Abstract
We on report an eco-friendly molecularly imprinted material based on carbon dots (C-dots) via a facile and efficient sol–gel polymerization for selective fluorescence detection of 4-nitrophenol (4-NP). The amino-modified C-dots were firstly synthesized by a hydrothermal process using citric acid as the carbon source and poly(ethyleneimine) as the surface modifier, and then after a sol–gel molecular imprinting process, the molecularly imprinted fluorescence material was obtained. The material (MIP-C-dots) showed strong fluorescence from C-dots and high selectivity due to the presence of a molecular imprint. After the detection conditions were optimized, the relative fluorescence intensity (F0/F) of MIP-C-dots presented a good linearity with 4-NP concentrations in the linear range of 0.2 − 50 μmol L-1 with a detection limit (3σ/k) of 0.06 μmol L-1. In addition, the correlation coefficient was 0.9978 and the imprinting factor was 2.76. The method was applicable to the determination of trace 4-NP in Yangtze River water samples and good recoveries from 92.6–107.3 % were obtained. The present study provides a general strategy to fabricate materials based on C-dots with good fluorescence property for selective fluorescence detection of organic pollutants.

An eco-friendly molecularly imprinted fluorescence sensor based on carbon dots (C-dots) (poly(ethyleneimine) (PEI) as the surface modifier) was prepared via a facile and efficient sol–gel polymerization (3-aminopropyltriethoxysilane (APTES) as the functional monomer and tetramethoxysilane (TEOS) as the cross linker) for selective fluorescence detection of 4-nitrophenol (4-NP).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
4-Nitrophenol (4-NP) has been listed by the U.S. Environmental Protection Agency (U.S.EPA) as the priority environmental pollutant due to its wide use in agriculture and drug production [1, 2]. Thus, developing effective analytical methods for the detection of the 4-NP is very important. Until now, many traditional detection methods, such as electrophoresis and electrochemical methods, [3] chromatographic techniques [4] and high performance liquid chromatography (HPLC) [5] have been applied to detect trace 4-NP in real samples. However, these methods suffer from expensive reagents, time consuming, tedious sample pretreatment and possible production of secondary pollutants. Therefore, it is still a challenge to develop a simple, rapid and selective method for the determination of the 4-NP in real samples.
Quantum dots (QDs) have attracted much attention in the scientific community because of their unique properties, such as good photostability, narrow emission spectra and broad absorption spectrum [6, 7]. However, most traditional QDs contain heavy metals, such as Cd, and their applications are thus limited due to the toxicity and potential environmental risk of the heavy metals. As one type of QD substitutes, carbon dots (C-dots) were discovered in single-walled carbon nanotubes in 2004 [8]. Compared with the traditional QDs, C-dots exhibit advantages, including low toxicity, excellent photostability, easy preparation, and good water solubility [9–11]. Substantial research has been reported on analytical and detecting applications of C-dots based on the excellent fluorescence property. Lu et al. used pomelo peel as the carbon source to synthesize C-dots by hydrothermal treatment for the detection of Cu2+ [12]. Huang et al. prepared a new C-dots material by a one-pot microwave-assisted hydrothermal treatment using histidine as the carbon source for the detection of Fe3+ [13]. Gao et al. used water-soluble amino-functionalized C-dots as a fluorescence probe for the detection of Hg2+ in aqueous solution [14]. However, fluorescent detection based on QDs often confronts with the interference of the coexisting substances. A feasible method to enhance the selectivity of fluorescent detection is the use of molecular imprinting technology (MIT) [15].
MIT is a versatile and well-established strategy to obtain three-dimensional cross-linked polymers with tailor-made recognition sites [16]. The resulting molecularly imprinted polymers (MIPs) are prepared by the polymerization of functional monomers, cross-linkers and initiators in the presence of the template molecules [17]. Subsequently, the removal of template molecules from the three-dimensional cross-linked polymers leaves behind specific recognition sites with complementarity to the original template [18]. Due to the good stability, high selectivity and satisfactory practicability, MIPs have been widely used in many applications, including drug delivery and controlled release, [19] extraction and separation, [20] chemical material [21] and catalysis [22]. Recently, many effective MIPs-based QDs materials have been reported, which combined the advantages of high selectivity of MIPs and high sensitivity of fluorescence detecting from QDs [23]. We reported previously on the use of MIPs on Mn-doped ZnS QDs for selective fluorescence detection of 2,4,5-trichlorophenol, 2,6-dichlorophenol and 2,4-dichlorophenol, [24–26] and the use of MIPs on Octadecyl-4-vinylbenzyl-dimethyl-ammonium chloride-modified CdTe QDs for specific recognition and fluorescence detection of bifenthrin and λ-cyhalothrin [27, 28]. Here, we synthesized a novel MIPs fluorescence material based on polyamine-functionalized C-dots using a simple room-temperature sol–gel method for the fluorescence detection of target molecules.
In the present work, we report on a facile approach for the formation of a fluorescent material by coating an imprinted polymer layer on the surface of C-dots by a sol–gel process. Polyamine-functionalized C-dots were first synthesized by hydrothermal process using citric acid as the carbon source and poly (ethylenimine) as the surface modifier. Then 4-NP, 3-aminopropyltriethoxysilane (APTES) and tetramethoxysilane (TEOS) were chosen as the template molecule, functional monomer and cross linker, respectively. After a sol–gel molecular imprinting process and a solvent extraction process, the MIP-C-dots fluorescence material was obtained. The synthesized nanomaterial was characterized by transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FTIR) and spectrofluorometer, and then used for selective recognition and fluorescence detection of the target molecule 4-NP. To the best of our knowledge, MIPs-capped C-dots for fluorescence detection of 4-NP has not been reported. Finally, this material was demonstrated as a simple, rapid and selective detection system for determination of 4-NP in real samples.
Experimental
Materials
All chemicals were of at least analytical grade. Citric acid, branched poly(ethyleneimine) (PEI) (MM 1800 Da), ammonia solution (25–28 %), tetraethoxysilane (TEOS) and 3-aminopropyltriethoxysilane (APTES) 4-nitrophenol (4-NP), 2-nitrophenol (2-NP), 2,4,6-trinitrotoluene (TNT), 2,4,5-trichlorophenol (2,4,5-TCP) were all purchased from Aladdin reagent Co., Ltd. (Shang Hai, China, www.aladdin-reagent.com). Ethanol was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shang Hai, China, www.sinoreagent.com). Double distilled water was used during the whole experiment process.
Instrumentation
The morphologies were observed by a transmission electron microscope (TEM, JEOL, JEM-2100). Fourier transform infrared (FT-IR) spectra were recorded using Nicolet NEXUS-470 FTIR apparatus (USA). The fluorescence spectra of the C-dots and eco-friendly fluorescence material were obtained by a spectrofluorometer (Cary Eclipse) equipped with a quartz cell and a plotter unit.
The synthesis of polyamine-functionalized C-dots
Polyamine-functionalized C-dots were synthesized based on the procedure described in the literature with some modifications [29]. Briefly, 2.0 g of citric acid and 1.0 g of PEI were dissolved in 30 mL of deionized water, and the mixture was then transferred into a 50 mL Teflon-lined autoclave and heated at 200 °C for 5 h. After the reaction, the autoclave was cooled to room temperature, and the mixture was centrifugated at 12,000 rpm for 15 min to remove the large dots. Finally, the C-dots were dried and re-dispersed in deionized water at the concentration of 50 g/L.
Synthesis of MIP-C-dots and NIP-C-dots
0.02 mmol of 4-NP template was dissolved in 10 mL of ethanol, mixed with 0.08 mmol of APTES (functional precursor), and then 100 μL of C-dots was added and stirred for 1.0 h. Subsequently, 0.4 mmol of TEOS (cross-linker) and 100 μL of NH3 · H2O (catalyst) were added to the reaction flask, and the mixture solution was kept stirring for another 12 h. The resultant MIP-C-dots were collected by centrifugation (8000 rpm, 5 min) and washed with ethanol several times to remove any free reagents. The 4-NP templates in the imprinted polymer were extracted with ethanol, until no 4-NP can be detected by using UV spectrometer. Finally, the MIP-C-dots were dried at 50 °C under a vacuum overnight. As a control, the non-imprinted fluorescence materials (NIP-C-dots) were also prepared using the same method but without the addition of 4-NP.
Fluorescence measurement
All the fluorescence measurements were performed under the same conditions: the slit widths of the emission and excitation were both 10 nm, and the excitation wavelength was set at 350 nm with a recording emission range of 390–540 nm. The MIP-C-dots were dispersed in deionized water to get the stock solution (100 mg L-1). 4-NP and other phenols were dissolved in ethanol to get the analyte stock solutions (1.0 mmol L-1), respectively. In a 5.0 mL tube, a given concentration of 4-NP solution was added to a certain volume of a solution of the MIP-C-dots, and then the mixture was diluted to volume with deionized water. After incubation, the testing solution was transferred to a quartz cell for the following fluorescence detection.
Results and discussion
Preparation and characterization of C-dots and MIP-C-dots
The general scheme for the preparations of C-dots and MIP-C-dots are illustrated in Fig. 1. Firstly, amino-functionalized C-dots (see Figure S1) with high fluorescence property were synthesized via a facile and effective hydrothermal method. Citric acid and poly (ethylenimine) were used as the carbon source and surface modifier, respectively. Subsequently, the sol–gel molecular imprinting method was chosen as a simple and appropriate way to fabricate the MIP-C-dots. As shown in Fig. 1, 4-NP, APTES, TEOS and NH3 · H2O were used as template molecule, functional monomer, cross-linking agent and catalyst, respectively. After a copolymerization of the C-dots, APTES, 4-NP, and TEOS, the polymeric networks around the 4-NP molecules and C-dots were formed. The C-dots and APTES were interacted with 4-NP via the hydrogen bond interaction and van der Waals force. After the removal of the templates by solvent extraction, the MIP-C-dots with specific imprinted cavities were obtained.
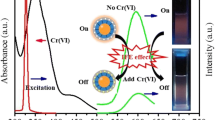
The fluorescence spectra of the amino-functionalized C-dots are shown in Fig. 2a. It can be found that when the excitation wavelength was set at 365 nm, the maximum emission wavelength was at 460 nm. Thus, excitation wavelength at 365 nm and emission wavelength at 460 nm were used in the further experiments. As shown in Fig. 2b, the fluorescence intensity of 4-NP@MIP-C-dots was about 18 % of that of NIP-C-dots; after 4-NP was removed by solvent extraction, the fluorescence intensity of MIP-C-dots was restored to 96.3 % of that of NIP-C-dots, which indicated that 4-NP was removed cleanly from the polymer matrix.
The morphology of C-dots and MIP-C-dots were investigated by TEM. As shown in Fig. 3a, the amino-functionalized CQDs are uniform and microspherical particles with the size about 4.5 nm. As shown in Fig. 3b, MIPs-CQDs exhibited spherical structure and the diameters were about 20 nm, indicating that the CQDs were coated with silica and the MIPs-CQDs were successfully synthesized.
FT-IR spectra of MIP-C-dots and NIP-C-dots were shown in Fig. 4. It can be found that the MIP-C-dots and NIP-C-dots showed similar spectrum owing to the same composition. The broad peak around 1097 cm-1 and the characteristic peaks at 800 and 465 cm–1 are attributed to the Si–O–Si asymmetric stretching and the Si-O vibrations, respectively. The bands at 3385, 1647 and 1556 cm–1 were assigned to the stretching vibration of N-H, indicating the presence of amino group. All the bonds further confirmed that the material was successfully synthesized by the sol–gel condensation of the silane reagents.
Fluorescence detection of 4-NP by MIP-C-dots
The following parameters were optimized: the concentration of MIP-C-dots and the detection time. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) the optimum concentration of MIP-C-dots was about 2.0 mg L-1; (b) 2 min was determined as the optimal detection time. Under the optimal condition, the capability of the MIP-C-dots for quantitative determination of 4-NP was further studied. After incubation of MIP-C-dots with different concentrations of 4-NP for 2 min, the test was implemented. As a control, NIP-C-dots with different concentrations of 4-NP were also researched. The detection system was based on the fluorescence quenching between C-dots and 4-NP. Moreover, the quenching mechanism of this fluorescence detection system can be described as follows: In the synthesis process of MIP-C-dots, many tailor-made recognition sites with complementarity to 4-NP (template molecule) were produced, and the specific imprinted cavities can generate a strong adsorption of 4-NP; based on the hydrogen bonding interactions between the template molecules and polymer matrix, the template 4-NP can easily close to the fluorescent material and lead to the fluorescence quenching behavior which can be explained as the electron transfer from C-dots to 4-NP. The fluorescence quenching followed the Stern − Volmer equation.
F 0 and F are the fluorescence intensities of the MIP-C-dots in the absence and presence of the target molecule 4-NP, respectively. K SV is the quenching constant, and [c] is the concentration of 4-NP. In addition, the ratio of K SV,MIP and K SV,NIP is defined as the imprinting factor (IF) to evaluate selectivity. The Stern-Volmer plots of MIP-C-dots and NIPs-C-dots with different concentrations of 4-NP were shown in Fig. 5a and b, respectively. The plot shown in Fig. 7a can be described by the following equation: F0/F = 0.01605 [c] + 1.01593, the K SV,MIP was 16,050 M-1 and the correlation coefficient was 0.9978. The linear calibration curve was obtained in the range of 0.2–50 μmol L-1 with a detection limit (3σ/k) of 0.06 μmol L-1. As shown in Fig. 7b, the K SV,NIP was about 5820 M-1 and the correlation coefficient was 0.9970. After being calculated, a high imprinting factor (IF) of 2.76 was obtained, indicating that MIP-C-dots can selectively recognize the template molecule 4-NP.
Selectivity study
Firstly, the interference of potentially interfering ions was studied. It can be found from the Table S1 that all of the potentially interfering ions have no effect on the FL intensity of the MIP-C-dots. Then the other three substances (2-NP, TNT and 2,4,5-TCP) were chosen to evaluate the selectivity of MIP-C-dots. As shown in Fig. 6, the MIP-C-dots have a strong response to the template molecule 4-NP, and the fluorescence quenching amount of the MIP-C-dots for 4-NP was larger than others. After being calculated, the difference in the quench efficiency ((F0-F)/F0) of MIP-C-dots and NIP-C-dots were 0.2136, 0.0242, 0.0330, 0.0190 at 50 μmol L−1 for the four phenols (4-NP, 2-NP, TNT and 2,4,5-TCP), respectively. Based on the above results, it can be found that MIP-C-dots had specific recognition ability for 4-NP and the specificity was probably due to the existence of the imprinted cavities in the MIP-C-dots. In addition, other target molecules were not be bound firmly into the imprinted cavities, and there were no specific recognition sites in the NIP-C-dots.
Analytical applications in real sample
To prove the applicability of the method, the nano material was used for the determination of 4-NP in Yangtze River water samples. The samples were filtered and stored in clean containers. As no response to 4-NP was found in the Yangtze River water samples, a recovery study was implemented and the corresponding analysis results were listed in Table 1. Owing to the excellent performance of the MIP-C-dots, good recoveries from 92.6 to 107.3 % were obtained. It can be found that the MIP-C-dots had good recognition ability to provide accurate values of 4-NP concentrations on unknown water samples. Thus, this eco-friendly fluorescence material can be used as an effective tool for rapid and accurate analysis of real samples.
Many good works about analytical method for 4-NP detection have been reported and some of them were summarized and compared with our work in Table 2. It can be found that the methodology had wide linear range and low detection limit. Compared with electrochemical methods, fluorescence analysis method has a lot of advantages, such as good stability, low cost, simplicity, high sensitivity and test rapidity. For the MIP-C-dots detection system, the selectivity was significant improved but the sensitivity was low. The reason can be described as follow: during the polymerization process, cross-linkers formed the main polymer layer, and the interaction sites between functinal monomers and template molecules were comparatively far less.
Conclusions
In summary, we developed a simple and efficient strategy to fabricate the eco-friendly molecularly imprinted fluorescence material based on C-dots for selective fluorescence detection of 4-NP. The prepared MIP-C-dots integrated the merits of the fluorescence property of C-dots and selectivity of MIPs. The material has a good linear range and detection limit, which provided a reliable method to detection of 4-NP in a real environment. Furthermore, it is suggested that a novel generation of eco-friendly fluorescence material based on C-dots can be fabricated by using this strategy.
References
Yin H, Zhou Y, Han R, Qiu Y, Ai S, Zhu L (2012) Electrochemical oxidation behavior of 2,4-dinitrophenol at hydroxylapatite film-modified glassy carbon electrode and its determination in water samples. J Solid State Electrochem 16:75–82
Hu S, Xu C, Wang G, Cu D (2001) Voltammetric determination of 4-nitrophenol at a sodium montmorillonite-anthraquinone chemically modified glassy carbon electrode. Talanta 54:115–123
Li S, Du D, Huang J, Tu H, Yang Y, Zhang A (2013) One-step electrodeposition of a molecularly imprinting chitosan/phenyltrimethoxysilane/AuNPs hybrid film and its application in the selective determination of p-nitrophenol. Analyst 138:2761–2768
Liu X, Ji Y, Zhang Y, Zhang H, Liu M (2007) Oxidized multiwalled carbon nanotubes as a novel solid-phase microextraction fiber for determination of phenols in aqueous samples. J Chromatogr A 1165:10–17
Guo XF, Wang ZH, Zhou SP (2004) The separation and determination of nitrophenol isomers by high-performance capillary zone electrophoresis. Talanta 64:135–139
Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, Akbarzadeh A, Davaran S (2012) Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett 7:480
Yuan C, Zhang K, Zhang Z, Wang S (2012) Highly selective and sensitive detection of mercuric ion based on a visual fluorescence method. Anal Chem 84:9792–9801
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49:6726–6744
Zuo PL, Lu XH, Sun ZG, Guo YH, He H (2016) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183(2):519–542
Yan X, Cui X, Li B, Li L (2010) Large, solution-processable graphene quantum dots as light absorbers for photovoltaics. Nano Lett 10:1869–1873
Lu WB, Qin XY, Liu S, Chang GH, Zhang YW, Luo YL, Asiri AM, Al-Youbi AO, Sun XP (2012) Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of Mercury(II) ions. Anal Chem 84:5351–5357
Huang H, Li CG, Zhu SJ, Wang HL, Chen CL, Wang ZR, Bai TY, Shi Z, Feng SH (2014) Histidine-derived nontoxic nitrogen-doped carbon dots for sensing and bioimaging applications. Langmuir 30:13542–13548
Gao ZH, Lin ZZ, Chen XM, Lai ZZ, Huang ZY (2016) Carbon dots-based fluorescent probe for trace Hg2+ detection in water sample. Sens Actuat B: Chem 222:965–971
Chen LX, Xu SF, Li JH (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942
Alexander C, Andersson HS, Andersson LI, Ansell RJ, Kirsch N, Nicholls IA, O’Mahony J, Whitcombe MJ (2006) Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003. J Mol Recognit 19:106–180
Ye L, Haupt K (2004) Molecularly imprinted polymers as antibody and receptor mimics for assays, sensors and drug discovery. Anal Bioanal Chem 378:1887–1897
Li Y, Ding MJ, Wang S, Wang RY, Wu XL, Wen TT, Yuan LH, Dai P, Lin YH, Zhou XM (2011) Preparation of imprinted polymers at surface of magnetic nanoparticles for the selective extraction of tadalafil from medicines. ACS Appl Mater Interfaces 3:3308–3315
Yin JF, Cui Y, Yang GL, Wang HL (2010) Molecularly imprinted nanotubes for enantioselective drug delivery and controlled release. Chem Commun 46:7688–7690
Xu SF, Chen LX, Li JH, Qin W, Ma J (2011) Molecularly imprinted core-shell nanoparticles for determination of trace atrazine by reversible addition-fragmentation chain transfer surface imprinting. J Mater Chem 21:12047–12053
Alizadeh T, Zare M, Ganjali MR, Norouzi P, Tavana B (2010) A new molecularly imprinted polymer (MIP)-based electrochemical sensor for monitoring 2,4,6-trinitrotoluene (TNT) in natural waters and soil samples. Biosens Bioelectron 25:1166–1172
Orozco J, Cortés A, Cheng GZ, Sattayasamitsathit S, Gao W, Feng XM, Shen YF, Wang J (2013) Molecularly imprinted polymer-based catalytic micromotors for selective protein transport. J Am Chem Soc 135:5336–5339
Liu HL, Fang GZ, Li CM, Pan MF, Liu CC, Fan C, Wang S (2012) Molecularly imprinted polymer on ionic liquid-modified C-dotse/ZnS quantum dots for the highly selective and sensitive optosensing of tocopherol. J Mater Chem 22:19882–19887
Wei X, Zhou ZP, Hao TF, Li HJ, Dai JD, Gao L, Zheng XD, Wang JX, Yan YS (2015) Simple synthesis of thioglycolic acid-coated CdTe quantum dots as probes for Norfloxacin lactate detection. J Lumin 161:47–53
Wei X, Zhou ZP, Hao TF, Li HJ, Yan YS (2015) Molecularly imprinted polymer nanospheres based on Mn-doped ZnS QDs via precipitation polymerization for room-temperature phosphorescence probing of 2,6-dichlorophenol. RSC Adv 5:19799–19806
Wei X, Zhou ZP, Hao TF, Li HJ, Xu YQ, Lu K, Wu YL, Dai JD, Pan JM, Yan YS (2015) Highly-controllable imprinted polymer nanoshell at the surface of silica nanoparticles based room-temperature phosphorescence probe for detection of 2,4-dichlorophenol. Anal Chim Acta 870:83–91
Wei X, Hao TF, Xu YQ, Lu K, Li HJ, Yan YS, Zhou ZP (2015) Swelling technique inspired synthesis of fluorescence composite sensor for highly selective detection of Bifenthrin. RSC Adv 5:79511–79518
Wei X, Hao TF, Xu YQ, Lu K, Li HJ, Yan YS, Zhou ZP (2016) Facile polymerizable surfactant inspired synthesis of fluorescent molecularly imprinted composite sensor via aqueous CdTe quantum dots for highly selective detection of λ-cyhalothrin. Sens Actuat B: Chem 224:315–324
Wei X, Zhou ZP, Hao TF, Xu YQ, Li HJ, Lu K, Dai JD, Zheng XD, Gao L, Wang JX, Yan YS, Zhu YZ (2015) Specific recognition and fluorescent determination of aspirin by using core-shell CdTe quantum dot-imprinted polymers. Microchim Acta 182:1527–1534
Rounaghi G, Kakhki RM, Azizi-toupkanloo H (2012) Voltammetric determination of 4-nitrophenol using a modified carbon paste electrode based on a new synthetic crown ether/silver nanoparticles. Mat Sci Eng C-Mater 32:172–177
Gu YE, Zhang Y, Zhang F, Wei J, Wang C, Du Y, Ye W (2010) Investigation of photoelectrocatalytic activity of Cu2O nanoparticles for p-nitrophenol using rotating ring-disk electrode and application for electrocatalytic determination. Electrochimi Acta 56:953–958
Xiao W, Xiao D, Yuan H (2007) A functionalized mesoporous silica sensor for the determination of p-nitrophenol or 2,4-dinitrophenol based on fluorescence quenching. Sens Lett 5:445–449
Moraes FC, Tanimoto ST, Salazar-Banda GR, Machado SAS, Mascaro LH (2009) A new indirect electroanalytical method to monitor the contamination of natural waters with 4-nitrophenol using multiwall carbon nanotubes. Electroanalysis 21:1091–1098
Yin H, Zhou Y, Ai S, Liu X, Zhu L, Lu L (2010) Electrochemical oxidative determination of 4-nitrophenol based on a glassy carbon electrode modified with a hydroxyapatite nanopowder. Microchim Acta 169:87–92
Ahmed GHG, Laíño RB, Calzón JAG, García MED (2015) Highly fluorescent carbon dots as nanoprobes for sensitive and selective determination of 4-nitrophenol in surface waters. Microchim Acta 182:51–59
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21277063, No. 21407057 and No. 21407064), National Basic Research Program of China (973 Program, 2012CB821500), Natural Science Foundation of Jiangsu Province (No. BK20140535), National Postdoctoral Science Foundation (No. 2014 M561595), Postdoctoral Science Foundation funded Project of Jiangsu Province (No. 1401108C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 250 kb)
Rights and permissions
About this article
Cite this article
Hao, T., Wei, X., Nie, Y. et al. An eco-friendly molecularly imprinted fluorescence composite material based on carbon dots for fluorescent detection of 4-nitrophenol. Microchim Acta 183, 2197–2203 (2016). https://doi.org/10.1007/s00604-016-1851-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1851-2