Abstract
The authors describe the preparation of molecularly imprinted nanoparticles (MINPs) for the solid phase extraction of hippuric acid (HA). The MINPs consists of a water-compatible organic-inorganic silica composite which was obtained from a functionalized silica by the sol-gel method. HA acted as the template, 3-aminopropyl trimethoxysilane as the functional monomer, and tetraethoxysilane as the crosslinker. Subsequently, methacryloxypropyltrimethoxysilane was used as a coupling agent to deposit a hydrophilic acrylamide coating onto the surface of the MINPs. The morphology and structure of the resulting restricted access material (referred to as RAM-MINP) were characterized by scanning electron microscopy, transmission electron microscopy and Fourier transform infrared spectroscopy. Solid phase extraction of HA was accomplished by passing urine samples through a RAM-MINP-packed SPE cartridge. Following elution, HA was quantified by HPLC using UV detection at 228 nm. The effects of sample pH, amount of sorbent and eluent and washing solvent volumes were optimized by experimental design methodology under response surface methodology. Under optimized conditions, the mean extraction efficiency of HA from spiked samples is adequately repeatable, with relative standard deviations of <6.1%. The limits of detection and quantitation are 0.15 and 0.25 μg.L−1, respectively.

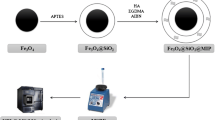
Schematic of the the preparation of molecularly imprinted nanoparticles (MINP) for the solid phase extraction of hippuric acid (HA). Solid phase extraction of HA was accomplished by passing urine samples through a RAM-MINP-packed SPE cartridge. After solid phase extraction and elution, HA was quantified by HPLC using UV detection at 228 nm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Toluene is a toxic colorless and widely used material which may cause anatomical changes in the brain and neurobehavioral impairments [1–3]. Such indications can be observed at exposures even less than 50 ppm [4, 5]. More than 80% of toluene inhaled by humans is oxidized to benzoic acid, which is then conjugated with glycine and converted to hippuric acid (HA) followed by excretion in urine [6, 7]. Therefore, monitoring of HA as a marker for toluene breathing by simple, accurate, cost-effective methods with a low detection limit is of great interest.
Pretreatment techniques based on molecular imprinting have been applied to the determination of trace amounts of various chemicals due to advantages in simplicity, accuracy, cost-effectiveness and selectivity [8–11]. For instance, molecularly imprinted polymers (MIP) have attracted attention due to their potential ability to recognise target molecules [12–15]. However, they cannot be used efficiently in the case of low molecular weight compounds due to the presence of interfering macromolecules such as proteins and lipids in biological samples. These interferences result from the strong adsorption of such hydrophobic materials onto the surface of MIP, thus preventing the target from being recognised [16, 17]. This problem can be resolved by using restricted access materials (RAM) for the direct extraction of target molecules from biological fluids [18–21]. RAM-MIP has an interior phase for small molecule retention and a hydrophilic layer, which is non-adsorptive for proteins and lipids. This is due to the chemical diffusion barrier created by hydrophilic network shielding. A limited number of applications of RAM-MIP for such purposes has been reported. Du et al. have developed a RAM-MIP with a methylmethacrylate-based MIP inner core and a hydrophilic RAM layer [17] using polymerization precipitation for MIP preparation. Subsequently, they hydrolyzed the epoxide rings present on the polymer surface leading to the formation of the hydrophilic network. Another kind of RAM-MIP was developed by Sambe and his colleagues [22], in which MIP was synthesized using a multi-step swelling and polymerization method. They prepared hydrophilic RAM as the external layer using hydrophilic monomers including glycerol monomethacrylate and Glycerol dimethacrylate. Manesiotis and co-workers [23] have developed “water-compatible” MIP using a hydrophilic co-monomer by hydrolytically hydrophilizing the polymer matrix. Nevertheless, the applied organic polymer-based MIPs have low diffusion of analytes. Additionally, shrinking or swelling of MIPs under exposure to different organic solvents can deform the selective cavities and decrease recognition ability towards target molecules.
It is well-known that urine contains hydrophobic organic compounds including proteins, hormones and other metabolites. Therefore, it is necessary to design a preliminary sample pretreatment procedure for the removal of such interfering concomitants as well as to concentrate HA. Here, an efficient approach for the sol-gel-based synthesis of molecularly imprinted nanoparticles using inorganic materials is used to resolve the problems of organic-based MIPs. The performance of the method was significantly enhanced by functionalizing the surface of the MIP nanoparticles using acrylamide (AC) as a hydrophilic monomer.
Experimental
Reagents and apparatus
Tetraethyl-orthosilicate (TEOS), 3-aminopropyl trimethoxysilane (APTMS), acetic acid, methacryloxypropyltrimethoxysilane (MPS), 2,2-Azobisisobutyronitrile (AIBN), acrylamide, phosphoric acid (H3PO4), sodium hydroxide (NaOH) and ammonium hydroxide (NH4OH), were purchased from Merck (Darmstadt, Germany www.merck.com). Hippuric acid (N-benzoylglycine, 98%) powder was acquired from Acros (Geel, Belgium). Ultrapure water was obtained using a MilliQ gradient water purification system. Methanol, ethanol, acetone, hexane, toluene and acetonitrile (HPLC grade) were provided by J.T. Baker (Deventer, Holland). The solutions were filtered before use through 0.45 μm nylon filters from Análisis Vínicos (Tomelloso, Spain).
The shape and surface morphology of RAM-MINP were investigated using a field emission scanning electron microscope (FE-SEM, VEGA TESCAN, USA). Fourier transform infrared (FT-IR) spectroscopy was performed by FTIR-8300, Shimadzu, Japan (www.shimadzu.com). The transmission electron microscopy (TEM) images were recorded on a Hitachi S-570 microscope for the determination of sample dimensions. A 130 W ultrasonic bath with a heating system (Tecno-GAZ SPA Ultra Sonic System) at a frequency of 40 kHz was used to disperse nanoparticles. High-performance liquid chromatography analysis was performed using an Agilent 1100 liquid chromatography apparatus (Wilmington, DE, USA www.agilent.com) equipped with a micro vacuum degasser (model G1379A), a quaternary pump (model G1311A), a multiple wavelength detector (model G13658: 228 nm for HA), a sample injection valve with a 20 μL sample loop, and a Knauer C18 column (4.6 mm i.d. 250 mm, 5 μm). Data were collected and analyzed using Agilent Chemstation software. Acetonitrile: H3PO4 buffer (10: 90, v/v) adjusted to pH 3.0 mixed with 1.0 mol.L−1 of sodium hydroxide was used as the mobile phase, set to a flow rate of 1.0 mL.min−1. The mobile phase was filtered through a 0.45 μm filter and degassed under vacuum before use. The system was operated at ambient temperatures.
Synthesis of imprinted and non-imprinted silica nanoparticles
The imprinted and non-imprinted silica nanoparticles were prepared by the hydrolysis of TEOS in aqueous ammonia as reported in previous work [24] with small modifications. In summary, 93.0 mg of HA was dissolved in 50.0 mL of ethanol under stirring followed by mixing with 0.61 mL of APTMS and 3.0 mL of TEOS. Then, 1.6 mL of aqueous solution of 25% (w/w) ammonia was added as a catalyst under vigorous stirring at room temperature for 24 h. The nanoparticles thus produced were collected by centrifugation for 10 min. Finally, the product was rinsed with methanol and distilled water used to remove unreacted materials. Non-imprinted nanoparticles (NINP) were made using the same procedure except no template (HA) was used.
Preparation of RAM-MINP and RAM-NINP
Surface modifications of nanoparticles were carried out as follows: 250 mg of nanoparticles were dispersed in 50.0 mL of toluene/DMSO (7:1 v/v) solution containing 200 mg of AC by sonication for 10 min. Next, 5.0 mL of MPS and 0.20 g of AIBN were added to the mixture and the reaction continued for 24 h at 120 °C under reflux in a nitrogen atmosphere. The material thus obtained was removed from the flask and subjected to centrifugation-assisted washing for many cycles via decantation and re-suspension in methanol/acetic acid (90/10 v/v) by sonication until the removal of the template. Finally, the nanoparticles were thoroughly rinsed with deionized water and dried at room temperature for further use.
Preparation of working samples
A standard stock solution of HA was prepared in methanol at a concentration of 1.0 mg.mL−1. Subsequently, the working HA standard solutions were obtained by the appropriate level of dilution with double distilled water (pH adjusted to 5.0 with phosphate buffer). The calibration curve for the urine analysis was constructed by spiking HA-free human urine with HA over a specific concentration range. All solutions were stored in the dark at +4 °C. Urine samples voluntarily donated by students were filtered through Whatman paper No. 42 and stored at +4 °C in the dark before the analysis.
Adsorption isotherm study
To investigate the binding property of the prepared sorbent, several samples were prepared by suspending 200 mg of either RAM-MINP or RAM-NINP in 10.0 mL of HA aqueous solutions with concentrations over the range of 0.05–1.5 mg.mL−1. The flasks were thoroughly sealed and each mixture was shaken for 12 h at room temperature. Then, aliquots of the supernatant were collected after centrifugation and this was followed by the quantification of HA by HPLC. The equilibrium adsorption contents (Q (mg.g−1)) were calculated by the following equation:
where V is the volume (mL) of solution; m is the mass (g) of the RAM-MINP or RAM-NINP; C0 and Ce are the initial and the equilibrium concentrations (mg.L−1) of HA in aqueous solution, respectively.
Extraction procedure
200 mg of nanoparticles (RAM-MINP or RAM-NINP) was packed into an empty 4 mL SPE cartridge. The nanoparticles in the cartridge were secured by porous polyethylene frits at the top and the bottom. After the packing process, the cartridge was sequentially conditioned with 5.0 mL of methanol and 5.0 mL of doubly distilled water (at pH 5.0) at a constant flow rate. Extraction experiments were performed by passing 15.0 mL of each urine sample through the cartridge at 1.0 mL min−1. Then, the column was washed with 4.0 mL of double distilled water followed by the elution, which was performed by passing 2.0 mL of methanol: acetonitrile: acetic acid (45:45:10 v/v/v). Next, the extract was collected in a vial and dried at 35 °C under N2 flow. The residue was reconstituted by its dissolution in 50 μL of mobile phase, 20 μL of which was analyzed by HPLC to achieve a pre-concentration factor of 300.
Results and discussion
Optimization of preparation conditions and studies of the recognition mechanism
HA has several functional groups, such as carboxylic acid and amide, which allow the formation of hydrogen-bonds with the amine group of APTMS. Thus, a stable donor–receptor complex between HA and APTMS is required for the polymerization process to result in the formation of specific cavities with unique shape, size and functional groups. MINP was synthesized using the sol-gel method at mild temperature. Various factors, such as the proportion of any pair of the functional monomer, cross-linker and template, affecting the selectivity and biding capacity of MINP were optimized. To this end, the effects of the molar ratios of the functional monomer to cross-linker in the range of 1:2 to 1:9 were investigated. It was shown that ratios lower than 1:4 lead to poor recognition ability, which may be due to the deformation of specific cavities. This is, in turn, is due to the low molar rate of cross-links and poor mechanical stability of polymer structure in solution. On the other hand, at ratios higher than 1:7, recognition ability decreased due to the excessive depth of embedding of specific cavities in the polymer network and thus decreased analyte mass-transfer. The optimized ratio of the functional monomer and cross-linker was found to be 1:5.
Furthermore, the molar ratio of template to functional monomer was evaluated and optimized. As mentioned above, non-covalent interactions between HA and APTMS may predominantly construct binding sites. The amine group of APTMS, which is the best site for hydrogen bond donors, makes the extraction procedure selective. The scheme of the MINP and its molecular recognition procedure are shown in Fig. 1. As can be seen, four sites available in each HA molecule form four hydrogen bonds, which correspond with each of four APTMS molecules. In other words, the ratio of template to functional monomer was observed to be 1:4, which is in good agreement with experimental results (1:5 ratio). Such a faint discrepancy may be attributed to partial failure of some functional monomers to form self-assembled complex of monomer and template.
The hydrophilic network was prepared on the surface of the MINP, which acts as a barrier against hydrophobic compounds and enhances analyte transfer into the specific cavities. The presence of C = C in AC as a water-soluble monomer made it suitable for coupling with MPS in the presence of AIBN as initiator via radical polymerization. The chain structure of hydrophilic sites (Fig. 1) prevents the hydrophobic macromolecules from penetrating and/or approaching the MIP. In addition, the high ability of AC to form hydrogen bonds with the target molecule leads to an increase in recognition ability.
Characteristic of the FT-IR spectra
To investigate the presence of functional sites in binding cavities and in the organic–inorganic hybrid hydrophilic external layer, FT-IR was employed to characterize the RAM-MINP, MINP and silica nanoparticles. Silica nanoparticles were prepared by the hydrolysis of TEOS with ammonium hydroxide according to the method used by Zhu et al. [25]. A broad absorption band observed at 3300 cm−1 is attributed to the stretching mode of −O − H [26]. As shown in Fig. S1, the observed feature around 1100 cm−1 corresponds to −Si − O − Si − stretching vibrations. The bands around 793 and 460 cm−1 are attributed to −Si − O vibrations. The characteristic peak of the −O − H group appears at 2955 cm−1 and the band around 2946 cm−1 refers to the −N − H group (Figs. S1b and S1c) [27]. The band observed around 1668 cm−1 is related to amide −C = O stretching vibrations (Fig. S1c) [28]. These results suggest that AC was covalently bonded to the MINP skeleton through the MPS.
SEM and TEM analysis
The SEM (Fig. S2) study of the MINP and RAM-MINP shows similar particles of spherical shape with a low degree of aggregation and low surface roughness. The TEM images show the spherical structure of RAM-MINP with an average diameter of about 100 nm, which is confirmed by the results obtained using SEM. Such features are of paramount importance with respect to packing the material for solid phase extraction, resulting in higher mass transfer and increased extraction efficiency. In addition, the small size of RAM-MINP enhances template removal and thus more recognition cavities are obtained [29]. The hydrophilic sites on the surface of RAM-MINP allow the small analyte molecules to pass through these sites, while the hydrophobic biomolecules are retained on the surface.
Binding study and Freundlich analysis
The static equilibrium adsorption experiments for the RAM-MINP and RAM-NINP were carried out by changing the initial concentrations of HA across the range of 0.05–1.5 mg.mL−1. The adsorption data can be fitted using the following Freundlich isotherm model to evaluate adsorption parameters [30].
where Q (mg.g−1) is the amount of adsorbed HA per unit polymer mass, Ce (g.L−1) is the equilibrium concentration of HA in solution. The constant α is the measure of capacity and average affinity. m with values from 0 to 1 is the Freundlich constant and is an indication of heterogeneity or homogeneity of the sites. The higher the m value the more homogeneous the sites. The extreme m value of 1 indicates that the system is homogeneous. The investigation on the isotherm of HA binding to RAM-MINP and RAM-NINP (Fig. 2) showed that the adsorption capacity of RAM-MINP was 68.5 mg.g−1, which is much higher than that of RAM-NINP (20.9 mg.g−1). This is due to the high-affinity of highly available binding sites in the polymer structure, which enables the analyte to be easily adsorbed by such sites. As mentioned above, RAM-NINP preparation was similar to that of RAM-MINP except that the template was not added during the synthesis. The AC on the surface of RAM-NINP and APTMS in the polymer structure can react with HA through hydrogen bonding and thus RAM-NINP may also bind some HA molecules.
Optimization of method
The use of different washing and desorption solvents was initially investigated by a univariate method. Double distilled water was found to be the most suitable washing solvent. The best recovery for the elution step was obtained using methanol/acetonitrile/acetic acid (45:45:10).
Additionally, the following parameters were optimized: (a) Sample pH value; (b) Sorbent amount; (c) Washing solvent volume; (d) Eluent volume. Relevant data and figures are given in the Electronic Supporting Material. We found the following experimental conditions to give the best results: (a) A sample pH value of 5.0; (b) 200 mg of sorbent; (c) 4.0 mL of washing solvent and (d) 2.0 mL of eluent.
Validation of analytical method
The calibration curve was constructed over the concentration range of 0.2–10,000 μg.L−1. It was revealed that there is a linear response over the range of 0.3–7500 μg.L−1 with a regression equation of Y = 74.932X − 1.3311 (R2 = 0.9996), where Y and X (μg.L−1) are the peak area and concentration of HA, respectively.
To evaluate the accuracy and precision of the developed method using the RAM-MINP as the extracting medium, the samples prepared for quality control (QC) were spiked to achieve HA concentrations of 0.0005, 0.01, 0.1, 1.0 and 5.0 μg.mL−1. Intra-assay precision was assessed by five repeated injections of each QC sample. The reasonable rates of recovery, which were in the range of 88.0–104.0 and the RSD %, which was in the interval of 1.1 to 6.1% demonstrate the high applicability of the method for the evaluation of analyte over a wide concentration range. The inter-day precision in terms of RSD % was found to be between 1.2 to 5.9% (Table 1), which is also acceptable. The results demonstrated the satisfactory recovery and precision of the RAM-MINP-SPE.
Limit of detection (0.15 μg.L−1) and limit of quantification (0.25 μg.L−1) were calculated using the ratios 3σ/slope and 10σ/slope, respectively, where σ is the standard deviation of the mean value for 10 chromatograms of the blank [31].
The reusability of the RAM-MINP-SPE was evaluated by comparing the recovery of HA from urine samples based on the following procedure: Following each extraction experiment, the cartridge was washed repeatedly using 5 mL of methanol and 5 mL of distilled water (at pH 5.0), the results of which are shown in Fig. S7. After eleven SPE cycles, no obvious decrease in the extraction efficiency of the cartridge was observed. All in all, it was confirmed that the RAM-MINP is reusable, satisfactorily stable in solvents and easy to synthesize.
The prepared RAM-MINP-SPE was compared with the MINP-SPE to determine HA in the urine sample spiked with HA standard based on loading 15 mL of sample into the cartridge according to the procedure described in section 2.6. The chromatograms of HA using RAM-MINP-SPE and MINP-SPE are shown in Fig. 3. Accordingly, the peak area corresponding to HA obtained using the RAM-MINP was higher than that of the MINP, which confirms the higher performance of the RAM-MINP. In addition, a more detailed observation of the chromatographic profile shows good clean-up of samples in chromatographic separation with no peak interference with HA.
Extraction of HA from human urine
The method developed was applied to two urine samples taken from our volunteer colleagues who had been subjected to toluene exposure in our laboratory. Each sample was subjected to the RAM-MINP-SPE and HPLC-UV procedure in triplicate. As shown in Table 2, all selected samples containing HA indicate that the current method can efficiently extract HA from urine samples.
Conclusions
A hydrophilic water-compatible RAM-MINP was successfully synthesized. This was efficiently applied for HA extraction and followed by the optimization of the procedure by response surface methodology. The applied method was compared with methods described in other works [32–34] what is shown in Table 3. The applied RAM-MINP-SPE method is simple, cost-effective, and easy to operate. Although the RSD value of the present work is higher than those of some methods used for the determination of HA [32, 36, 37, 41], it is still lower than or close to those of other methods [33, 34, 38]. This suggests that the present method has acceptable repeatability. Our method has a wide dynamic linear range over concentrations of 0.3–7500 (μg.L−1) which is higher than most reported methods [32–41]. Furthermore, the LOD value of 0.15 μg.L−1 is lower than that of most methods previously reported [32, 36–41] confirming highly sensitive detection of HA. It is shown that the method applied is sufficiently accurate and precise for the analysis of HA in urine samples, and more efficient than what has been found in other HPLC-based works.
References
Boor JW, Hurtig HI (1977) Persistent cerebellar ataxia after exposure to toluene. Ann Neurol 2(5):440–442
Rosenberg NL, Spitz MC, Filley CM, Davis KA, Schaumburg HH (1988) Central nervous system effects of chronic toluene abuse—clinical, brainstem evoked response and magnetic resonance imaging studies. Neurotoxicol Teratol 10(5):489–495
Feldman RG, Ratner MH, Ptak T (1999) Chronic toxic encephalopathy in a painter exposed to mixed solvents. Environ Health Perspect 107(5):417–422
Berenguer P, Soulage C, Perrin D, Pequignot J-M, Abraini JH (2003) Behavioral and neurochemical effects induced by subchronic exposure to 40 ppm toluene in rats. Pharmacol Biochem Behav 74(4):997–1003
Chouanière D, Wild P, Fontana JM, Héry M, Fournier M, Baudin V, Subra I, Rousselle D, Toamain JP, Saurin S (2002) Neurobehavioral disturbances arising from occupational toluene exposure. Am J Ind Med 41(2):77–88
Pierce CH, Chen Y, Dills RL, Kalman DA, Morgan MS (2002) Toluene metabolites as biological indicators of exposure. Toxicol Lett 129(1):65–76
Wiwanitkit V, Suwansaksri J, Soogarun S (2008) High urine hippuric acid level among police working close to traffic in an urban area, Thailand: a preliminary study. Stoch Env Res Risk A 22(2):281–283
Inoue N, Ooya T, Takeuchi T (2014) Erratum to: hydrophilic molecularly imprinted polymers for bisphenol a prepared in aqueous solution. Microchim Acta 181(15–16):2009–2009
Wang Y-Z, Li D-Y, He X-W, Li W-Y, Zhang Y-K (2015) Epitope imprinted polymer nanoparticles containing fluorescent quantum dots for specific recognition of human serum albumin. Microchim Acta 182(7–8):1465–1472
Wei X, Zhou Z, Hao T, Xu Y, Li H, Lu K, Dai J, Zheng X, Gao L, Wang J (2015) Specific recognition and fluorescent determination of aspirin by using core-shell CdTe quantum dot-imprinted polymers. Microchim Acta 182(7–8):1527–1534
Bates F, del Valle M (2015) Voltammetric sensor for theophylline using sol–gel immobilized molecularly imprinted polymer particles. Microchim Acta 182(5–6):933–942
Tan L, He R, Chen K, Peng R, Huang C, Yang R, Tang Y (2016) Ultra-high performance liquid chromatography combined with mass spectrometry for determination of aflatoxins using dummy molecularly imprinted polymers deposited on silica-coated magnetic nanoparticles. Microchim Acta 183(4):1469–1477
Li S, Wu X, Zhang Q, Li P (2016) Synergetic dual recognition and separation of the fungicide carbendazim by using magnetic nanoparticles carrying a molecularly imprinted polymer and immobilized β-cyclodextrin. Microchim Acta 183(4):1433–1439
Yang G, He Z, Liu X, Liu C, Zhan J, Liu D, Wang P, Zhou Z (2016) Polymer-coated magnetic nanospheres for preconcentration of organochlorine and pyrethroid pesticides prior to their determination by gas chromatography with electron capture detection. Microchim Acta 183(3):1187–1194
Li L, Lin Z-z, X-m C, H-y Z, Lin Y-d, Z-z L, Z-y H (2015) Molecularly imprinted polymers for extraction of malachite green from fish samples prior to its determination by HPLC. Microchim Acta 182(9–10):1791–1796
Bures P, Huang Y, Oral E, Peppas NA (2001) Surface modifications and molecular imprinting of polymers in medical and pharmaceutical applications. J Control Release 72(1):25–33
Du B, Qu T, Chen Z, Cao X, Han S, Shen G, Wang L (2014) A novel restricted access material combined to molecularly imprinted polymers for selective solid-phase extraction and high performance liquid chromatography determination of 2-methoxyestradiol in plasma samples. Talanta 129:465–472
He J, Song L, Chen S, Li Y, Wei H, Zhao D, Gu K, Zhang S (2015) Novel restricted access materials combined to molecularly imprinted polymers for selective solid-phase extraction of organophosphorus pesticides from honey. Food Chem 187:331–337
Santos MG, Moraes GOI, Nakamura MG, dos Santos-Neto ÁJ, Figueiredo EC (2015) Restricted access molecularly imprinted polymers obtained by bovine serum albumin and/or hydrophilic monomers’ external layers: a comparison related to physical and chemical properties. Analyst 140(22):7768–7775
Souza ID, Melo LP, Jardim IC, Monteiro JC, Nakano AMS, Queiroz MEC (2016) Selective molecularly imprinted polymer combined with restricted access material for in-tube SPME/UHPLC-MS/MS of parabens in breast milk samples. Anal Chim Acta 932:49–59
Moraes GOI, da Silva LMR, dos Santos-Neto ÁJ, Florenzano FH, Figueiredo EC (2013) A new restricted access molecularly imprinted polymer capped with albumin for direct extraction of drugs from biological matrices: the case of chlorpromazine in human plasma. Anal Bioanal Chem 405(24):7687–7696
Sambe H, Hoshina K, Haginaka J (2007) Molecularly imprinted polymers for triazine herbicides prepared by multi-step swelling and polymerization method: their application to the determination of methylthiotriazine herbicides in river water. J Chromatogr A 1152(1):130–137
Manesiotis P, Borrelli C, Aureliano CS, Svensson C, Sellergren B (2009) Water-compatible imprinted polymers for selective depletion of riboflavine from beverages. J Mater Chem 19(34):6185–6193
Arabi M, Ghaedi M, Ostovan A (2016) Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chem 210:78–84
Zhu R, Zhao W, Zhai M, Wei F, Cai Z, Na Sheng QH (2010) Molecularly imprinted layer-coated silica nanoparticles for selective solid-phase extraction of bisphenol a from chemical cleansing and cosmetics samples. Anal Chim Acta 658:209–216
Atakay M, Omr ÇÇ, Salih B (2012) Amine-functionalized sol–gel-based lab-in-a-pipet-tip approach for the fast enrichment and specific purification of phosphopeptides in MALDI-MS applications. Anal Chem 84(6):2713–2720
da Costa Silva RG, Augusto F (2006) Sol–gel molecular imprinted ormosil for solid-phase extraction of methylxanthines. J Chromatogr A 1114(2):216–223
Mehdinia A, Kayyal TB, Jabbari A, Ovais M, Aziz-Zanjani ZE (2013) Magnetic molecularly imprinted nanoparticles based on grafting polymerization for selective detection of 4-nitrophenol in aqueous samples. J Chromatogr A 1283:82–88
Ebrahimzadeh H, Dehghani Z, Asgharinezhad AA, Shekari N, Molaei K (2013) Determination of haloperidol in biological samples using molecular imprinted polymer nanoparticles followed by HPLC-DAD detection. Int J Pharm 453(2):601–609
Arabi M, Ostovan A, Ghaedi M, Purkait MK (2016) Novel strategy for synthesis of magnetic dummy molecularly imprinted nanoparticles based on functionalized silica as an efficient sorbent for the determination of acrylamide in potato chips: optimization by experimental design methodology. Talanta 154:526–532. doi:10.1016/j.talanta.2016.04.010
Committee AM (1987) Recommendations for the definition, estimation and use of the detection limit. Analyst 112(2):199–204
Toulabi P, Daneshfar A, Sahrai R (2010) Determination of hippuric acid in biological fluids using single drop liquid–liquid-liquid microextraction. Anal Methods 2(5):564–569
Penner N, Ramanathan R, Zgoda-Pols J, Chowdhury S (2010) Quantitative determination of hippuric and benzoic acids in urine by LC–MS/MS using surrogate standards. J Pharm Biomed Anal 52(4):534–543
Ohashi Y, Mamiya T, Mitani K, Wang B, Takigawa T, Kira S, Kataoka H (2006) Simultaneous determination of urinary hippuric acid, o-, m-and p-methylhippuric acids, mandelic acid and phenylglyoxylic acid for biomonitoring of volatile organic compounds by gas chromatography–mass spectrometry. Anal Chim Acta 566(2):167–171
Zinalibdin MR, Yacob AR, Sanagi MM (2015) A novel molecularly imprinted acrylonitrile-butadiene-styrene membrane for adsorption of hippuric acid. Procedia Chem 16:91–98
Ahmadi F, Asgharloo H, Sadeghi S, Gharehbagh-Aghababa V, Adibi H (2009) Post-derivatization procedure for determination of hippuric acid after extraction by an automated micro solid phase extraction system and monitoring by gas chromatography. J Chromatogr B 877(27):2945–2951
Wang JZ, Lu XY, Zhao NP, Cheng YY, Zeng S (2007) Simultaneous determination of phenylglyoxylic acid, mandelic acid, styrene glycol and hippuric acid in primary culture of rat hepatocytes incubate by high-performance liquid chromatography. Biomed Chromatogr 21(5):497–501
Zuppi C, Rossetti DV, Vitali A, Vincenzoni F, Giardina B, Castagnola M, Messana I (2003) Determination of urinary hippuric acid by micellar electrokinetic capillary chromatography. J Chromatogr B 793(2):223–228
Choi Y-B, Kim N-H, Kim S-H, Tae G-S, Kim H-H (2014) Heterogeneous electrochemical immunoassay of hippuric acid on the electrodeposited organic films. Sensors 14(10):18886–18897
Jeon WY, Choi YB, Kim HH (2013) Homogeneous electrochemical detection of hippuric acid in urine based on the osmium–antigen conjugate. Chem Phys Chem 14(10):2331–2337
Hao J-N, Yan B (2015) Recyclable lanthanide-functionalized MOF hybrids to determine hippuric acid in urine as a biological index of toluene exposure. Chem Commun 51(77):14509–14512
Acknowledgements
The authors express their appreciation to the Graduate School and Research Council of Yasouj University for their financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 2735 kb)
Rights and permissions
About this article
Cite this article
Arabi, M., Ghaedi, M. & Ostovan, A. Water compatible molecularly imprinted nanoparticles as a restricted access material for extraction of hippuric acid, a biological indicator of toluene exposure, from human urine. Microchim Acta 184, 879–887 (2017). https://doi.org/10.1007/s00604-016-2063-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2063-5