Abstract
A polybenzidine-modified Fe3O4@SiO2 nanocomposite was successfully synthesized through a chemical oxidation method and employed as a novel sorbent in dispersive magnetic solid phase extraction (DMSPE) for the preconcentration and determination of three triazole fungicides (TFs), namely diniconazole, tebuconazole, and triticonazole in river water, rice paddy soil, and grape samples. The synthesis method involved a polybenzidine self-assembly coating on Fe3O4@SiO2 magnetic composite. Characterization techniques such as FT-IR, XRD, FESEM, EDX, and VSM were used to confirm the correctness of the synthesized nano-sorbent. The target TFs were determined in actual samples using the synthesized nanocomposite sorbent in combination with gas chromatography–flame ionization detection (FID). Several variables were carefully optimized , including the sample pH, sorbent dosage, extraction time, ionic strength, and desorption condition (solvent type, volume, and time). Under the optimized experimental conditions, the method exhibited linearity in the concentration range 5–1000 ng mL−1 for triticonazole and 2–1000 ng mL−1 for diniconazole and tebuconazole. The limits of detection (LOD) for the three TFs were in the range 0.6–1.5 ng mL−1. The method demonstrated acceptable precision with intra-day and inter-day relative standard deviation (RSD) values of less than 6.5%. The enrichment factors ranged from 248 to 254. Finally, the method applicability was evaluated by determining TFs in river water, rice paddy soil, and grape samples with recoveries in the range 90.5–106, indicating that the matrix effect was negligible in the proposed DMSPE procedure.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triazole fungicides (TFs), the most used class of highly effective and broad-spectrum bactericidal pesticides, are used extensively in agriculture to prevent and treat a wide range of crop diseases [1]. Properties including high solubility in water, high resistance to light and heat, and the ability to endure prolonged exposure to very acidic and alkaline conditions are all characteristics of TFs [2]. Due to their non-degradability, TFs may cause residuals in agricultural products and have long-term environmental impacts, posing a hidden threat to the environment, food, and human health [3]. Consequently, it is important to monitor triazole fungicide residues in food samples and the environment.
Generally, several analytical methods such as high-performance liquid chromatography (HPLC) [4,5,6,7,8,9], gas chromatography (GC) [10, 11], GC-mass spectrometry [12,13,14,15], GC-tandem mass spectrometry [16], HPLC-MS [17], and voltammetry [18] have been used to simultaneously determine the levels of TFs and their metabolites in various types of samples. The sample preparation stage (clean-up and preconcentration) before analysis is one of the most crucial procedures in analytical operations due to the complexity of the sample matrix and the trace levels of the analytes present. So far, different methods such as liquid-liquid microextraction [19, 20] have been utilized in the determination and preconcentration of TFs in various samples. However, sorbent-based techniques are more adaptable and efficient than LLE in terms of ease of use, cleanness, low requirement for reagents and organic solvents, and high enrichment factor (EF). A technique based on sorbents called solid phase microextraction (SPME) has been developed and utilized for the preconcentration of TFs. The sorbents/coatings can determine the selectivity, extraction dynamics, and sensitivity of the applicable techniques [3]. To date, several sorbent materials have been used for the extraction of TFs, including metal-organic framework [21], molecularly imprinted polymer [22], metal oxide nanoparticles [23], graphene-based nanocomposite [24], carbon nanotubes [25], and poly(ionic liquid) [26].
Conductive polymers have attracted much interest as sorbent coatings used for analyte extraction by adsorption due to their unique properties such as functional groups, acid-base and π–π interactions, ion exchange, hydrogen bonding, and electroactivity [27, 28]. The synthesis of polymeric aromatic diamines such as poly(phenylenediamine) [29] and poly(aniline-naphthylamine) [30] and their application for analyte extraction is a popular subject in conductive polymers. These polymers have numerous benefits, including excellent redox reversibility, ease of synthesis, and high chemical stability in aqueous solution and air [31]. Although the aromatic amine polymers have received substantial study, there are limited articles on polybenzidine. The electrochemical polymerization has been used to synthesize the majority of known polybenzidines [32]. The chemical oxidation method for polybenzidine synthesis and the discussion about its mechanism are limited [33,34,35], and its application for solid phase extraction has rarely been reported.
Introducing magnetic properties into conducting polymers is an efficient strategy to address the issue of sorbent recovery. By incorporating magnetic nanoparticles (MNPs) into DMSPE extraction, a magnetic field positioned outside of the extraction container can swiftly and easily collect the MNPs without filtering or centrifuging the sample, thereby streamlining the sampling and collection process [36].
In adsorbent applications, magnetic nanomaterials must maintain colloidal stability, which is why analytical chemistry researchers have been exploring methods to enhance the stability of iron oxide nanoparticles. One promising approach is to chemically graft a silica layer onto Fe3O4 nanoparticles, which greatly improves their stability even in acidic solutions. This modified Fe3O4@SiO2 structure can be easily dispersed in the concentrated acetic acid, which is necessary for the formation of a polybenzidine layer.
This study is the first to investigate the attachment of benzidine polymer to the surface of silica-coated Fe3O4 nanoparticles via a self-assembly approach. The resulting material is abbreviated as Fe3O4@SiO2@PBenz. In the subsequent stage, the ability of this nano-sorbent to preconcentrate and determine model compounds of TFs (tebuconazole, triticonazole, and diniconazole) simultaneously using a DMSPE method was evaluated. The applicability of the proposed method for extracting and analyzing TFs diverse in real samples was also investigated.
Experimental
Reagents and materials
Benzidine; tetraethyl orthosilicate (TEOS); acetone; n-octane; methanol; sodium chloride; sodium hydroxide; hydrochloric acid (37%); glacial acetic acid (100%); triazole fungicides containing diniconazole, tebuconazole, and triticonazole (purities > 98%); ferrous chloride (FeCl2·4H2O); and ferric chloride (FeCl3·6H2O) were purchased from Merck (Darmstadt, Germany). Acetonitrile and aqueous ammonia solution (NH4OH, 30% w/w) was prepared from Panreac (Spain). Pure ethanol (EtOH, 99%) was bought from Kimia Alcohol in Zanjan, Iran. With the use of a Millipore Milli-Q system (Bedford, USA), ultrapure water was prepared.
Instrumentation
Agilent 7890A gas chromatography (USA) with an FID detector and a split/splitless injector was used to determine the standard and test samples. Chromatographic separations were performed on a BP-5 fused silica capillary column (30 m × 0.32 mm I.D. and thickness film: 0.25 μm). With a split ratio of 1:10, 1 μL of sample solution was injected into the instrument. One milliliter per minute of ultra-high purity nitrogen was used as the carrier gas, while H2 (35 mL/min) and air (350 mL/min) were used to feed the FID detector. The oven temperature was programmed as follows: the initial temperature was 55 °C (held 1 min), from 55 to 200 °C at a rate of 30 °C min−1 (held 1 min) and from 200 to 280 °C at a rate of 30 °C min−1 and then maintained at 280 °C for 3.5 min. The injector and detector temperatures were set at 280 and 300 °C, respectively. The pH of each solution was measured using a digital Metrohm ion analyzer (Herisau, Switzerland) outfitted with a combined glass-calomel electrode.
Using a Bruker IFS-66 FT-IR instrument, the FT-IR spectra of a KBr pellet between 4000 and 400 cm−1 were captured (Bruker Optics, Karlsruhe, Germany). The morphology and dimension of the nanoparticle were explored by a field emission scanning electron microscopy (FESEM) model FEI ESEM QUANTA 200 (FELMI, USA). An EDAX EDS silicon drift 2017 instrument was used for the energy-dispersive X-ray (EDX). Using a Philips-PW 12C diffractometer apparatus from Amsterdam, the Netherlands, which was outfitted with a Cu K radiation source, X-ray powder diffraction (XRD) patterns of the nanocomposites were obtained. Magnetization was measured using a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co., Kashan, Iran) in a 1-T magnetic field at room temperature.
Preparation of standard solutions
To prepare a stock solution (1000 mg L−1) of triazole fungicides (purities > 98%), the required amount of fungicides was dissolved in ethanol. The standard stock solutions were stored at 4 °C and shielded from light. Daily preparation of working solutions involved dilution of the stock solutions with deionized water. Ethanol was used to prepare all standard solutions for GC-FID apparatus calibration.
Sample collection and pretreatment
River water samples were collected from the Jajrood River in Tehran, Iran, and filtered through a 0.45-mm membrane. The filtered samples were then stored at 4 °C in brown glass bottles and used without any pretreatment.
The soil sample was obtained from rice agricultural land in Fereydunkenar (Mazandaran, Iran). Any materials such as plants, seeds, roots, and stones were removed, and the soil sample was air-dried, ground into powder, passed through an 80-mesh sieve, and homogenized according to a previously reported method [37] with minor modifications.
Two grams of homogenized soil sample was weighed into a conical test tube, and a desired concentration of mixed TF standard solution was added to the tube. Subsequently, the spiked soil sample was allowed to stand for 24 h at room temperature in a dark space before analysis. After that, 5 mL of acetonitrile was added to the prepared spiked or unspiked soil sample, and the mixture was sonicated for 30 min. The mixture was then centrifuged for 5 min at 10,000 rpm, and the supernatant was collected and transferred to another test tube. Next, 5 mL of acetonitrile was added to the remaining sample, and the aforementioned procedure was repeated. For the DMSPE process, the obtained supernatants from two stages were mixed and diluted to a final volume of 40 mL.
The grape sample was purchased from a local supermarket in Tehran, Iran. The grape sample treatment was carried out according to the method described in Ref. [38] with minor modifications. Briefly, the sample was ground, mashed, and homogenized in a blender. Then, 2 g of the sample was spiked with various concentrations of the TF standard solution. After that, the sample was mixed with 8 mL of acetonitrile and 2 mL of distilled water and ultrasonicated for 30 min. The mixture was then centrifuged for 5 min at 10,000 rpm. For the DMSPE procedure, the supernatant was collected, diluted, and loaded onto the sorbent. The same procedure was conducted for unspiked samples without the addition of the standard solution of TFs.
Synthesis procedures
Figure 1 illustrates the sequential steps for synthesizing the Fe3O4@SiO2@PBenz nanocomposite. In the first step, Fe3O4 nanoparticles were synthesized using a chemical co-precipitation method [39]. Next, a silica shell was formed around the Fe3O4 nanoparticles via hydrolysis of tetraethyl orthosilicate (TEOS). Finally, Fe3O4@SiO2@PBenz was prepared using a self-assembly strategy previously reported [34]. The detailed synthesis procedures are presented in the Electronic Supplementary Material (ESM).
DMSPE procedure
An appropriate amount of TF standards was spiked into a 40 mL aqueous solution at neutral pH in a conical test tube. Then, 20 mg of the Fe3O4@SiO2@PBenz magnetic sorbent was added to the sample. The solution containing the sorbent was stirred using a mechanical stirrer with a glass rod for 25 min to disperse the sorbent in the sample media. In the next step, the magnetic sorbent was thoroughly separated from the sample by exposing the conical tube wall to a strong magnet, and the solution was decanted. In the final step, 150 μL of ethanol was added to the sorbent, and the mixture was ultrasonicated for 5 min to desorb the target analytes. The resulting solution was monitored by GC-FID.
Results and discussion
Characterization of the sorbent
FT-IR spectra (Fig. S1), vibrating sample magnetometer (VSM) results (Fig. S2), and further details on the characterization of nano-sorbent were presented in the ESM.
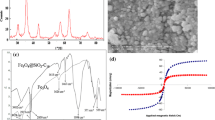
XRD pattern of Fe3O4@SiO2 MNPs and Fe3O4@SiO2@PBenz nanocomposite is shown in Fig. 2(a) and (b), respectively. The peaks appearing at 2Ө = 30.4°, 35.7°, 43.2°, 53.8°, 57.4°, and 63.1° that correspond to (220), (311), (400), (422), (511), and (440) crystal planes are characteristic peaks of Fe3O4 (according to the JCPDS number 01-088-0866). Therefore, the crystal structure of the Fe3O4 nanospheres is integrity. In the XRD pattern of Fe3O4@SiO2 nanocomposite (Fig. 2(a)), the Fe3O4 nanospheres is covered by an amorphous silica layer while the crystal structure of the Fe3O4 nanospheres has been preserved. In the XRD pattern of Fe3O4@SiO2@PBenz nanocomposite (Fig. 2(b)), several sharp peaks at 24.4°, 28.2°, 30.2°, and 33.6° suggest more crystallinity in nanocomposite due to PBenz shell.
Surface morphology and the average size of particles are shown by the FESEM technique. The FESEM image of Fe3O4@SiO2@PBenz nanocomposite is depicted in Fig. 3 in two scale bars of 1 μm and 200 μm. As shown, fine spherical particles of Fe3O4@SiO2 with an average diameter of 45–74 nm are well-dispersed and also have a smooth and uniform surface morphology.
For semi-quantitative investigation of the chemical elements contained in the Fe3O4@SiO2 and Fe3O4@SiO2@PBenz nanocomposites, the energy-dispersive X-ray spectroscopy (EDX) technique is applied (Fig. S3). The EDX spectra of Fe3O4@SiO2 demonstrated the peaks were associated with C, O, Si, and Fe with mass ratios (w%) of 15.32%, 41.70%, 7.26%, and 35.71% respectively. The mass ratios of carbon, oxygen, nitrogen, silicon, and iron are 16.85%, 25.00%, 7.80%, 11.67%, and 38.69%, respectively, according to the EDX spectrum of Fe3O4@SiO2@PBenz. It shows that Fe3O4@SiO2 nanoparticles were successfully coated with polybenzidine.
Optimization
In this study, triazole fungicides were simultaneously extracted from aqueous samples, preconcentrated, and determined using DMSPE combined with GC-FID. Respective data and figures of experimental variables influencing the extraction procedure such as the amount of sorbent, sample pH, extraction time, elution solvent, the volume of desorption solvent, ionic strength, and desorption time are given in the ESM. The chromatographic peak area was used to evaluate the effect of these parameters on extraction efficiency. The following experimental conditions were found to give best results: pH of sample solution, 7 (Fig. S4); sorbent amount, 20 mg (Fig. S5); sorption time, 25 min (Fig. S6); desorption solvent type, ethanol (Fig. S7); desorption solvent volume, 150 μL (Fig. S8); desorption time, 6 min (Fig. S9); salt concentration, 0.0% w/v (Fig. S10).
Reusability of the Fe3O4@SiO2@PBenz particles
The reusability of a magnetic nano-sorbent is crucial in determining its efficiency. Before use, the synthesized sorbent was washed twice with a desorption solvent (ethanol), each time with 3 mL, to ensure that no fungicide remained on the sorbent. The results showed that the Fe3O4@SiO2@PBenz sorbent could be employed at least five times without a substantial loss in extraction recoveries (<5%) or magnetic properties. This indicates that the sorbent is highly efficient, cost-effective, and sustainable for magnetic sorption applications
Analytical performance
Analytical figures of merit, including linearity, repeatability (RSD), reproducibility, limit of detection (LOD), limit of quantification (LOQ), and EF for triazole fungicides, were determined using the current method under optimized extraction conditions. The summary of the findings is presented in Table 1.
The linearity of the method was evaluated using seven different concentrations of triazole fungicide in the range of 2–1000 ng mL−1. External calibration plots showed satisfactory linearity with correlation coefficients ranging from 0.996 to 0.997. The limit of detection (LOD) for each fungicide was defined at a concentration where the signal-to-noise ratio was 3, with LODs ranging between 0.6 and 1.5 ng mL−1. The repeatability of the method for the determination of the target analytes was assessed by performing five consecutive analyses of the same sample (50 ng mL−1), which ranged from 3 to 6.5% (Table 1). The following formula is used to calculate the extraction recovery:
where Ci and Cf represent the aqueous solution concentration and the final concentration of analytes present in the eluent, respectively. Va is the sample volume and Vf is the eluent final volume. To assess the impact of varying batches of the sorbent on the method precision, RSD between batches was examined. For this purpose, three distinct batches of the sorbent were synthesized and utilized to quantify the target analytes (100.0 ng mL−1) in the sample solutions. The resulting RSDs were found to be less than 9.6% indicating that the method is relatively robust and consistent despite variations in the batches of sorbent.
EF for each fungicide was determined by calculating the analyte final concentration ratio in the eluted phase to its initial concentration in the source phase. A summary of the analytical merits of the suggested method can be found in Table 1.
Analysis of the real samples
The suggested method was used to determine the TF traces in river water, rice paddy soil, and grape. The findings indicated that none of the investigated TFs was present in any of the samples. The suggested method accuracy was examined by determining the relative recovery of river water (%) at concentrations of 5.0, 50.0, and 100.0 ng mL−1 and for soil and grape samples at concentrations of 20.0, 50.0, and 100.0 ng g−1. The relative recovery (RR) was calculated using the following equation:
where Cfound is the analyte concentration determined after adding a given quantity of standard, Creal is the initial analyte concentration in the actual sample, and Cadded is the standard known concentration that was added into the actual sample.
Additionally, the value of the matrix effect (ME%) was evaluated to analyze all real samples under the optimized conditions by using Eq. (3):
R m and Rw are the recovery of each analyte in the real matrix and ultrapure water, respectively. The results are summarized in Table 2, which indicates that the DMSPE treatment procedure can be utilized to extract and quantify triazole fungicides from real samples with slightly matrix effect. Furthermore, Fig. 4(a) and (b) show typical chromatograms of the grape sample (blank) and grape sample after spiking with TFs at a concentration of 50 ng mL−1, respectively, under optimal conditions.
Comparison of DMSPE with other reported methods
The figures of merit of the DMSPE method were compared with other MSPE methods for the extraction and determination of triazole fungicides in various real samples. The sorbents listed in Table 3 [3, 4, 7, 8, 40,41,42,43,44] have different natures and are used in field sample preparation. The methods [41, 43, 44] exhibited lower LOD in comparison with our developed method, which may be attributed to the use of expensive and sensitive detection instruments such as UHPLC-MS/MS, GC-MS, and HPLC-MS/MS for TF detection. The method provided in [40] exhibited limited linearity and low EF, although it has a shorter extraction time and lower relative standard deviations (RSD) compared to our method. In general, the results showed that the proposed method, which utilizes Fe3O4@SiO2@PolyBenzidine as a nano-sorbent, offers wider linearity, higher EF, and lower RSDs compared to other methods. These findings indicate that the method has suitable sensitivity and repeatability for the analysis of pesticides in soil and grape samples.
Conclusions
The DMSPE method utilizing the novel Fe3O4@SiO2@PBenz nano-sorbent was effectively employed to extract and preconcentrate triazole fungicides from various real samples. Coating the magnetic nanoparticles with polybenzidine improves the nano-sorbent ability of target analytes due to hydrophobic and π–π interactions and enhances the stability and dispersibility of the nanoparticles in aqueous environments. The proposed method for determining trace triazole fungicides exhibited low limits of detection, good repeatability, wide linear range, high enrichment factor, and satisfactory spiked recoveries. These characteristics confirm the outstanding performance of this method for monitoring of TFs in a wide variety of real samples. Furthermore, the sorbent could be regenerated at least five times without significantly decreasing extraction recoveries (<5%).
Although the synthesized sorbent was effective in extracting triazole fungicides from various real samples using the DMSPE method, however, the sorbent could not be directly applied to soil and grape matrices due to obstruction of the sorbent separation and reduced reusability. Therefore, pretreatment of these matrices was required before analysis, which is the limitation of this method.
References
Han X, Chen J, Li Z, Quan K, Qiu H (2020) Magnetic solid-phase extraction of triazole fungicides based on magnetic porous carbon prepared by combustion combined with solvothermal method. Analytica Chimica Acta 1129:85–97. https://doi.org/10.1016/j.aca.2020.06.077
Buerge IJ, Poiger T, Müller MD, Buser H-R (2006) Influence of pH on the stereoselective degradation of the fungicides epoxiconazole and cyproconazole in soils. Environ Sci Technol 40:5443–5450. https://doi.org/10.1021/es060817d
Li D, He M, Chen B, Hu B (2019) Magnetic porous organic polymers for magnetic solid-phase extraction of triazole fungicides in vegetables prior to their determination by gas chromatography-flame ionization detection. J Chromatogr A 1601:1–8. https://doi.org/10.1016/j.chroma.2019.04.062
Kadivar M, Aliakbar A (2019) A new composite based on graphene oxide-poly 3-aminophenol for solid-phase microextraction of four triazole fungicides in water and fruit juices prior to high-performance liquid chromatography analysis. Food Chem 299:125127. https://doi.org/10.1016/j.foodchem.2019.125127
Li L, Gao B, Zhang Z, Yang M, Li X, He Z, Wang M (2018) Stereoselective separation of the fungicide bitertanol stereoisomers by high-performance liquid chromatography and their degradation in cucumber. J Agric Food Chem 66:13303–13309. https://doi.org/10.1021/acs.jafc.8b04594
Pang J, Mei M, Yuan D, Huang X (2018) Development of on-line monolith-based in-tube solid phase microextraction for the sensitive determination of triazoles in environmental waters. Talanta 184:411–417. https://doi.org/10.1016/j.talanta.2018.03.005
Seebunrueng K, Tamuang S, Ruangchai S, Sansuk S, Srijaranai S (2021) In situ self-assembled coating of surfactant-mixed metal hydroxide on Fe3O4@ SiO2 magnetic composite for dispersive solid phase microextraction prior to HPLC analysis of triazole fungicides. Microchem J 168:106396. https://doi.org/10.1016/j.microc.2021.106396
Majd M, Nojavan S (2021) Magnetic dispersive solid-phase extraction of triazole and triazine pesticides from vegetable samples using a hydrophilic-lipophilic sorbent based on maltodextrin-and β-cyclodextrin-functionalized graphene oxide. Microchim Acta 188:1–12. https://doi.org/10.1007/s00604-021-05039-x
Senosy IA, Zhang X-Z, Lu Z-H, Guan X-Y, Yang Z-H, Li J-H, Guo H-M, Abdelrahman TM, Mmby M, Gbiliy A (2021) Magnetic metal-organic framework MIL-100 (Fe)/polyethyleneimine composite as an adsorbent for the magnetic solid-phase extraction of fungicides and their determination using HPLC-UV. Microchim Acta 188:1–9. https://doi.org/10.1007/s00604-020-04648-2
Xue J, Li H, Liu F, Jiang W, Hou F (2016) Vortex-assisted matrix solid–liquid dispersive microextraction for the analysis of triazole fungicides in cotton seed and honeysuckle by gas chromatography. Food Chem 196:867–876. https://doi.org/10.1016/j.foodchem.2015.09.093
Wang Y-X, Shen X-F, Feng Y-W, Pang Y-H (2023) Covalent organic framework in situ grown on Fe3O4 hollow microspheres for stir bar sorptive-dispersive microextraction of triazole pesticides. Microchim Acta 190:34. https://doi.org/10.1007/s00604-022-05613-x
Lv H, Jin X, Zhang Z, Chen Y, Zhu G, Li Z, Lee M (2022) Ultrasound-assisted switchable hydrophilic solvent-based homogeneous liquid–liquid microextraction for the determination of triazole fungicides in environmental water by GC-MS. Anal Methods 14:1187–1193. https://doi.org/10.1039/D1AY02109E
Machado SC, Souza BM, de Aguiar Marciano LP, Pereira AFS, de Carvalho DT, Martins I (2019) A sensitive and accurate vortex-assisted liquid-liquid microextraction-gas chromatography-mass spectrometry method for urinary triazoles. J Chromatogr A 1586:9–17. https://doi.org/10.1016/j.chroma.2018.11.082
Wang P, Zhao Y, Wang X, Yu GW, Wang J, Li ZG, Lee MR (2018) Microwave-assisted-demulsification dispersive liquid–liquid microextraction for the determination of triazole fungicides in water by gas chromatography with mass spectrometry. J Sep Sci 41:4498–4505. https://doi.org/10.1002/jssc.201800860
Charlton AJA, Jones A (2007) Determination of imidazole and triazole fungicide residues in honeybees using gas chromatography–mass spectrometry. J Chromatogr A 1141:117–122. https://doi.org/10.1016/j.chroma.2006.11.107
Celeiro M, Facorro R, Dagnac T, Llompart M (2018) Simultaneous determination of trace levels of multiclass fungicides in natural waters by solid-phase microextraction-gas chromatography-tandem mass spectrometry. Anal Chim Acta 1020:51–61. https://doi.org/10.1016/j.aca.2018.03.014
Liu G, Tian M, Lu M, Shi W, Li L, Gao Y, Li T, Xu D (2021) Preparation of magnetic MOFs for use as a solid-phase extraction absorbent for rapid adsorption of triazole pesticide residues in fruits juices and vegetables. J Chromatogr B 1166:122500. https://doi.org/10.1016/j.jchromb.2020.122500
Šelešovská R, Schwarzová-Pecková K, Sokolová R, Krejčová K, Martinková-Kelíšková P (2021) The first study of triazole fungicide difenoconazole oxidation and its voltammetric and flow amperometric detection on boron doped diamond electrode. Electrochim Acta 381:138260. https://doi.org/10.1016/j.electacta.2021.138260
Wang C, Wu Q, Wu C, Wang Z (2011) Application of dispersion–solidification liquid–liquid microextraction for the determination of triazole fungicides in environmental water samples by high-performance liquid chromatography. J Hazard Mater 185:71–76. https://doi.org/10.1016/j.jhazmat.2010.08.124
Zhang Y, Zhang Y, Zhao Q, Chen W, Jiao B (2016) Vortex-assisted ionic liquid dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography for the determination of triazole fungicides in fruit juices. Food Anal Methods 9:596–604. https://doi.org/10.1007/s12161-015-0223-6
Sun M, Sun H, Feng J, Feng J, Fan J, Sun M, Feng Y (2022) Carbonized metal-organic framework-74/carbon aerogel composites for the efficient extraction of triazole fungicides from fruits and vegetables. J Chromatogr A 1683:463552. https://doi.org/10.1016/j.chroma.2022.463552
He Y, Zhao F, Zhang C, Abd Ei-Aty AM, Baranenko DA, Hacimüftüoğlu A, She Y (2019) Assessment of magnetic core-shell mesoporous molecularly imprinted polymers for selective recognition of triazoles residual levels in cucumber. J. Chromatogr B 1132:121811. https://doi.org/10.1016/j.jchromb.2019.121811
Zendegi-Shiraz A, Sarafraz-Yazdi A, Es' haghi Z (2016) Polyethylene glycol grafted flower-like cupric nano oxide for the hollow-fiber solid-phase microextraction of hexaconazole, penconazole, and diniconazole in vegetable samples. J Sep Sci 39:3137-3144. https://doi.org/10.1002/jssc.201600429
Aladaghlo Z, Fakhari AR, Alavioon SI, Dabiri M (2019) Ultrasound assisted dispersive solid phase extraction of triazole fungicides by using an N-heterocyclic carbene copper complex supported on ionic liquid-modified graphene oxide as a sorbent. Microchim Acta 186:1–8. https://doi.org/10.1007/s00604-019-3276-1
Sun P, Gao Y, Lian Y (2019) Determination of triazole fungicides in environmental water by magnetic solid-phase extraction coupled with UHPLC-MS/MS. J Iran Chem Soc 16:1483–1489. https://doi.org/10.1007/s13738-019-01614-5
Zhang Y, Zhang Y, Nie J, Jiao B, Zhao Q (2016) Determination of triazole fungicide residues in fruits by QuEChERS combined with ionic liquid-based dispersive liquid-liquid microextraction: optimization using response surface methodology. Food Anal Methods 9:3509–3519. https://doi.org/10.1007/s12161-016-0548-9
Asgharinezhad AA, Ebrahimzadeh H, Mirbabaei F, Mollazadeh N, Shekari N (2014) Dispersive micro-solid-phase extraction of benzodiazepines from biological fluids based on polyaniline/magnetic nanoparticles composite. Anal Chim Acta 844:80–89. https://doi.org/10.1016/j.aca.2014.06.007
Bagheri H, Ayazi Z, Naderi M (2013) Conductive polymer-based microextraction methods: a review. Anal Chim Acta 767:1–13. https://doi.org/10.1016/j.aca.2012.12.013
Mehrabian M, Noroozian E, Maghsoudi S (2021) Preparation and application of Fe3O4@ SiO2@ poly (o-phenylenediamine) nanoparticles as a novel magnetic sorbent for the solid-phase extraction of tellurium in water samples and its determination by ET-AAS. Microchem J 165:106104. https://doi.org/10.1016/j.microc.2021.106104
Bagheri H, Daliri R, Roostaie A (2013) A novel magnetic poly (aniline-naphthylamine)-based nanocomposite for micro solid phase extraction of rhodamine B. Anal Chim Acta 794:38–46. https://doi.org/10.1016/j.aca.2013.07.066
D’Eramo F, Zón MA, Fernández H, Sereno L, Arévalo AH (2008) Studies of a novel conducting polymer by cyclic and square wave voltammetries: its synthesis and characterization. Electrochim Acta 53:7182–7190. https://doi.org/10.1016/j.electacta.2008.05.013
Muslim A, Malik D, Rexit AA (2012) Effects of monomer concentration on the structure and properties of polybenzidine micro rods. Polym Sci Ser B 54:518–524. https://doi.org/10.1134/S1560090412100077
do Nascimento GM, Pradie NA (2021) Resonance Raman characterization of poly (benzidine) in different oxidation states. J Mol Struct 1242:130751. https://doi.org/10.1016/j.molstruc.2021.130751
Durgaryan R, Durgaryan N (2021) Chemical oxidative condensation of benzidine in non-aqueous medium: synthesis and investigation of oligomers and polymer with benzidine diimine units. Polymers 14:34. https://doi.org/10.3390/polym14010034
Naveen Kumar M, Nagabhooshanam M, Anand Rao M, Bhagvanth Rao M (2001) Preparation and characterization of doped polybenzidine. Crystal Res Technol: J Exp Ind Crystallogr 36:309–317. https://doi.org/10.1002/1521-4079(200103)36:3
Tahmasebi E, Yamini Y (2012) Facile synthesis of new nano sorbent for magnetic solid-phase extraction by self assembling of bis-(2, 4, 4-trimethyl pentyl)-dithiophosphinic acid on Fe3O4@ Ag core@ shell nanoparticles: characterization and application. Anal Chim Acta 756:13–22. https://doi.org/10.1016/j.aca.2012.10.040
He X, Zhou Y, Yang W, Li S, Liu T, Wang T, Hou X (2019) Microwave assisted magnetic solid phase extraction using a novel amino-functionalized magnetic framework composite of type Fe3O4-NH2@ MIL-101 (Cr) for the determination of organochlorine pesticides in soil samples. Talanta 196:572–578. https://doi.org/10.1016/j.talanta.2018.12.019
Teixeira RA, Dinali LAF, de Oliveira HL, da Silva ATM, Borges KB (2021) Efficient and selective extraction of azamethiphos and chlorpyrifos residues from mineral water and grape samples using magnetic mesoporous molecularly imprinted polymer. Food Chem 361:130116. https://doi.org/10.1016/j.foodchem.2021.130116
Fahimirad B, Rajabi M, Elhampour A (2019) A rapid and simple extraction of anti-depressant drugs by effervescent salt-assisted dispersive magnetic micro solid-phase extraction method using new adsorbent Fe3O4@SiO2@N3. J Anal Chim Acta 1047:275–284. https://doi.org/10.1016/j.aca.2018.10.028
Kachangoon R, Vichapong J, Santaladchaiyakit Y, Srijaranai S (2022) Green fabrication of Moringa oleifera seed as efficient biosorbent for selective enrichment of triazole fungicides in environmental water, honey and fruit juice samples. Microchem J 175:107194. https://doi.org/10.1016/j.microc.2022.107194
Yi X, Liu C, Liu X, Wang P, Zhou Z, Liu D (2019) Magnetic partially carbonized cellulose nanocrystal-based magnetic solid phase extraction for the analysis of triazine and triazole pesticides in water. Microchim Acta 186:825. https://doi.org/10.1007/s00604-019-3911-x
Senosy IA, Guo H-M, Ouyang M-N, Lu Z-H, Yang Z-H, Li J-H (2020) Magnetic solid-phase extraction based on nano-zeolite imidazolate framework-8-functionalized magnetic graphene oxide for the quantification of residual fungicides in water, honey and fruit juices. Food Chem 325:126944. https://doi.org/10.1016/j.foodchem.2020.126944
Chen F, Song Z, Nie J, Yu G, Li Z, Lee M (2016) Ionic liquid-based carbon nanotube coated magnetic nanoparticles as adsorbent for the magnetic solid phase extraction of triazole fungicides from environmental water. RSC Adv 6:81877–81885. https://doi.org/10.1039/C6RA16682B
Su H, Lin Y, Wang Z, Wong YLE, Chen X, Chan TWD (2016) Magnetic metal–organic framework–titanium dioxide nanocomposite as adsorbent in the magnetic solid-phase extraction of fungicides from environmental water samples. J Chromatogr A 1466:21–28. https://doi.org/10.1016/j.chroma.2016.08.066
Acknowledgements
Financial support from the Research Affairs of Shahid Beheshti University is gratefully acknowledged.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Abolfath Shahsavani: conceptualization, methodology, investigation, formal analysis, validation, writing original draft, supervision. Zolfaghar Aladaghlo: methodology, writing, review and editing. Alireza Fakhari: supervision, resources, writing, review and editing, project administration. All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) the approval of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2.29 mb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahsavani, A., Aladaghlo, Z. & Fakhari, A.R. Dispersive magnetic solid phase extraction of triazole fungicides based on polybenzidine/magnetic nanoparticles in environmental samples. Microchim Acta 190, 377 (2023). https://doi.org/10.1007/s00604-023-05948-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05948-z