Abstract
Magnetic polymer nanospheres were prepared and used as adsorbents for the extraction of organochlorine and pyrethroid pesticides from water samples. The adsorbents were synthesized by miniemulsion polymerization of N-vinylimidazole and divinylbenzene and simultaneous encapsulation of oleic acid-coated Fe3O4 nanoparticles. Following desorption with ethyl acetate, the target analytes β-hexachlorocyclohexane, δ-hexachlorocyclohexane, p,p’-DDE, heptachlor, trans-chlordan, cis-chlordan, bifenthrin, β-cypermethrin, δ-methrin, λ-cyhalothrin and esfenvalerate were determined by gas chromatography with electron capture detection. Desorption conditions, extraction times and sample volume were screened by Plackett-Burman design and optimized by Box-Behnken design. Under the optimum conditions, the organochlorines can be quantified in the 20 to 400 ng L−1 concentration range, and the pyrethroids in the 400 to 4000 ng L−1 concentration range. The recoveries of organochlorines and pyrethroids from spiked real water samples are between 77.6 and 97.3 %, with relative standard deviations between 0.9 and 10.0 %. The method for magnetic solid phase extraction described here is fast, simple and friendly to the environment.

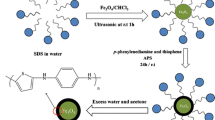
Fe3O4 nanoparticles coated with oleic acid (OA) were covered with a copolymer prepared from N-vinylimidazole (VI) and divinylbenzene (DB), and then used for magnetic solid phase extraction of organochlorine and pyrethroid pesticides. Recoveries from spiked water samples range from 77.6 to 97.3 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs) such as hexachlorocyclohexanes (HCHs), DDTs, aldrin, dieldrin had been widely used all over the word, well known as the persistence and significant magnification in food chain [1]. Due to their chemical and physical stability, the residue of OCPs in the environment matrices can last for years or even decades [2]. Although OCPs were prohibited since 1970s in many countries, they can still be widely detected in various kinds of environmental matrices, including water [3], soil [4], animals [5] and plants [6]. OCPs can transport globally because of the grasshopper effect. Water can be contaminated by OCPs by deposition, leaching or rainfall runoff [7], and which are frequently detected in many kinds of waters [8, 9]. Zhang H [10] et al. detected the OCPs in Singapore’s coastal waters and the concentrations of analyzed OCPs ranged from 2.2 to 455 pg L−1. Derbalah A [11] et al. monitored 10 kinds of organochlorine pesticides in drinking water. The result showed the concentrations of the analyzed OCPs were under 1 μg L−1 and lindane was the highest detected compound.
Pyrethroids are kinds of bionic pesticides which are synthesized derivates of pyrethrin [12]. Because of the broad spectrum and high activity, pyrethroids have been widely used in agriculture as well as household [13] since 1970s. Pyrethroids have caused contamination for the environment for the wide use throughout the world. A lot of works have been conducted for the residue analysis of pyrethroids and contamination monitoring. Feo M L [14] et al. measured the concentrations of pyrethroid insecticides in surface water of the Ebro River Delta. The results showed cypermethrin was detected in most water samples at concentrations ranging from 0.73 to 57.2 ng L−1. Hladik M L [15] et al. detected pyrethroids in American urban creek water and the concentrations of the detected targets ranged from 5.4 to 15 ng L−1.
European Union Directive 98/83/EC stipulated that the maximum level for each individual pesticide in drinking water is 0.10 μg L−1, and the maximum level for total pesticides is 0.50 μg L−1. Effective and environmentally friendly pretreatment and determination methods are needed for the trace amount of pesticides in water.
Varieties of pretreatment techniques have been developed to meet the requirements of complex environmental matrices, such as accelerated solvent extraction (ASE) [16], dispersive liquid-liquid microextraction (DLLME) [17], solid phase microextraction (SPME) [18], matrix solid phase dispersion (MSPD) [19], magnetic solid-phase extraction (MSPE) and so on. MSPE is a pretreatment technique in which magnetic or magnetizable adsorbents were used to achieve the separation and preconcentration of target analytes from large volumes of solution. It was first introduced by Safarikova and Safarik at 1999, and then used for the determination of organic dyes in water [20]. In MSPE procedure, the adsorbent is completely dispersed in the sample solution, instead of being packed into the SPE cartridge [21] which increases the rate of diffusion and mass transfer of the analytes [22]. After the analytes are adsorbed onto the adsorbent, an external magnetic field is used to achieve the phase separation without laborious procedures like centrifugations or filtrations. Most magnetic nanoparticles can be easily recycled by a simple washing operation [23]. However, MSPE is a pretreatment technique without cleaning step and can be only used for aqueous samples.
Developing valid magnetic adsorbents is a key point of MSPE. Procedures applied in preparing SPME materials can also be used in forming MSPE materials. Aziz-Zanjani M O [24] et al. reviewed the state of the art in methods for the preparation of SPME materials. The coating procedures mainly include dipping and physical agglutinating methods, Sol–gel technology, chemical grafting, electrochemical methods, electrospinning, liquid-phase deposition, and hydrothermal method.
Polymer is widely used to fabricate hybrid nanocomposite. He Z [21] et al. developed a hydrophilic–lipophilic balanced magnetic material, which was used to extract two kinds of pesticides with opposite hydrophily. Huang X [25] et al. has synthesized magnetic polymer nanoparticles, using N-vinylimidazole and divinylbenzene (1:3 w/w) as monomers for fluoroquinolones extraction. In addition, molecularly imprinted polymer and rattle-type nanoparticles have been developed and used in these years. Molecularly imprinted polymer is a kind of intelligent polymer which was first used as MSPE material in 2007 [26]. These materials are widely used in extracting dyes [27], peptides [28], pesticides [29] and so on [30, 31]. Rattle-type polymer nanoparticle is a kind of low density material with a void space between the core and the shell. It also can be used as a adsorbent to remove organic dyestuff in water [32]. Besides polymer, other materials or even use-patterns make magnetic nanoparticles more applicative. Magnetic nanoparticles physically adsorb oleate can be used as MSPE adsorbents in the preconcentration of polychlorinated biphenyls [33]. After triazine herbicides adsorbed by supramolecular solvent, Fe3O4 nanoparticles can be added to accelerate the phase separation process for the solid phase extraction [34].
A magnetic polymer nanosphere was designed and synthesized. N-vinylimidazole and divinylbenzene were used as functional monomer and structural monomer. Miniemulsion polymerization was employed to encapsulate Fe3O4 nanoparticles. The prepared magnetic polymer nanosphere was used as a MSPE adsorbent to extract organochlorine and pyrethroid pesticides in water sample. Organochlorine and pyrethroid pesticides were determined by gas chromatography with electron capture detector (ECD). The factors affecting the MSPE procedure were optimized. Plackett–Burman design was used to screen the influencing factors and Box–Behnken design was used for optimization.
Experimental
Chemicals and reagents
FeCl2 · 4H2O, FeCl3 · 6H2O, ammonium hydroxide (25 %, w/w), sodium dodecyl sulfate (SDS), n-hexadecane (HD), oleic acid (OA), hydroxyethyl cellulose (HEC), azodiisobutyronitrile (AIBN), polyvinylpyrrolidone (PVP), n-hexane, acetone, methanol, ethyl acetate, methylene chloride, acetonitrile were analytical grade and purchased from Beijing Chemical Reagents Company (Beijing, China; http://www.bjgqhg.com.cn/). HLPC-grade isooctane was purchased from Fisher Scientific (USA; http://www.thermo.com.cn/). Divinylbenzene 80 % (DB) and N-vinylimidazole 99 % (VI) were purchased from Aladdin Industrial Corporation (Shanghai, China; http://aladdin.company.lookchem.cn/).
Respective standard stock solutions (100 mg L−1) of six OCPs (beta-HCH, delta-HCH, heptachlor, trans-chlordane, cis-chlordane and p,p’-DDE) were obtained from J&K Scientific Ltd. (Beijing, China; http://www.jkchemical.com/). The working standard solutions were prepared by appropriate dilution of the above standard solution in analytical grade n-hexane to suitable concentration levels. Bifenthrin (99 %), beta-cypermethrin (99 %), lambda-cyhalothrin (99 %), esfenvalerate (99 %), deltamethrin (99 %) were obtained from Agricultural Environmental Protection Institution (Tianjin, China; http://www.aepi.org.cn/sites/iapmoa/). Stock solution of each standard was prepared by dissolving the substances in analytical grade n-hexane to suitable concentration levels. All of the standard solutions were stored at 4 °C in dark.
Ultrapure water was prepared by Milli-Q water purification system (Millipore, Billerica, MA, USA; http://www.merckmillipore.com/). It was confirmed there were no any target compounds.
Instruments
OCPs and pyrethroids were analyzed on an Agilent-7890A gas chromatography equipped with electron capture detector (GC–ECD). Chromatographic separation was accomplished with an HP-5 fused silica capillary column (30 m × 0.32 mm × 0. 25 μm; Agilent; http://www.agilent.com/home). The temperature of injector and detector was set at 270 and 300 °C, respectively. Ultrapure nitrogen was used as carrier gas with a flow rate of 1 mL min−1. The oven temperature started at 90 °C for 1 min, raised to 180 °C at a rate of 15 °C min−1 and held for 5 min, raised to 220 °C at 5 °C min−1, raised to 250 °C at 2 °C min−1, and then to 270 °C at 4 °C min−1 and held for 5 min.
Characterization of magnetic polymer nanospheres was carried out by a transmission electron microscopy (Tecnai F30 transmission electron microscopy, USA; http://www.fei.com/) and a fourier infrared spectrometer (Perkin-Elmer, Inc. CA, USA; http://www.perkinelmer.com.cn/).
Synthesis of magnetic polymer nanospheres
Synthesis of oleic acid coated Fe3O4 nanoparticles
Oleic acid coated Fe3O4 (OA-Fe3O4) nanoparticles were prepared by an improved co-precipitation method [35]. Exactly 10.8 g of FeCl3 · 6H2O, 3.98 g of FeCl2 · 4H2O were dissolved in 200 mL of degassed deionized water with vigorous stirring. Then 25 mL of acetone with 4.0 g of oleic acid was added into the above mentioned solution. After being stirred for 30 min, 25 mL of ammonium hydroxide (25 %, w/w) was dropwise added. The three-neck flask was placed in an 85 °C oil bath for 1 h. At last, the reaction system was cooled down to room temperature and the pH was adjusted to 2. The OA-Fe3O4 nanoparticals were separated from the suspension by magnetic decantation and washed with 200 mL of ultrapure water three times. The particles were vacuum dried at 60 °C for 24 h.
Synthesis of magnetic polymer nanospheres
Magnetic polymer nanospheres were prepared by a modified miniemulsion polymerization reported by Shulai L and Forcada J [35]. Firstly, 0.42 g of SDS, 0.7 g of PVP and 0.14 g of HEC were dissolved into 60 mL of water. Secondly, 0.7 g of OA-Fe3O4 nanoparticles, 3.98 g of DB, 2.32 g of VI and 0.5 g of AIBN were homogeneously mixed and added into the above-mentioned aqueous phase. After mixing, vigorous stirring and ultrasound were used for miniemulsion. After 10 min, the three-neck flask was placed in a 70 °C oil bath for 24 h. At last, the final magnetic material was washed with ultrapure water, acetone, methanol, ethyl acetate, methylene chloride, acetonitrile three times respectively. The washed magnetic polymer nanospheres were vacuum dried at 60 °C for 24 h.
MSPE procedure
Under optimized conditions, the MSPE was performed as follows. After activated by 50 μL of methanol, 50 mg of magnetic adsorbent was dispersed in 50 mL of water sample (15 % NaCl) with ultrasonic irradiation and vigorous stirring. After 30 min, external magnet field was used to isolate magnetic adsorbent from the suspension. The solution was decanted and the adsorbent was moved to a centrifuge tube (5 mL). A syringe was employed to remove the residue water after the magnetic adsorbent aggregated in the centrifuge tube. After removing the water, 0.5 mL of methanol was used to elute the target analytes first, then the magnetic adsorbent was eluted by 1 mL of ethyl acetate with vortexing for 0.5 min twice. The eluants were combined in a centrifuge tube (5 mL) for evaporation to dryness with mild nitrogen stream at 35 °C. The residues were redissolved in 200 μL of isooctane and filtered through a PTEE filter (0.22 μm) before GC analysis. The filtrate was placed in a 200 μL-insert before GC analysis.
Preparation of real water samples
Environmental reservoir and river water samples were analyzed. Two kinds of reservoir water were collected from Miyun reservoir (Beijing) and Shangzhuang reservoir (Beijing). River water was collected from Xiaoqinghe river (Beijing). All the samples were vacuum-filtered through medium-speed qualitative filter papers and stored in dark containers at 4 °C.
Results and discussion
Synthesis strategy
Miniemulsion is aqueous dispersion system which contains oil, water, emulsifier, costabilizer, hydrophobe, surfactant and initiator. By the application of high shear forces, a relatively stable system with small and narrowly distributed miniemulsion droplets is formed. After reaching certain reaction temperature, polymerization will happen in these droplets. Miniemulsion polymerization is a conventional method to encapsulate magnetic particles inside polymers. It is a supplement of the above mentioned coating method for
Divinylbenzene is a common crosslinking agent which can be used as a hydrophobe. N-vinylimidazole is a hydrophilic monomer and can be dispersed in DB to constitute oil phase. However, Fe3O4 nanoparticles are either hydrophilic or hydrophobic and they cannot be directly dispersed in DB. Oleic acid is the most used unsaturated carboxylic acid which can make the surface of Fe3O4 nanoparticles hydrophobic and helps Fe3O4 nanoparticles disperse in DB.
Huang X [25] et al. synthesized magnetic silica particles using sol–gel polymerization and based on which magnetic polymer nanoparticles were prepared using N-vinylimidazole and divinylbenzene (1:3 w/w) as monomers via reflux-precipitation polymerization. The material was used as adsorbent to concentrate fluoroquinolones which were lyophobic compounds in water. Compared with Huang’s work, oleic acid coated Fe3O4 nanoparticles were synthesized and miniemulsion polymerization was used to form core-shell structure materials. Furthermore, the ratio of N-vinylimidazole and divinylbenzene was different. The materials synthesized were applied to adsorb hydrophobic pesticides.
N-vinylpyridine as a hydrophilic precursor monomer has also been tried to design another nanospheres. However, the self-polymeric reaction of N-vinylpyridine happened in the mixing process before the miniemulsion polymerization showing N-vinylpyridine cannot be used in the miniemulsion polymerizations.
Characterization of magnetic polymer nanospheres
The functional groups were identified by FT-IR spectra, and the size and morphological features of the prepared nanospheres were determined by transmission electron microscopy (TEM).
The FT-IR spectra of the nanospheres was shown in Fig. 1. The peaks at 570 cm−1 (Fe-O-Fe), 3450 cm−1 (O-H) indicated Fe3O4 was successfully encapsulated. The absorption peaks at 2924 and 2853 cm−1 were typical stretching vibrations of CH2 groups. The stretching vibration of C = N was at 1598 cm−1, which indicated the existence of imidazole groups. The peaks at 1400–1500 and 800–900 cm−1 were contributed to the benzene ring. The FT-IR spectra results suggested that the polymer was successfully coated on Fe3O4 nanoparticles.
The TEM images of the nanospheres were shown in Fig. 2. The nanosphere seemed like an egg cell with diameter of about 100 nm and unsmooth surface (Fig. 2b).
Optimization of method
Copolymer based on N-vinylimidazole–divinylbenzene combines hydrophilic monomers and hydrophobic monomers. This feature made the copolymer easily disperse in water and have interactions with hydrophobic compound. Organochlorine and pyrethroid were selected as model compounds for the microextraction Fig. 3.
GC chromatograms of the OCPs spiked at 400 ng L−1 and for the pyrethroids at 4000 ng L−1. Chromatographic peaks: (1) beta-HCH, (2) delta-HCH, (3) heptachlor, (4) trans-chlordane, (5) cis-chlordane, (6) p,p’-DDE, (7) bifenthrin, (8) lambda-cyhalothrin, (9) beta-cypermethrin, (10) esfenvalerate, (11) deltamethrin
In order to evaluate the feasibility of the method , the following parameters were screened and optimized: (a) Eluting solvent; (b) NaCl concentration; (c) Sample volume; (d) Extraction time; (e) Volume of desorption solvent; (f) Desorption time. Respective data and figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: Eluting solvent was ethyl acetate; NaCl concentration was 15 %; sample volume was 50 mL; extraction time was 30 min.
Method validation
Under the optimal conditions the method was validated by linearity, recoveries, repeatability and limits of quantitation.
Milli-Q water was used for method validation. The results of method validation were listed in Table 3. Calibration curves for all the OCPs were in the range of 20–400 ng L−1 (20, 100, 200, 300 and 400 ng L−1) with correlation coefficients ranging from 0.9925 to 0.9994. The limit of quantitation (LOQ) of the OCPs ranged from 4.0 to 29.4 ng L−1 calculated at a signal-to-noise ratio of 10.
Good linearities were obtained for the pyrethroids in the range of 400–20,000 ng L−1 (400, 4000, 1000, 14,000 and 20,000 ng L−1) with correlation coefficients ranging from 0.9930 to 0.9996. The LOQs for the pyrethroids ranged from 41.6 to 190.5 ng L−1 Table 1.
Application to real water
Three kinds of environmental water samples were selected and used to validate the method. First, blank environmental water samples were analyzed at the optimal conditions to make sure free of the target analytes. The chromatograms of three water samples showed no target analyte residues. The water samples were spiked with the OCPs at concentration levels of 100 and 400 ng L−1 and with the pyrethroids at 400 and 4000 ng L−1. Recoveries and relative standard deviations were investigated. The results were listed in Table 3 showing the recoveries were between 77.6 and 97.3 %, and the relative standard deviations were between 0.9 and 10.0 %. The results showed that the MSPE method can be successfully applied for the analysis of real samples Table 2.
Comparison of the MSPE method with other methods
The analytical method was compared with other methods (Table 3). This method determined 11 kinds of hydrophobic pesticides simultaneously. The LOQs of the presented method is comparable, and the consumption of solvent is relatively lower.
Conclusions
Magnetic polymer nanospheres has been synthesized and used in the MSPE for OPCs and pyrethroids in the environment water. NaCl concentration, sample volume and extraction time are most important for this adsorbents in the extraction of hydrophobic compounds in aqueous sample. The method has comparable LODs and recoveries. However, the RSD indicated the stability and accuracy of the method are relatively poor. This adsorbents have a potential application in practical samples, such as beverage, vegetable juice, fruit juice. Generally speaking, this method is simple, sensitive and environmentally friendly.
References
Borga K, Gabrielsen GW, Skaare JU (2001) Biomagnification of organochlorines along a Barents Sea food chain. Environ Pollut 113(2):187–198
Longanathan BG, Kannan K (1994) Global oganochlorine contamination trends: an overview. Ambio 23(3):187–191
Zhou R, Zhu L, Yang K, Chen Y (2006) Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. J Hazard Mater 137(1):68–75
X-h L, Wang W, Wang J, Cao X-l, Wang X-f, Liu J-c, Liu X-f, Xu X-b, Jiang X-n (2008) Contamination of soils with organochlorine pesticides in urban parks in Beijing, China. Chemosphere 70(9):1660–1668
Berzas Nevado JJ, Rodriguez Martin-Doimeadios RC, Guzman Bernardo FJ, Rodriguez Farinas N, Gonzalez Cogolludo JM, Castro Osma JA (2010) Multiresidue determination of organochlorines in fish oil by GC MS: a new strategy in the sample preparation. Talanta 81(3):887–893
Li J, Liu D, Wu T, Zhao W, Zhou Z, Wang P (2014) A simplified procedure for the determination of organochlorine pesticides and polychlorobiphenyls in edible vegetable oils. Food Chem 151:47–52
Yu Y, Li Y, Shen Z, Yang Z, Mo L, Kong Y, Lou I (2014) Occurrence and possible sources of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) along the Chao River, China. Chemosphere 114:136–143
Grung M, Lin Y, Zhang H, Steen AO, Huang J, Zhang G, Larssen T (2015) Pesticide levels and environmental risk in aquatic environments in China - A review. Environ Int 81:87–97
Yadav IC, Devi NL, Syed JH, Cheng Z, Li J, Zhang G, Jones KC (2015) Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: a comprehensive review of India. Sci Total Environ 511:123–137
Zhang H, Bayen S, Kelly BC (2015) Multi-residue analysis of legacy POPs and emerging organic contaminants in Singapore’s coastal waters using gas chromatography-triple quadrupole tandem mass spectrometry. Sci Total Environ 523:219–232
Derbalah A, Ismail A, Hamza A, Shaheen S (2014) Monitoring and remediation of organochlorine residues in water. Water Environ Res 86(7):584–593
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann Rev of Entomol: 45–66
Feo ML, Eljarrat E, Barcelo D (2010) A rapid and sensitive analytical method for the determination of 14 pyrethroids in water samples. J Chromatogr A 1217(15):2248–2253
Feo ML, Ginebreda A, Eljarrat E, Barcelo D (2010) Presence of pyrethroid pesticides in water and sediments of Ebro River Delta. J Hydrol 393(3–4):156–162
Hladik ML, Kuivila KM (2009) Assessing the occurrence and distribution of pyrethroids in water and suspended sediments. J Agric Food Chem 57(19):9079–9085
Richter BE, Jones BA, Ezzell JL, Porter NL, Avdalovic N, Pohl C (1996) Accelerated solvent extraction: a technique for sample preparation. Anal Chem 68(6):1033–1039
Herrera-Herrera AV, Asensio-Ramos M, Hernandez-Borges J, Rodriguez-Delgado MA (2010) Dispersive liquid-liquid microextraction for determination of organic analytes. Trac-Trends Anal Chem 29(7):728–751
Beltran J, Lopez FJ, Hernandez F (2000) Solid-phase microextraction in pesticide residue analysis. J Chromatogr A 885(1–2):389–404
Capriotti AL, Cavaliere C, Lagana A, Piovesana S, Samperi R (2013) Recent trends in matrix solid-phase dispersion. Trac-Trends Anal Chem 43:53–66
Safarikova M, Safarik I (1999) Magnetic solid-phase extraction. J Magn Magn Mater 194(1–3):108–112
He Z, Wang P, Liu D, Zhou Z (2014) Hydrophilic-lipophilic balanced magnetic nanoparticles: preparation and application in magnetic solid-phase extraction of organochlorine pesticides and triazine herbicides in environmental water samples. Talanta 127:1–8
Gao Q, Zheng H-B, Luo D, Ding J, Feng Y-Q (2012) Facile synthesis of magnetic one-dimensional polyaniline and its application in magnetic solid phase extraction for fluoroquinolones in honey samples. Anal Chim Acta 720:57–62
Yu X, Sun Y, Jiang C, Sun X, Gao Y, Wang Y, Zhang H, Song D (2012) Magnetic solid-phase extraction of five pyrethroids from environmental water samples followed by ultrafast liquid chromatography analysis. Talanta 98:257–264
Aziz-Zanjani MO, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta 181(11–12):1169–1190
Huang X, Wang Y, Liu Y, Yuan D (2013) Preparation of magnetic poly(vinylimidazole-co-divinylbenzene) nanoparticles and their application in the trace analysis of fluoroquinolones in environmental water samples. J Sep Sci 36(19):3210–3219
Yan S, Gao Z, Fang Y, Cheng Y, Zhou H, Wang H (2007) Characterization and quality assessment of binding properties of malachite green molecularly imprinted polymers prepared by precipitation polymerization in acetonitrile. Dyes Pigments 74:572–577
Huang B, Zhou X, Chen J, Wu G, Lu X (2015) Determination of malachite green in fish based on magnetic molecularly imprinted polymer extraction followed by electrochemiluminescence. Talanta 142:228–234
Wang C, Hu X, Guan P, Qian L, Wu D, Li J (2015) Superparamagnetic molecularly imprinting polymers for adsorbent and separation pentapeptides by surface ATRP. Sep Sci Technol 50(12):1768–1775
Zuo HG, Zhu JX, Zhan CR, Shi L, Xing M, Guo P, Ding Y, Yang H (2015) Preparation of malathion MIP-SPE and its application in environmental analysis. Environ Monit Assess 187(7)
Lin S, Gan N, Zhang J, Chen X, Cao Y, Li T (2015) A novel reductive graphene oxide-based magnetic molecularly imprinted poly (ethylene-co-vinyl alcohol) polymers for the enrichment and determination of polychlorinated biphenyls in fish samples. J Mol Recog 28(6):359–368
Chen F, Zhang J, Wang M, Kong J (2015) Magnetic molecularly imprinted polymers synthesized by surface-initiated reversible addition-fragmentation chain transfer polymerization for the enrichment and determination of synthetic estrogens in aqueous solution. J Sep Sci 38(15):2670–2676
Shao Y, Zhou L, Bao C, Ma J (2015) A facile approach to the fabrication of rattle-type magnetic carbon nanospheres for removal of methylene blue in water. Carbon 89:378–391
Pérez RA, Albero B, Tadeo JL, Sánchez-Brunete C (2015) Oleate functionalized magnetic nanoparticles as sorbent for the analysis of polychlorinated biphenyls in juices. Microchimica Acta
Safari M, Yamini Y, Tahmasebi E, Ebrahimpour B (2015) Magnetic nanoparticle assisted supramolecular solvent extraction of triazine herbicides prior to their determination by HPLC with UV detection. Microchimica Acta
Shulai L, Forcada J (2006) Preparation and characterization of magnetic polymeric composite particles by miniemulsion polymerization. J Polym Sci A Polym Chem 44(13):4187–4203
Li C, Chen L (2013) Determination of pyrethroid pesticides in environmental waters based on magnetic titanium dioxide nanoparticles extraction followed by HPLC analysis. Chromatographia 76(7–8):409–417
Fang G, Chen W, Yao Y, Wang J, Qin J, Wang S (2012) Multi-residue determination of organophosphorus and organochlorine pesticides in environmental samples using solid-phase extraction with cigarette filter followed by gas chromatography–mass spectrometry. J Sep Sci 35(4):534–540
Ozcan S, Tor A, Aydin ME (2012) Application of magnetic nanoparticles to residue analysis of organochlorine pesticides in water samples by GC/MS. J Aoac Int 95(5):1343–1349(1347)
Acknowledgments
Supported by the National Natural Science Foundation of China (J1210064,21277171,21307155), Chinese Universities Scientific Fund 2015LX005 and Beijing Nova program YETP0323.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1032 kb)
Rights and permissions
About this article
Cite this article
Yang, G., He, Z., Liu, X. et al. Polymer-coated magnetic nanospheres for preconcentration of organochlorine and pyrethroid pesticides prior to their determination by gas chromatography with electron capture detection. Microchim Acta 183, 1187–1194 (2016). https://doi.org/10.1007/s00604-015-1725-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1725-z