Abstract
The authors describe a method for enhancing the hybridization chain reaction (HCR) by using gold nanoparticles (AuNPs). This can considerably improve the sensitivity of electrochemical immunoassays as demonstrated for the carbohydrate antigen 125 (CA125), a biomarker for ovarian cancer. Compared to previous HCR based assays, the DNA acting as fuel strands were immobilized onto AuNPs, so that dendrimeric like chains were formed on the electrode after HCR. The improved signal is due to the reaction of DNA on the electrode. Specifically, the reaction of the phosphate groups of DNA with molybdate forms redox-active molybdophosphate, and this generates a strong electrochemical current. The immunosensor was prepared by sequential capturing, on the electrode, (a) antibody against CA125, (b) analyte (CA125), and (c) an aptamer against CA125 to form a sandwich structure. The primer on the aptamer sequence initiates HCR by annealing to one strand of DNA on the AuNPs and to another DNA in solution. The increased loading of DNA molecules onto the electrode increases the amount of phosphate groups and subsequently increases the electrical signal. The sensitivity of the assay is found to be significantly improved compared to assays without HCR and when using conventional HCR. The immunosensor was successfully applied to the determination of CA125 in human serum samples. The detection limit (based on an S/N ratio of 3) is 50 μU.mL−1. This indicates that this signal amplification strategy has a large potential in terms of clinical applications. It may be modified such that it also can be applied to the determination of other analytes for which proper aptamers are available.

Gold nanoparticle (AuNP) enhanced hybridization chain reaction is reported to improve the sensitivity of electrochemical immunosensor. Hybridization chain reaction is carried out by annealing of H1 DNA strand immobilized on AuNP to the sticky end primer sequence of the aptamer and H2 strand to the complementary sequence of H1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoassays are traditionally used for the detection of different protein biomarkers, however, their sensitivity is often limited for the detection of low level of analyte targets due to the difficulties for signal amplification [1,2,3,4,5,6]. Signal tags usually need to be conjugated onto antibodies, which is rather complex and can affect the bioactivity of antibodies [7]. On the other hand, DNA based signal amplification are versatile and can be simply amplified. For example, widely reported DNA signal amplification strategies include polymerase chain reaction (PCR) [8, 9], rolling circle amplification (RCA) [10, 11], hybridization chain reaction (HCR) [12, 13] and et al.
Among the above mentioned DNA signal amplification strategies, HCR has been extensively reported for the detection of different analytes in connection with various detection techniques [14, 15]. HCR is a rapid linear amplification method that is enzyme-free and can be performed at room temperature. Typically, two stable species of DNA hairpins (H1 and H2) coexist in the solution until a primer strand is introduced [16, 17]. The two hairpin DNA molecules were triggered by the primer to start autonomously a cascade of hybridization events in which both hairpins assemble into long nicked double helices analogous to alternating copolymers [18].
Previously, we reported electrochemicalassays for the detection of different protein biomarkers based on DNA molecules generated electrochemical current [19, 20]. The generation of electrochemical current was due to the reaction of DNA phosphate backbone with molybdate to form redox molybdophosphate [21, 22]. The electrochemical current intensity was proportional to the amount of DNA molecules on the electrode surface. So DNA signal amplification strategies, such as RCA and HCR can also be combined with the DNA generated current technique to further enhance the assay sensitivity.
Here, we describe a gold nanoparticle (AuNP) enhanced HCR as a means for signal amplification in electrochemical immunoassays. In contrary to traditional HCR that H1 and H2 DNA strands are coexist in the solution, we immobilized H1 onto AuNP surface. After construction of the immunoassay based on sandwich protocol, for signal amplification, HCR was carried out by annealing of H1 immobilized on AuNP to the sticky end primer sequence of the aptamer. Then each H1 strand on AuNP can anneal H2 strand to its complementary sequence. Through this way, each aptamer molecule can initiate dendrimeric like chain reaction, not just the formation of linear double helices strand. Thus, the loading of DNA molecules were significantly increased and then the sensitivity of the immunoassay was enhanced after reaction with molybdate. Protein biomarker carbohydrate antigen 125 (CA125), which is the clinical gold standard biomarker for ovarian cancer, was chosen as a model analyte to test this signal amplification strategy [23, 24]. The resulted electrochemical immunoassay display high sensitivity, low limit of detection, and was successfully applied for detection of CA125 in human serum samples.
Experimental
Reagents and instrumentations
The DNA strands used in our study were synthesized and purified by Sangon Biotech Co., Ltd. (Shanghai, China, http://www.sangon.com/). The detailed sequences of DNA used in this work are listed below. Anti-CA125 antibody and CA125 protein were obtained from Abcam (Cambridge, UK, http://www.abcam.cn/). Sodium molybdate dihydrate (Na2MoO4.2H2O) and chitosan were purchased from Sigma-Aldrich (http://www.sigmaaldrich.com). The serum samples were obtained from the Second Xiangya hospital that attached to Central South University (http://www.xyeyy.com/). All other reagents were of analytical grade and deionized water with a conductivity of 18.2 MΩ.cm was used throughout the experiment.
CA125 aptamer-primer sequence: TAATACGA CTCACTATAGGGAGACAAGAATAAACGCTCAATATCGTTAATTCGGTCG (the bold part is the primer sequence).
H1: 5’-SH- TACGTGGCTTGGACCGACCGAATTAACGATA-3’.
H2: 5′- GTCCAAGCCACGTATATCGTTAATTCGGTCG-3′.
Electrochemical measurements were performed on a CHI-650D electrochemical workstation (Shanghai CH Instruments Co., China, http://www.chinstr.com/lxwm). A conventional three-electrode system was used with glassy carbon electrode (GCE, 3 mm in diameter) as the working electrode, an Ag/AgCl electrode as the reference electrode and a platinum wire as the auxiliary electrode.
Preparation of the immunoassay
The immunoassay was prepared based on the sandwich protocol. To conjugate CA125 antibodies onto GCE surface, graphene oxide (GO) was dispersed into chitosan solution (0.2%) to reach a concentration of 3 mg.mL−1 and 5 μL of the composite solution was dropped onto electrode surface. After dried at room temperature, the electrode was immersed into glutaraldehyde (0.25%) mixture for 30 min and then into anti-CA125 antibody solution (1 μg.mL−1) for 1 h. After gentle wash, the electrode was incubated with BSA (1%) solution for 30 min to block nonspecific binding sites. Then, the modified electrode was incubated with different concentrations of CA125 standard solution for 1 h. With another round of wash, subsequently, the electrode was incubated with 50 μM CA125 aptamer for 1 h. After wash again, the immunosensor was finished.
HCR reaction
To carry out HCR reaction, H1 DNA strand was immobilized onto 13 nm AuNP surface via the thiol moiety on the end of H1 DNA. Subsequently, 5 μL DNA hybridization buffer (10 mM Tris-HCl, 1 mM EDTA, 500 mM NaCl, 1 mM MgCl2, pH 7.4) containing H1 modified AuNPs and 50 μM of H2 was dropped on electrode and reacted at 37 °C for 2 h to accomplish the HCR. After extensive wash, the electrode was ready for measurement.
Electrochemical measurements
For electrochemical testing, 5 μL of Na2MoO4 solution (6 mM) was dropped onto electrode and reacted for 20 min. The electrode was then measured in 0.5 M H2SO4. Cyclic voltammetry (CV) measurements were performed at scan rate of 0.1 V/s. For square wave voltammetry (SWV) testing, the amplitude applied was 0.025 V with frequency of 15 Hz. The SWV current at a peak potential of 0.18 V was recorded to draw the calibration plot.
Results and discussion
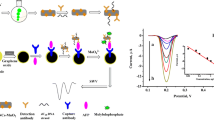
Figure 1 shows the schematic representation of the immunoassay preparation and detection processes. The idea of the work is utilizing AuNP to enhance the HCR and improve the sensitivity of the immunosasssay. After formation of the sandwich structure, HCR is carried out to induce dendrimeric like chain reaction due to the immobilization of H1 DNA strands on AuNPs. This is in contrary to traditional HCR that linear chain reaction is performed. The signal of the immunoassay is due to the reaction of DNA molecules on electrode with molybdate to form redox molybdophosphate and generate electrochemical current. Since the HCR increased the amount of DNA molecules on the electrode surface, the sensitivity of the assay is significantly increased.
Before characterization of the performance of the immunoassay, initially, the immunoassay preparation and AuNP based HCR process were characterized by electrochemical impedance spectroscopy (EIS) measurements in 5 mM [Fe(CN)6]3−/4- solution. As shown in Fig. 2, compared to the bare electrode (curve a), the electron-transfer resistance of the electrode is increased after the antibody immobilization (curve b), the capture of CA125 as well as aptamer (curve c), the HCR (curve d) and the reaction with molybdate (curve e). Especially, there is a significant increase of the resistance values after HCR and after the reaction with molybdate, indicating the successful carry out of HCR and the formation of molybdophosphate precipitation on the electrode.
Electrochemical impedance characterization of the immunoassay preparation process. Curve a to e represent the electron-transfer resistance of the bare electrode (curve a), the electrode after the antibody immobilization (curve b), after the capture of CA125 as well as aptamer (curve c), after HCR (curve d) and after the reaction with molybdate (curve e)
Then, the effect of the AuNP enhanced HCR on the improvement of the sensitivity of the immunoassay was studied in detail. Three immunoelectrodes were prepared for the detection of 1 U.mL−1 of CA125. The first immunoelectrode was based on aptamer along for signal amplification, the second was based on traditional HCR (with H1 and H2 DNA strands in solution) for signal amplification and the third was based on AuNP enhanced HCR for signal amplification. The square wave voltammetry (SWV) responses of the three immunoelectrodes are shown in Fig. 3. As can be seen, of the first immunoelectrode, two small current peaks are observed at around 0.20 and 0.32 V (curve a). The generated electrochemical current is ascribed to the reaction of aptamer phosphate backbone with molybdate that generated redox molybdophosphate [25,26,27,28,29]. The peak current is relatively small is because the amount of aptamer captured onto electrode surface and then the amount of phosphate groups is limited. After the traditional HCR was carried out, for the second immunoelectrode, the current intensity is increased about 3 times (curve b). This result proved the successful assemble of the DNA molecules onto electrode surface via HCR. The increased loading of DNA onto electrode resulted in enhanced electrochemical current intensity [30, 31]. Of the third immunoelectrode based on AuNP enhanced HCR, it can be seen that the electrochemical current intensity is about 3 times to that based on traditional HCR. This data demonstrated that compared to traditional HCR, the AuNP based HCR further increased the loading of DNA molecules onto electrode, probably due to the formation of dendrimeric like DNA network.
After proved the AuNP based HCR can enhance the sensitivity of the immunoassay, we studied the effect of the HCR time on the sensitivity of the immunoassay. For the detection of 1 U.mL−1 of CA125, with the increase of the HCR time, the sensitivity of the immunoassay is increased continually. However, after 2 h, further increase of the reaction time resulted in no obvious enhancement of the sensitivity (Fig. 4). So the HCR time of 2 h was selected for the following experiments.
Under the optimized experimental conditions, different concentrations of CA125 standard solutions were then analyzed by the immunoassay to test the linear range of the immunoassay. From Fig. 5, we can see that the SWV peak current of the immunoassay at 0.2 V is increased with the increase of CA125 concentrations. A linear range between the current response of the immunoassay and the logarithm of concentrations of CA125 in the range from 100 μU.mL−1 to 10 U.mL−1 is obtained (inset of Fig. 5) with sensitivity of 198 μA·U·mL−1 ·cm−2. The detection limit of the immunoassay that calculated based on a S/N ratio of 3 is 50 μU.mL−1.
When comparing the performance of the immunoassay with literature reports for the detection of CA125, we can see the performance of our immunoassay is better than literature reports (Table 1). The good performance of our immunoassay, including high sensitivity and wide linear range is mainly ascribed to the AuNP enhanced HCR and the DNA molecules generated electrochemical current.
Besides high sensitivity and low limit of detection, good selectivity and reproducibility are also of great importance for application of the immunoassay in real sample analysis. Good selectivity is to minimize interference of other substances in the serum sample on the precise detection of target analyte. The responses of the immunoassay to potential interfering proteins, such as α-fetoprotein (AFP), carcinoembryonic antigen (CEA), human IgG, human epidermal growth factor receptor 2 (HER2) and human serum albumin (HSA) were tested. The 1 U.mL−1 of CA125 were mixed with 10 ng. mL−1 of the above mentioned interfering proteins and then analyzed by the immunoassay. The signal variation due to the presence of interfere proteins were within 10% of that without interfering proteins, demonstrating good selectivity of the immunoassay (Fig. 6). In addition, to study the reproducibility of the immunoassay, five immunoassays were prepared for the detection of 1 mU.mL−1 and 1 U.mL−1 of CA125. The relative standard deviation (RSD) of the testing result is 5.6 and 4.5%, respectively, indicating good reproducibility of the immunoassay.
Finally, to test potential clinical application of the immunoassay, clinical serum samples were obtained and then analyzed by the immunoassay. Eight human serum samples were collected. Before tested by the immunoassay, the serum samples were diluted with phosphate buffer. The level of CA125 in the serum samples that measured by the immunoassay were compared with those obtained by ELISA kit. From Fig. 7, we can see that the two results agreed well. A straight line was obtained by plotting the two CA125 values with a correlation coefficient of 0.991, demonstrating reliability of the immunoassay testing results.
Conclusion
In summary, we studied a new HCR, that is AuNP enhanced HCR as signal amplification strategy for electrochemical immunoassays. The new signal amplification strategy was tested for detection of CA125 as a model analyte. The reaction of DNA molecules on electrode with molybdate leads to formation of molybdophosphate and generation of electrochemical current. Experimental data indicated that AuNP enhanced HCR improved sensitivity of the immunoassay compared to traditional HCR. This method expanded HCR as signal amplification strategy and will find wide applications in different bioassays. However, the detailed mechanism of the AuNP enhanced HCR was not studied in this work, which needs further research.
References
Zeng K, Tian S, Wang Z, Shen C, Luo J, Yang M, Liu Y-N (2017) An ELISA for the determination of human IgG based on the formation of a colored iron(II) complex and photometric or visual read-out. Microchim Acta 184(8):2791–2796
Tang D, Su B, Tang J, Ren J, Chen G (2010) Nanoparticle-based sandwich electrochemical immunoassay for carbohydrate antigen 125 with signal enhancement using enzyme-coated nanometer-sized enzyme-doped silica beads. Analy Chem 82(4):1527–1534
Tang Z, Ma Z (2017) Multiple functional strategies for amplifying sensitivity of amperometric immunoassay for tumor markers: a review. Biosens Bioelectron 98:100–112
Liu W, Ma C, Yang H, Zhang Y, Yan M, Ge S, Yu J, Song X (2014) Electrochemiluminescence immunoassay using a paper electrode incorporating porous silver and modified with mesoporous silica nanoparticles functionalized with blue-luminescent carbon dots. Microchim Acta 181(11–12):1415–1422
Shan J, Ma Z (2017) A review on amperometric immunoassays for tumor markers based on the use of hybrid materials consisting of conducting polymers and noble metal nanomaterials. Microchim Acta 184(4):969–979
Zhao Y, Zheng Y, Kong R, Xia L, Qu F (2016) Ultrasensitive electrochemical immunosensor based on horseradish peroxidase (HRP)-loaded silica-poly(acrylic acid) brushes for protein biomarker detection. Biosens Bioelectron 75:383–388
Wang Z, Zong S, Wu L, Zhu D, Cui Y (2017) SERS-activated platforms for immunoassay: probes, encoding methods, and applications. Chem Rev 117(12):7910–7963
Kieu The Loan T, Lee NY (2017) A portable microreactor with minimal accessories for polymerase chain reaction: application to the determination of foodborne pathogens. Microchim Acta 184(11):4225–4233
Kieu The Loan T, Wu W, Lee NY (2014) Bent polydimethylsiloxane-polycarbonate hybrid microdevice for on-chip flow-through polymerase chain reaction employing a single heater. Microchim Acta 181(13–14):1697–1705
Cheng X, Yu X, Chen L, Zhang H, Wu Y, Fu F (2017) Visual detection of ultra-trace levels of uranyl ions using magnetic bead-based DNAzyme recognition in combination with rolling circle amplification. Microchim Acta 184(11):4259–4267
Li X, Xu X, Song J, Xue Q, Li C, Jiang W (2017) Sensitive detection of T4 polynucleotide kinase activity based on multifunctional magnetic probes and polymerization nicking reactions mediated hyperbranched rolling circle amplification. Biosens Bioelectron 91:631–636
Liu H, Bei X, Xia Q, Fu Y, Zhang S, Liu M, Fan K, Zhang M, Yang Y (2016) Enzyme-free electrochemical detection of microRNA-21 using immobilized hairpin probes and a target-triggered hybridization chain reaction amplification strategy. Microchim Acta 183(1):297–304
Qing Y, Li X, Chen S, Zhou X, Luo M, Xu X, Li C, Qiu J (2017) Differential pulse voltammetric ochratoxin a assay based on the use of an aptamer and hybridization chain reaction. Microchim Acta 184(3):863–870
Chen Y-X, Huang K-J, He L-L, Wang Y-H (2017) Tetrahedral DNA probe coupling with hybridization chain reaction for competitive thrombin aptasensor. Biosens Bioelectron 100:274–281
Bi S, Yue S, Zhang S (2017) Hybridization chain reaction: a versatile molecular tool for biosensing, bioimaging, and biomedicine. Chem Soc Rev 46(14):4281–4298
Guo J, Wang J, Zhang J, Zhang W, Zhang Y (2017) Ultrasensitive non enzymatic multiple immunosensor for tumor markers detection by coupling DNA hybridization chain reaction with intercalated molecules. Biosens Bioelectron 90:159–165
Guo Q, Bian F, Liu Y, Qu X, Hu X, Sun Q (2017) Hybridization chain reactions on silica coated Qbeads for the colorimetric detection of multiplex microRNAs. Chem Commun 53(36):4954–4957
Zhang B, Liu B, Tang D, Niessner R, Chen G, Knopp D (2012) DNA-based hybridization chain reaction for amplified bioelectronic signal and ultrasensitive detection of proteins. Anal Chem 84(12):5392–5399
Shen C, Zeng K, Luo J, Li X, Yang M, Rasooly A (2017) Self-assembled DNA generated electric current biosensor for HER2 analysis. Anal Chem 89(19):10264–10269
Hu L, Hu S, Guo L, Shen C, Yang M, Rasooly A (2017) DNA generated electric current biosensor. Anal Chem 89(4):2547–2552
Jiang W, Tian D, Zhang L, Guo Q, Cui Y, Yang M (2017) Dual signal amplification strategy for amperometric aptasensing using hydroxyapatite nanoparticles. Application to the sensitive detection of the cancer biomarker platelet-derived growth factor BB. Microchim Acta 184(11):4375–4381
Si Z, Xie B, Chen Z, Tang C, Li T, Yang M (2017) Electrochemical aptasensor for the cancer biomarker CEA based on aptamer induced current due to formation of molybdophosphate. Microchim Acta 184(9):3215–3221
Zheng Y, Wang H, Ma Z (2017) A nanocomposite containing Prussian blue, platinum nanoparticles and polyaniline for multi-amplification of the signal of voltammetric immunosensors: highly sensitive detection of carcinoma antigen 125. Microchim Acta 184(11):4269–4277
Babamiri B, Hallaj R, Salimi A (2018) Ultrasensitive electrochemiluminescence immunoassay for simultaneous determination of CA125 and CA15-3 tumor markers based on PAMAM-sulfanilic acid-Ru(bpy)(3)(2+) and PAMAM-CdTe@CdS nanocomposite. Biosens Bioelectron 99:353–360
Huang Y, Tang C, Liu J, Cheng J, Si Z, Li T, Yang M (2017) Signal amplification strategy for electrochemical immunosensing based on a molybdophosphate induced enhanced redox current on the surface of hydroxyapatite nanoparticles. Microchim Acta 184(3):855–861
Shen C, Li X, Rasooly A, Guo L, Zhang K, Yang M (2016) A single electrochemical biosensor for detecting the activity and inhibition of both protein kinase and alkaline phosphatase based on phosphate ions induced deposition of redox precipitates. Biosens Bioelectron 85:220–225
Xie B, Zhou N, Ding R, Zhao Y, Zhang B, Li T, Yang M (2017) Dual signal amplification strategy for electrochemical detection of platelet-derived growth factor BB. Anal Methods 9(46):6569–6573
Jiang W, Liu L, Zhang L, Guo Q, Cui Y, Yang M (2017) Sensitive immunosensing of the carcinoembryonic antigen utilizing aptamer-based in-situ formation of a redox-active heteropolyacid and rolling circle amplification. Microchim Acta 184(12):4757–4763
Qu F, Yang M, Rasooly A (2016) Dual signal amplification electrochemical biosensor for monitoring the activity and inhibition of the Alzheimer's related protease beta-secretase. Anal Chem 88(21):10559–10565
Feng K, Liu J, Deng L, Yu H, Yang M (2018) Amperometric detection of microRNA based on DNA-controlled current of a molybdophosphate redox probe and amplification via hybridization chain reaction. Microchim Acta 185 (1). Unsp 28
Li X, Shen C, Yang M, Rasooly A (2018) Polycytosine DNA electric-current-generated Immunosensor for electrochemical detection of human epidermal growth factor Receptor2 (HER2). Anal Chem 90(7):4764–4769
Zhao L, Han H, Ma Z (2018) Improved screen-printed carbon electrode for multiplexed label-free amperometric immuniosensor: addressing its conductivity and reproducibility challenges. Biosens Bioelectron 101:304–310
Al-Ogaidi I, Aguilar ZP, Suri S, Gou H, Wu N (2013) Dual detection of cancer biomarker CA125 using absorbance and electrochemical methods. Analyst 138(19):5647–5653
Torati SR, Kasturi KCSB, Lim B, Kim C (2017) Hierarchical gold nanostructures modified electrode for electrochemical detection of cancer antigen CA125. Sensor Actuator B Chem 243:64–71
Wu L, Sha Y, Li W, Wang S, Guo Z, Zhou J, Su X, Jiang X (2016) One-step preparation of disposable multi-functionalized g-C3N4 based electrochemiluminescence immunosensor for the detection of CA125. Sensor Actuators B Chem 226:62–68
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Nie, Y., Yang, M. & Ding, Y. Gold nanoparticle enhanced hybridization chain reaction as a method for signal amplification. Application to electrochemical immunodetection of the ovarian cancer biomarker carbohydrate antigen 125. Microchim Acta 185, 331 (2018). https://doi.org/10.1007/s00604-018-2869-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2869-4