Abstract
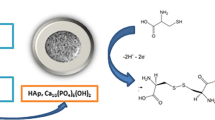
A glassy carbon electrode was modified with hydroxyapatite nanopowder (HA-NP) and characterized in terms of electrochemical oxidation of 4-nitrophenol (4-NP) via cyclic voltammetry, differential pulse voltammetry, chronoamperometry, and chronocoulumetry. The oxidation peak current of 4-NP at the modified electrode was increased (compared to the bare GCE), thus indicating that the HA-NP exhibits a remarkable enhancement effect on the electrochemical oxidation of 4-NP. The effects of loading with HA-NP, pH value, scan rate and accumulation time were examined. The oxidation peak current of 4-NP is proportional to its concentration in the range from 1.0 μM to 300 μM, with a correlation coefficient of 0.9996. The detection limit is 0.6 μM (at an S/N = 3). The method is simple, selective and sensitive. It was successfully applied to the determination of 4-NP in water samples, with recoveries ranging from 96% to 104%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
4-Nitrophenol (4-NP) is considered to be a hazardous waste by the U.S. Environmental Protection Agency [1, 2], which has a high environmental impact due to its toxicity and persistence. Unfortunately, 4-NP is widely used as intermediates in the production of pharmaceuticals, pesticides and dyestuffs, such as parathion insecticide which can also reversely hydrolyze to form 4-NP. In addition, 4-NP can also be used as leather fungicide and acid-base indicator. Therefore, 4-NP will be inevitably released into environment to cause pollution in the process of fabrication and application. Based on the above description, it is important to develop simple and reliable method for determination of trace amounts of 4-NP in environments.
Up to now, various techniques have been investigated to determine 4-NP, such as spectrophotometry [3], fluorescence [4], high-performance liquid chromatography [5], liquid chromatography with electrochemical detection [6], capillary zone electrophoresis [7], fiber optode [8] and electrochemical method [9–12]. Among them, electrochemical methods have received considerable attention because of the advantage of fast response, cheap instrument, low cost, simple operation, timesaving, high sensitivity and selectivity, real-time detection in situ condition. However, until now, many electrochemical determination techniques mainly focus on utilizing the reduction of nitryl in 4-NP, not the oxidation of hydroxide radical. Thus, the determination would be interfered by the oxygen molecule dissolved in solvent. In order to eliminate it, purging nitrogen for a certain time was employed. But this will make the determination more complicated and time-consuming. Moreover, the reduction of nitryl is more complicated than the oxidation of hydroxide radical. Therefore, electrochemical determination of 4-NP using the oxidation signal should be an appropriate alternative. Nevertheless, the use of bare electrode has revealed the drawback of weak electrochemical response and low sensitivity. Consequently, chemically modified electrodes (CMEs) have been widely investigated with various modification materials, especially nanophase materials, such as MWNT [9], SWNT [10], gold nanoparticles [11], TiO2 [12], zeolite [13], sodium montmorillonite [14] and hydroxyapatite (HAP) [15]. Among them, hydroxyapatite nanopowder (Ca10(PO4)6(OH)2, HA-NP), a kind of inorganic nanoporous material, has attracted many interest for possessing high biocompatibility and excellent adsorption property, which have been widely used as bone fillers, implant material, drug carrier, protein adsorbent, and removal reagent for heavy metal ions and organic molecules [16]. In addition, compared with other modified materials, HA-NP has advantages of abundant in nature, cheap, readily available, and stable in water and nontoxicity. Due to the high surface area and strong adsorption ability, a film of silica/HRP-HAP has been deposited on GCE surface to investigate the direct electron transfer of immobilized HRP and H2O2 determination [17]. El Mhammedi et al. have evaluated the analytical performance of HA-NP-CPE for the detection of trace lead (II), paraquat and 4-NP. They found that HAP can effectively improve the electrochemical response due to its excellent adsorption property [15, 18, 19]. So, HA-NP should be a suitable electrode modified material for pollution determination. However, to the best of our knowledge, electrochemical determination of 4-NP by HA-NP modified glassy carbon electrode using the oxidation process has not yet been reported.
The aim of the present work is to develop a simple, reliable and sensitive electrochemical method for the determination of 4-NP based on the unusual properties of HA-NP modified electrode. Here, the electrochemical oxidation of 4-NP on HA-NP modified glassy carbon electrode was reported. The modified electrode showed good electrochemical oxidation to 4-NP with an increase of the oxidation peak current. The effect factors and some kinetic parameters were investigated. Based on the electrochemical oxidation response of 4-NP on the modified electrode, a new differential pulse voltammetric technique was developed to determine 4-NP in real water samples.
Experimental
Reagents and apparatus
HA-NP (10–40 nm) and 4-NP were purchased from Aladdin (Shanghai, China http://www.aladdin-reagent.com). 0.1 M stock solution of 4-NP was prepared by dissolving 4-NP into absolute ethanol, and then stored at 4°C in a refrigerator. 4-NP working solutions were freshly prepared by appropriate dilution of the stock solution. Phosphate buffer solutions (PBS) from pH 4–11 was prepared by mixing the stock solutions of 0.1 M NaH2PO4 and 0.1 M Na2HPO4 and adjusting the pH with 0.1 M H3PO4 or 0.1 M NaOH. All the chemicals were of analytical grade and were used without further purification. All the solutions were prepared with redistilled deionized water from quartz.
All the electrochemical measurements were carried out using a CHI660C electrochemical workstation (Shanghai Chenhua Co., China http://chi.instrument.com.cn) with a conventional three-electrode cell. A bare GCE (CHI104, d = 3 mm) or HA-NP modified GCE was used as working electrode. A saturated calomel electrode (SCE) and a platinum wire were used as reference electrode and auxiliary electrode, respectively. All the measurements were carried out at room temperature (25 ± 0.5°C).
Fabrication of HA-NP modified GCE
The HA-NP suspension was prepared by dispersing 5 mg HA-NP into 1 mL redistilled deionized water with the aid of ultrasonication for 30 min. Prior to modification, GCE was polished on chamois leather with alumina slurry, washed successively with redistilled deionized water, anhydrous alcohol and redistilled deionized water in an ultrasonic bath for 3 min and dried in air. Then 5 μL HA-NP suspension was deposited on the surface of GCE and dried under an infrared lamp to obtain the uniform modifying layer. The obtained modified electrode was noted as HA-NP/GCE.
Analytical procedure
Unless otherwise stated, 0.1 M PBS (pH 7.0) was used as the supporting electrolyte for 4-NP determination. A certain volume of 4-NP working solution and 10 mL 0.1 M PBS were transferred into an electrochemical cell, and then the three-electrode system was installed on it. After an accumulation of 7 min at open-circuit under stirring and following quiet for 2 s, the cyclic voltammograms were recorded from 0 V to 1.2 V at scan rate of 100 mV/s. The differential pulse voltammograms were recorded from 0.5 V to 1.2 V. The same procedure was carried out for the water sample analysis.
Results and discussion
Characterization of HA-NP/GCE
The HA-NP/GCE was characterized by cyclic voltammetry and AC impedance in 5 mM [Fe(CN)6]3−/4− solution containing 1 M KCl (Supplementary Section Figure 1S and Figure 2S).
Electrochemical oxidation behavior of 4-NP
The electrochemical oxidation behaviors of 4-NP were investigated at GCE and HA-NP/GCE by cyclic voltammetry. The results were shown in Fig. 1. No redox signals were observed in PBS without 4-NP at HA-NP/GCE, suggesting that HA-NP is an electrochemically inactive material in the selected potential range. After 4-NP was added into PBS, an obvious oxidation peak was obtained at both electrodes. It is no doubt that the oxidation peak should be attributed to the oxidation of 4-NP. However, no corresponding reduction peak was observed in the following reverse scan from 1.2 V to 0 V, implying that the oxidation of 4-NP is a totally irreversible electrode process under the above experimental conditions. As expected, the oxidation peak current obtained at HA-NP/GCE was higher and the oxidation peak potential was a little lower than those at bare electrode, indicating that HA-NP has good electrocatalytic activity for 4-NP oxidation, which can obviously increase the current response and decrease the detection limit. Therefore, HA-NP was chosen as the working electrode.
It must be highlighted that the oxidation peak current gradually decreased and the peak potential shifted positively as increasing the number of cyclic potential sweeps (shown in Figure 3S). This phenomenon may be attributed to that the oxidative product of 4-NP or its dimer, which could deposit on the electrode surface and retard the electrochemical oxidation of 4-NP [20–22]. Considering the determination sensitivity and accuracy, the oxidation peak current in the first anodic sweep was recorded for 4-NP analysis in the following studies. In order to ascertain the electron number (n) involved in 4-NP oxidation process at HA-NP/GCE, the n value was determined by cyclic voltammogram using the equation of αn = 47.7/(E p−E p/2) [23]. In this work, E p = 914 mV, E p/2 = 850 mV. Therefore, αn was calculated to be 0.84. Generally, α (charge transfer coefficient) is assumed to be 0.5 in totally irreversible electrode process. So, n was calculated to be 2.
Optimum conditions of the modified electrode
The effect of the amount of HA-NP on the voltammetric response of 4-NP was shown in Figure 4S in Supplementary Section. The optimized concentration of HA-NP was chosen as 5 mg/mL.
The effect of pH on the oxidation of 0.2 mM 4-NP at HA-NP/GCE was also investigated by cyclic voltammetry in the pH range from 4.0 to 11.0. As shown in Fig. 2, the oxidation peak current of 4-NP increased gradually with increasing pH from 4.0 to 7.0, and the maximum current was achieved at pH 7.0. With further increasing pH, the oxidation peak current conversely decreased. Therefore, pH 7.0 was chosen for the subsequent analytical experiments. With the solution pH increasing from 4.0 to 11.0, the E pa shifted negatively and linearly. The linear regression equation can be expressed as E pa (V) =−0.031pH + 1.13 (R = 0.9931). According to the following formula of dE pa/dpH = (2.303mRT)/(nF) [24], the proton number (m) intervening in the oxidation process was calculated to be 1.05 ≈ 1. Therefore, the electrochemical oxidation of 4-NP at HA-NP/GCE should be a two-electron and one-proton process.
Open-circuit accumulation was investigated (Figure 5S in Supplementary Section). The accumulation time of 7 min was chosen in this work.
Figure 3 showed the influence of scan rate (v) on the electrochemical oxidation behavior of 4-NP. The oxidation current increased with increasing the scan rate. As can be seen from the insert of Fig. 3, the oxidation peak current increased linearly with the square root of scan rate in the range of 20–400 mV/s, indicating that the oxidation of 4-NP on HA-NP/GCE is a diffusion-controlled process. It seemed to be inconsistent with the accumulation characteristics of 4-NP mentioned above. This may relate to the diffusion of 4-NP accumulated in the HA-NP film to the electrode surface to undergo electrochemical reaction. The similar phenomenon was also observed in previous report for cathodic reduction of methylparathion [25].
Chronoamperometry and chronocoulumetry
Chronoamperometry was used for the evaluation of the catalytic rate constant k cat and the results were shown in Fig. 4. The rate constant for the electrochemical reaction between 4-NP and HA-NP/GCE was determined according to the equation of I cat/I L = π1/2(k cat c 0 t)1/2 [26], where I L was the current of HA-NP/GCE in the absence of 4-NP, and I cat was the catalytic current due to the addition of 4-NP. k cat, c 0 and t were the catalytic rate constant (M−1 s−1), the bulk concentration (M) of 4-NP and time elapsed (s), respectively. According to the slope of I cat/I L versus t 1/2 plot (inset of Fig. 4), the average value of the calculated k cat value was 8.78 × 103 M−1 s−1, indicating that HA-NP has an excellent catalytic effect towards 4-NP oxidation.
The diffusion coefficient D of 4-NP at HA-NP/GCE can be determined using chronocoulometry based on the equation of Q = (2nFAcD 1/2 t 1/2)π1/2 + Q ads [27]. After background subtraction, the plot of charge (Q) against the square root of time (t 1/2) showed a linear relationship with slope of 7.13 × 10−6 C/s1/2 and Q ads of 1.14 × 10−6 C (Figure 6S in Supplementary Section). As n = 1, A = 0.07 cm2 (geometric area) and c = 0.5 mM, it was calculated that D = 4.32 × 10−5 cm2/s. In addition, according to the equation of Q ads = nFAГ s, the adsorption capacity, Г s, can be obtained as 7.12 × 10−10 mol/cm2, indicating that HA-NP has excellent adsorption property towards 4-NP.
Calibration curve
Figure 5 showed the differential pulse voltammograms of 4-NP with the concentration ranging from 1 μM to 300 μM under the optimized experimental conditions. The linear regression equation was I pa (μA) = 0.0295c (μM)–0.123 with a correlation coefficient of 0.9996 (Figure 7S in Supplementary Section). The detection limit (S/N = 3) was 0.6 μM, which was lower than those of 17.1 μM at Nafion/GCE [28], 8.23 μM at Pt/PAZ modified electrode [29], 1.38 μM at BDD film electrode [30], 1.1 μM at mercury meniscus-modified silver solid amalgam electrode [31] and 0.7 μM at RTIL/CPE [32]. However, the detection of limit is higher than that of 0.288 μM at zeolite-modified CPE [13], 0.11 μM at Ti/TiO2/Au/HRP-MB electrode [12], 0.04 μM at MWNT-Nafion/GCE [9], 0.011 μM at SWCNH/GCE [33], 0.0025 μM at SMWNT/GCE [10] and 0.0075 μM at Lithium tetracyanoethylenide modified GCE [34]. The relative low detection limit should be attributed to excellent adsorption ability of HA-NP towards 4-NP.
The kinetic reaction order of 4-NP at HA-NP/GCE was also investigated. The results implies that the electrooxidation of 4-NP at nano-HAP/GCE follows the first-order kinetics with respect to 4-NP [35] (Figure 8S in Supplementary Section).
The fabrication reproducibility was estimated by determining the electrochemical response of 8 μM 4-NP at ten modified electrodes prepared independently. The RSD was calculated to be 3.54%, revealing that this method has excellent reproducibility for the determination of 4-NP. After the electrode was stored for 30 days, it still retained 82% of the original response of 8 μM 4-NP, suggesting acceptable storage stability. In addition, the 500 μM of Na+, Ca2+, Mg2+, Fe3+, Ba2+,Al3+, Zn2+, Ni2+, Cu2+, F−, Cl−, SO 2−4 , CO 2−3 , PO 3−4 and NO −3 ; 200 μM of 2,4-dinitrophenol, pyrocatechol, hydroquinone, o-nitrobenzoic acid, m-nitrobenzoic acid and p-nitrobenzoic acid do not interfere with the oxidation signal of 10 μM 4-NP (peak current change < 5%). However, it is also found that 100 μM of phenol, 2-nitrophenol and hydroxyphenol interfere the determination. Nevertheless, these results indicated that HA-NP/GCE has an excellent selectivity for 4-NP, and the fabricated modified electrode might be applied to determine 4-NP in real samples.
Analytical application
The possibility of applying the present electrochemical method for 4-NP determination in water samples was evaluated. No signals for 4-NP were observed when these water samples were analyzed, which may be attributed to that no 4-NP is in water samples or the concentration of 4-NP is lower than the detection limit. Thus, the determination of 4-NP concentration was performed by the standard addition method. The results were listed in Table 1. It is clear that the recoveries of this method were in the range from 95.86% to 104.38%. These results indicate that this method should be reliable, effective and sufficient for 4-NP determination. Additionally, the interferences in water samples can be almost neglected.
Conclusion
A sensitive and reliable electrochemical oxidation method was investigated for determination of 4-NP in water samples using HA-NP/GCE. Due to its unique properties of high specific surface area and strong adsorptive ability, HA-NP could effectively enhance the electrochemical response of 4-NP, increase the determination sensitivity and decrease the limit of detection. In addition, the fabrication of HA-NP/GCE is very simple, cheap and easy to prepare. Based on this, a promising electrochemical method was developed for 4-NP determination and applied to water samples.
References
Agency UEP (1979) Fed Regist 44
Agency UEP (1989) Fed Regist 52
Niazi A, Yazdanipour A (2007) Spectrophotometric simultaneous determination of nitrophenol isomers by orthogonal signal correction and partial least squares. J Hazard Mater 146:421
Nistor C, Oubi A, Marco MP, Barceló D, Emnéus J (2001) Competitive flow immunoassay with fluorescence detection for determination of 4-nitrophenol. Anal Chim Acta 426:185
Thompson MJ, Ballinger LN, Cross SE, Roberts MS (1996) High-performance liquid chromatographic determination of phenol, 4-nitrophenol, β-naphthol and a number of their glucuronide and sulphate conjugates in organ perfusate. J Chromatogr B 677:117
Galeano-Diaz T, Guiberteau-Cabanillas A, Mora-Diez N, Parrilla-Vazquez P, Salinas-Lopez F (2000) Rapid and sensitive determination of 4-nitrophenol, 3-methyl-4-nitrophenol, 4, 6-dinitro-o-cresol, parathion-methyl, fenitrothion, and parathion-ethyl by liquid chromatography with electrochemical detection. J Agric Food Chem 48:4508
Guo X, Wang Z, Zhou S (2004) The separation and determination of nitrophenol isomers by high-performance capillary zone electrophoresis. Talanta 64:135
Yang X, Shen G, Yu R (2001) A fiber optode for p-nitrophenol based on covalently bound 9-allylaminoacridine. Microchim Acta 136:73
Huang W, Yang C, Zhang S (2003) Simultaneous determination of 2-nitrophenol and 4-nitrophenol based on the multi-wall carbon nanotubes Nafion-modified electrode. Anal Bioanal Chem 375:703
Yang C (2004) Electrochemical determination of 4-nitrophenol using a single-wall carbon nanotube film-coated glassy carbon electrode. Microchim Acta 148:87
Liu Z, Du J, Qiu C, Huang L, Ma H, Shen D, Ding Y (2009) Electrochemical sensor for detection of p-nitrophenol based on nanoporous gold. Electrochem Commun 11:1365
Kafi AKM, Chen A (2009) A novel amperometric biosensor for the detection of nitrophenol. Talanta 79:97
Del Mar Cordero-Rando M, Barea-Zamora M, Barberá-Salvador JM, Naranjo-Rodríguez I, Muñoz-Leyva JA, Hidalgo-Hidalgo de Cisneros JL (1999) Electrochemical study of 4-nitrophenol at a modified carbon paste electrode. Microchim Acta 132:7
Hu S, Xu C, Wang G, Cui D (2001) Voltammetric determination of 4-nitrophenol at a sodium montmorillonite-anthraquinone chemically modified glassy carbon electrode. Talanta 54:115
El Mhammedi MA, Achak M, Bakasse M, Chtaini A (2009) Electrochemical determination of para-nitrophenol at apatite-modified carbon paste electrode: application in river water samples. J Hazard Mater 163:323
Lin K, Pan J, Chen Y, Cheng R, Xu X (2009) Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders. J Hazard Mater 161:231
Wang B, Zhang JJ, Pan ZY, Tao XQ, Wang HS (2009) A novel hydrogen peroxide sensor based on the direct electron transfer of horseradish peroxidase immobilized on silica-hydroxyapatite hybrid film. Biosens Bioelectron 24:1141
El Mhammedi MA, Achak M, Chtaini A (2009) Ca10(PO4)6(OH)2-modified carbon-paste electrode for the determination of trace lead (II) by square-wave voltammetry. J Hazard Mater 161:55
El Mhammedi MA, Bakasse M, Chtaini A (2007) Square-wave voltammetric determination of paraquat at carbon paste electrode modified with hydroxyapatite. Electroanalysis 19:1727
Agboola B, Nyokong T (2007) Electrocatalytic oxidation of chlorophenols by electropolymerised nickel (II) tetrakis benzylmercapto and dodecylmercapto metallophthalocyanines complexes on gold electrodes. Electrochim Acta 52:5039
Eickhoff H, Jung G, Rieker A (2001) Oxidative phenol coupling-tyrosine dimers and libraries containing tyrosyl peptide dimers. Tetrahedron 57:353
Koile RC, Johnson DC (1979) Electrochemical removal of phenolic films from a platinum anode. Anal Chem 51:741
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Zhou C, Liu Z, Dong Y, Li D (2009) Electrochemical behavior of o-nitrophenol at hexagonal mesoporous silica modified carbon paste electrodes. Electroanalysis 21:853
Fan S, Xiao F, Liu L, Zhao F, Zeng B (2008) Sensitive voltammetric response of methylparathion on single-walled carbon nanotube paste coated electrodes using ionic liquid as binder. Sensor Actuat B 132:34
Andrieux CP, Saveant JM (1978) Heterogeneous (chemically modified electrodes, polymer electrodes) versus homogeneous catalysis of electrochemical reactions. J Electroanal Chem 93:163
Anson F (1964) Application of potentiostatic current integration to the study of the adsorption of cobalt (III)-(ethylenedinitrilo (tetraacetate) on mercury electrodes. Anal Chem 36:932
Calvo-Marzal P, Rosatto SS, Granjeiro PA, Aoyama H, Kubota LT (2001) Electroanalytical determination of acid phosphatase activity by monitoring p-nitrophenol. Anal Chim Acta 441:207
Lupu S, Lete C, Marin M, Totir N, Balaure PC (2009) Electrochemical sensors based on platinum electrodes modified with hybrid inorganic-organic coatings for determination of 4-nitrophenol and dopamine. Electrochim Acta 54:1932
Zhao GH, Tang YT, Liu MC, Lei YZ, Xiao XE (2007) Direct and simultaneous determination of phenol, hydroquinone and nitrophenol at boron-doped diamond film electrode. Chin J Chem 25:1445
Fischer J, Vanourkova L, Danhel A, Vyskocil V, Cizek K, Barek J, Peckova K, Yosypchuk B, Navratil T (2007) Voltammetric determination of nitrophenols at a silver solid amalgam electrode. Int J Electrochem Sci 2:226
Sun W, Yang MX, Jiang Q, Jiao K (2008) Direct electrocatalytic reduction of p-nitrophenol at room temperature ionic liquid modified electrode. Chin Chem Lett 19:1156
Zhu S, Niu W, Li H, Han S, Xu G (2009) Single-walled carbon nanohorn as new solid-phase extraction adsorbent for determination of 4-nitrophenol in water sample. Talanta 79:1441
de Cássia Silva Luz R, Damos FS, de Oliveira AB, Beck J, Kubota LT (2004) Voltammetric determination of 4-nitrophenol at a lithium tetracyanoethylenide (LiTCNE) modified glassy carbon electrode. Talanta 64:935
Cheng H, Scott K (2006) Determination of kinetic parameters for borohydride oxidation on a rotating Au disk electrode. Electrochim Acta 51:3429
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.20775044) and the Natural Science Foundation of Shandong province, China (Y2006B20).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material
(DOC 96 kb)
Rights and permissions
About this article
Cite this article
Yin, H., Zhou, Y., Ai, S. et al. Electrochemical oxidative determination of 4-nitrophenol based on a glassy carbon electrode modified with a hydroxyapatite nanopowder. Microchim Acta 169, 87–92 (2010). https://doi.org/10.1007/s00604-010-0309-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0309-1