used to obtain the optimum conditions for retention and desorption of lead ions. The obtained optimum conditions for the proposed method by experimental design methodology were pH of solution 7.5, sonication time for sorption 7.5 min, IIP amount 24 mg, type and concentration of eluent HCl 1.4 mol L−1, volume of eluent 2.1 mL, and sonication time for desorption 135 s. The method has several advantages such as low detection limit, good precision, and high selectivity. Furthermore, the applied ultrasound power in the present method was used to decrease the retention and desorption time of solid phase extraction. As a conclusion, the performance of the method for highly selective extraction of trace amounts of lead ions in different food and water samples was excellent.
References
Ansari F, Ghaedi M, Taghdiri M, Asfaram A (2016) Application of ZnO nanorods loaded on activated carbon for ultrasonic assisted dyes removal: experimental design and derivative spectrophotometry method. Ultrason Sonochem 33:197–209
Aladaghlo Z, Fakhari A, Behbahani M (2016) Solvent-assisted dispersive solid-phase extraction: a sample preparation method for trace detection of diazinon in urine and environmental water samples. J Chromatogr A 1462:27–34
Asfaram A, Ghaedi M, Dashtian K (2017a) Rapid ultrasound-assisted magnetic microextraction of gallic acid from urine, plasma and water samples by HKUST-1-MOF-Fe3O4-GA-MIP-NPs: UV–Vis detection and optimization study. Ultrason Sonochem 34:561–570
Asfaram A, Ghaedi M, Dashtian K (2017b) Ultrasound assisted combined molecularly imprinted polymer for selective extraction of nicotinamide in human urine and milk samples: spectrophotometric determination and optimization study. Ultrason Sonochem 34:640–650
Asfaram A, Ghaedi M, Hajati S, Goudarzi A, Dil EA (2017c) Screening and optimization of highly effective ultrasound-assisted simultaneous adsorption of cationic dyes onto Mn-doped Fe3O4-nanoparticle-loaded activated carbon. Ultrason Sonochem 34:1–12
Bagheri A, Ghaedi RM, Asfaram A, Bazrafshan AA, Jannesar R (2017) Comparative study on ultrasonic assisted adsorption of dyes from single system onto Fe 3O4 magnetite nanoparticles loaded on activated carbon: experimental design methodology. Ultrason Sonochem 34:294–304
Behbahani M, Abolhasani J, Amini MM, Sadeghi O, Omidi F, Bagheri A, Salarian M (2015a) Application of mercapto ordered carbohydrate-derived porous carbons for trace detection of cadmium and copper ions in agricultural products. Food Chem 173:1207–1212
Behbahani M, Bagheri A, Taghizadeh M, Salarian M, Sadeghi O, Adlnasab L, Jalali K (2013) Synthesis and characterisation of nano structure lead (II) ion-imprinted polymer as a new sorbent for selective extraction and preconcentration of ultra trace amounts of lead ions from vegetables, rice, and fish samples. Food Chem 138:2050–2056
Behbahani M, Ghareh Hassanlou P, Amini MM, Omidi F, Esrafili A, Farzadkia M, Bagheri A (2015b) Application of solvent-assisted dispersive solid phase extraction as a new, fast, simple and reliable preconcentration and trace detection of lead and cadmium ions in fruit and water samples. Food Chem 187:82–88
Behbahani M, Hassanlou PG, Amini MM, Moazami HR, Abandansari HS, Bagheri A, Zadeh SH (2015c) Selective solid-phase extraction and trace monitoring of lead ions in food and water samples using new lead-imprinted polymer nanoparticles. Food Anal Methods 8(3):558–568
Behbahani M, Salimi S, Abandansari HS, Omidi F, Salarian M, Esrafili A (2015d) Application of a tailor-made polymer as a selective and sensitive colorimetric sensor for reliable detection of trace levels of uranyl ions in complex matrices. RSC Adv 5(74):59912–59920
Cacho C, Turiel E, Martin-Esteban A, Pérez-Conde C, Cámara C (2004) Characterisation and quality assessment of binding sites on a propazine-imprinted polymer prepared by precipitation polymerisation. J Chromatogr B 802(2):347–353
Dashamiri S, Ghaedi M, Asfaram A, Zare F, Wang S (2017) Multi-response optimization of ultrasound assisted competitive adsorption of dyes onto Cu (OH) 2-nanoparticle loaded activated carbon: central composite design. Ultrason Sonochem 34:343–353
Divrikli U, Kartal AA, Soylak M, Elci L (2007) Preconcentration of Pb (II), Cr (III), Cu (II), Ni (II) and Cd (II) ions in environmental samples by membrane filtration prior to their flame atomic absorption spectrometric determinations. J Hazard Mater 145(3):459–464
Ebrahimzadeh H, Behbahani M (2013) A novel lead imprinted polymer as the selective solid phase for extraction and trace detection of lead ions by flame atomic absorption spectrophotometry: synthesis, characterization and analytical application. Arab J Chem. doi:10.1016/j.arabjc.2013.09.017
Ebrahimzadeh H, Behbahani M, Yamini Y, Adlnasab L, Asgharinezhad AA (2013) Optimization of Cu (II)-ion imprinted nanoparticles for trace monitoring of copper in water and fish samples using a Box–Behnken design. React Funct Polym 73(1):23–29
Elci L, Soylak M, Özcan B (2003) Coprecipitation of Cu (II), Ni (II), Fe (III), Cd (II), Pb (II), and Co (II) in wastewater, sediment, and metallic zinc samples with HMDTC–HMA for flame atomic absorption spectrometric determination. Anal letters 36(5):987–999
Esen C, Andac M, Bereli N, Say R, Henden E, Denizli A (2009) Highly selective ion-imprinted particles for solid-phase extraction of Pb 2+ ions. Mater Sci Eng C 29(8):2464–2470
Ghaedi M, Noormohamadi HR, Asfaram A, Montazerozohori M, Tashkhourian J, Soylak M (2016) Modification of platinum nanoparticles loaded on activated carbon and activated carbon with a new chelating agent for solid phase extraction of some metal ions. J Mol Liq 221:748–754
Ghorbani-Kalhor E, Behbahani M, Abolhasani J (2015) Application of ion-imprinted polymer nanoparticles for selective trace determination of palladium ions in food and environmental samples with the aid of experimental design methodology. Food Anal Methods 8(7):1746–1757
Giokas D, Paleologos E, Tzouwara-Karayanni S, Karayannis M (2001) Single-sample cloud point determination of iron, cobalt and nickel by flow injection analysis flame atomic absorption spectrometry—application to real samples and certified reference materials. J Anal At Spectrom 16(5):521–526
Hu Q, Yang G, Yang J, Yin J (2002) Study on determination of iron, cobalt, nickel, copper, zinc and manganese in drinking water by solid-phase extraction and RP-HPLC with 2-(2-quinolinylazo)-5-diethylaminophenol as precolumn derivatizing reagent. J Environ Monitor 4(6):956–959
Jamil M, Zia MS, Qasim M (2010) Contamination of agro-ecosystem and human health hazards from wastewater used for irrigation. J Chem Soc Pak 32(3):370–378
Jia K, Pan B, Lv L, Zhang Q, Wang X, Pan B, Zhang W (2009) Impregnating titanium phosphate nanoparticles onto a porous cation exchanger for enhanced lead removal from waters. J Colloid Interface Sci 331(2):453–457
Khan S, Cao Q, Zheng Y, Huang Y, Zhu Y (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China Environ Pollut 152(3):686–692
Kubáň P, Guchardi R, Hauser PC (2005) Trace-metal analysis with separation methods. Trends Anal Chem 24(3):192–198
Liu Y, Liu Z, Wang Y, Dai J, Gao J, Xie J, Yan Y (2011) A surface ion-imprinted mesoporous sorbent for separation and determination of Pb (II) ion by flame atomic absorption spectrometry. Microchim Acta 172(3–4):309–317
Mayer M, Wilson D (1998) Health and safety—the downward trend in lead levels. J Power Sources 73(1):17–22
Narin I, Surme Y, Bercin E, Soylak M (2007) SP70-α-benzoin oxime chelating resin for preconcentration–separation of Pb (II), Cd (II), Co (II) and Cr (III) in environmental samples. J Hazard Mater 145(1):113–119
Omidi F, Behbahani M, Bojdi MK, Shahtaheri SJ (2015) Solid phase extraction and trace monitoring of cadmium ions in environmental water and food samples based on modified magnetic nanoporous silica. J Magn Magn Mater 395:213–220
Stalikas C, Fiamegos Y, Sakkas V, Albanis T (2009) Developments on chemometric approaches to optimize and evaluate microextraction. J Chromatogr A 1216(2):175–189
Tamayo F, Casillas J, Martin-Esteban A (2003) Highly selective fenuron-imprinted polymer with a homogeneous binding site distribution prepared by precipitation polymerisation and its application to the clean-up of fenuron in plant samples. Anal Chim Acta 482(2):165–173
Yang G, Fen W, Lei C, Xiao W, Sun H (2009) Study on solid phase extraction and graphite furnace atomic absorption spectrometry for the determination of nickel, silver, cobalt, copper, cadmium and lead with MCI GEL CHP 20Y as sorbent. J Hazard Mater 162(1):44–49
Yılmaz E, Soylak M (2016) Preparation and characterization of magnetic carboxylated nanodiamonds for vortex-assisted magnetic solid-phase extraction of ziram in food and water samples. Talanta 158:152–158
Zhu X, Cui Y, Chang X, Zou X, Li Z (2009) Selective solid-phase extraction of lead (II) from biological and natural water samples using surface-grafted lead (II)-imprinted polymers. Microchim Acta 164(1–2):125–132
Acknowledgments
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical of Sciences for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mohammad Behbahani declares that he has no conflict of interest. Fariborz Omidi declares that he has no conflict of interest. Masoud Ghanbari Kakavandi declares that he has no conflict of interest. Masoud Ghanbari Kakavandi declares that he has no conflict of interest. Ghasem Hesam declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
It is not applicable in this study.
Rights and permissions
About this article
Cite this article
Kakavandi, M.G., Behbahani, M., Omidi, F. et al. Application of Ultrasonic Assisted-Dispersive Solid Phase Extraction Based on Ion-Imprinted Polymer Nanoparticles for Preconcentration and Trace Determination of Lead Ions in Food and Water Samples. Food Anal. Methods 10, 2454–2466 (2017). https://doi.org/10.1007/s12161-016-0788-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0788-8