Abstract
Novel magnetic multi-walled carbon nanotubes@Fe3O4 molecularly imprinted polymers (MWNTs@Fe3O4-MIPs) intended for bovine serum albumin (BSA) recognition were successfully developed. The MWNTs@Fe3O4-MIPs were characterized with scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FT-IR). Scanning electron microscopy images showed that the Fe3O4 nanoparticles (diameter: 50–60 nm) were coated with a layer of MIPs with an average thickness of 25–30 nm. The magnetic material was easily dispersed and retrieved through the application of an external magnetic field. Adsorption experiments showed that the estimated maximum amount of BSA that could be adsorbed onto the MWNTs@Fe3O4-MIPs was 52.8 mg/g, and the time taken to reach equilibrium was about 40 min. Meanwhile, the MWNTs@Fe3O4-MIPs exhibited excellent selectivity towards (i.e., recognition of) BSA. The feasibility of the use of the MWNTs@Fe3O4-MIPs as a solid-phase extraction (SPE) sorbent was evaluated, and the results showed that the MWNTs@Fe3O4-MIPs were able to separate the template protein BSA from a binary protein solution. The proposed sorbent based on MWNTs@Fe3O4-MIPs for BSA separation exhibited satisfactory recoveries ranging from 92.0% to 97.3% in real samples. It was also successfully used for the purification of BSA from bovine calf serum.

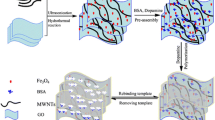
Procedure for preparing magnetic protein imprinted polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molecularly imprinted polymers (MIPs) are artificially synthesized polymeric materials, each of which contains a large number of cavities that are complementary in terms of shape, size, and functional groups present to a specific target molecule. Therefore, MIPs show the ability to recognize specific molecules, and high binding affinities for these target molecules [1, 2]. In recent years, MIPs have been widely used in various fields, such as separations [3], sensors [4], and catalysis [5]. The imprinting of small molecules onto polymers has been performed with great success ever since noncovalent and covalent methods of doing so were formulated by Mosbach and Wulff, respectively [6, 7]. However, the imprinting of macromolecules like proteins onto polymers has proven to be more problematic, and developments in this area of research have been rather slow. This is due to the challenges associated with the large molecular size of such macromolecules, their relatively unstable three-dimensional conformations, and limitations on their diffusion. In response to such limitations, surface imprinting has been proposed as a viable strategy for protein imprinting.
Surface imprinting can overcome not only the disadvantages mentioned above, but also those associated with the production of bulk MIPs by the traditional method, such as the time and labor required, long response times, and poor site accessibility to the template molecule [8, 9]. Many kinds of matrices have been used to synthesize MIPs, such as silica nanoparticles [10], silica nanotubes [11], and quantum dots [12]. Most recognition sites are situated on the surface of the material, which makes it easier and quicker to bind and remove template molecules due to easy accessibility and low mass transfer resistance. Multi-walled carbon nanotubes (MWNTs), which were first discovered in 1991, are widely considered the quintessential nanomaterial. Due to their high strength, extremely large surface areas, and unique chemical properties, MWNTs can serve as a reinforcing element or the core when fabricating core–shell structural MIPs. A thin layer of MIPs can be polymerized onto the surfaces of MWNTs. Thus, binding cavities in the thin MIPs outer layer can greatly improve the accessibility to template molecules.
During the past decade, the synthesis of nanosized magnetic materials has been the subject of intensive research, owing to their biocompatibility and potential applications. When Fe3O4 particles are encapsulated inside MIPs, the resulting polymers are magnetically susceptible. Thus, they can be easily—and rather usefully—isolated from the sample by applying an external magnetic field, without the need for complicated centrifugation steps or filtration. Successful applications of magnetic MIPs to the recognition of biomolecules have also been reported. Tong and coworkers reported the preparation of core–shell magnetic MIPs via miniemulsion polymerization for bovine serum albumin (BSA) recognition [13]. Yang and coworkers synthesized magnetic MIPs in a nanoporous alumina membrane for theophylline recognition [14]. Therefore, magnetic nanoparticle/multiwalled carbon nanotube composites could have potential applications as novel magnetic materials that combine the magnetism of Fe3O4 nanoparticles with the outstanding mechanical properties of MWNTs.
To the best of our knowledge, there is no report of the preparation and application of MWNTs@Fe3O4-MIPs for protein recognition. Therefore, this work focused on the synthesis of novel magnetic MIPs based on multi-walled carbon nanotubes and their application to protein recognition. The adsorption kinetics, static adsorption, and selective recognition of the magnetic MIPs were investigated in detail. This method was used to separate BSA from real serum samples.
Materials and methods
Materials and reagents
Multi-walled carbon nanotubes (MWNTs, 95%) were obtained from Shenzhen Bill Corporation (China). Bovine serum albumin (BSA), human serum albumin (HSA), bovine hemoglobin (BHb), ovalbumin (OB), and lysozyme (Lyz) were purchased from Shanghai Lanji Co. Ltd. (Shanghai, China). Bovine calf serum was purchased from RuiTe Biotech Company (Guangzhou, China). Methacrylic acid (MAA) and ethyleneglycol dimethacrylate (EGDMA) were purchased from Sigma (St. Louis, MO, USA). Sodium hydroxide (NaOH), 2,2′-azobisisobutyronitrile (AIBN), methanol, ethanol, acetic acid (HAc), ammonium persulfate ((NH4)2S2O8), N, N′-dimethylformamide (DMF), FeCl3·6H2O, nitric acid (HNO3), ethylene glycol (EG), polyethylene glycol (PEG, M W = 4000), sodium acetate (NaAc), and aniline were obtained from Changsha Chemical Reagent Company (Hunan, China). All of the chemicals used in this work were of analytical grade. Double-distilled water was used throughout this work.
Pretreatment of the multi-walled carbon nanotubes
Five hundred milligrams of MWNTs were dispersed in 50 mL of nitric acid solution under sonication for 10 min. Then the mixture was stirred continuously at 80 °C for 24 h. After being cooled to room temperature, the mixture was diluted tenfold with double-distilled water. The mixture was then filtered through a 0.45 μm polytetrafluoroethylene (PTFE) membrane and rinsed with double-distilled water until the pH was neutral. Finally, the filtered solid was dried under vacuum at 80 °C for 24 h to obtain MWNTs-COOH.
Synthesis of Fe3O4 nanoparticle coated MWNTs
In our experiment, Fe3O4 nanoparticles were generated by reduction reactions between FeCl3 and ethylene glycol (EG) in a hydrothermal system, as described in [15]. Typically, 0.81 g of FeCl3·6H2O were dissolved in 40 mL of EG to form an orange solution. Then 200 mg of MWNTs-COOH were dispersed in the above solution by sonicating for 3 h. After that, 3.6 g of NaAc and 1.0 g of polyethylene glycol (PEG) were added under constant stirring for 30 min. The mixture was sealed in a Teflon-lined stainless steel autoclave and maintained at 200 °C for 8 h, then cooled to ambient temperature. The MWNTs–Fe3O4 composites (designated MWNTs@Fe3O4) were collected using an external magnetic field and washed three times with alcohol and double-distilled water, respectively. There were finally dried in a vacuum at 60 °C for 10 h.
Synthesis of MWNTs@Fe3O4/polyaniline (PANI) nanotubes
MWNTs@Fe3O4/PANI composites were synthesized according a method used in previous studies [16, 17]. First, 100 mg of MWNTs@Fe3O4 were dispersed in 30 mL of double-distilled water by sonicating for 20 min. In a typical procedure, 0.14 g of aniline were added to 7.5 mL of the above mixture. The mixture was stirred for 30 min and cooled at 0–5 °C for 0.5 h. Then, 0.4 g of FeCl3·6H2O dissolved in 7.5 mL of double-distilled water at 0–5 °C were quickly added. The mixture was allowed to react at 0–5 °C for 15 h. Finally, the solid was collected using an external magnetic field and dried at 50 °C for 24 h after washing three times each with double-distilled water and ethanol.
Preparation of MWNTs@Fe3O4-MIPs
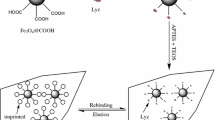
The multi-walled carbon nanotubes@Fe3O4 molecularly imprinted polymers (MWNTs@Fe3O4-MIPs) was prepared as follows: a solution of BSA (0.22 mmol) in Tris-HCl buffer solution (7.75 mL) and 200 mg of MWNTs@Fe3O4 were added to a 100 mL round flask. The mixture was magnetically stirred at 300 rpm for 3 h at 4 °C. Then the BSA-immobilized particles were washed three times with double-distilled water upon the completion of the reaction. Next, 0.88 mmol of MAA, 4.40 mmol of EGDMA, 30 mg of AIBN, and 30 mL of acetonitrile were added to this mixture. Flushing with nitrogen gas, the mixture was then polymerized at 40 °C for 12 h under magnetic stirring. After polymerization, the MWNTs@Fe3O4-MIPs were washed with Tris-HCl buffer solution (pH 7.0) containing 0.5 mol/L NaCl to remove the template molecules. Finally, the obtained polymer was dried at 50 °C for 24 h.
The corresponding molecularly non-imprinted polymers (MWNTs@Fe3O4-NIPs) were prepared in the same manner in the absence of template protein.
Effect of the pH on the rebinding of protein
The effect of the pH on protein adsorption experiments was investigated for the magnetic molecularly imprinted and non-imprinted polymers. Briefly, protein solutions (0.4 mg/mL) were prepared in Tris-HCl buffer solution with different pH values. Ten milliliters of the protein solution and 100 mg of the MWNTs@Fe3O4-MIPs were added to a 25 mL tube. The tube was then sealed and shaken at room temperature for 1 h. The MWNTs@Fe3O4-MIPs were isolated by an external magnetic field. The BSA concentration in the supernatant was measured by high-performance liquid chromatography (HPLC) analysis. The amount of protein adsorbed by the MWNTs@Fe3O4-MIPs was calculated based on the change in protein concentration before and after incubation. The amount of protein adsorbed by the polymer was determined by the following formula [18]:
where Q (mg/g) is the amount of protein adsorbed by the MWNTs@Fe3O4-MIPs, C (mg/mL) is the initial protein concentration, C f (mg/mL) is the final protein concentration, V (mL) is the volume of the protein solution, and m (g) is the mass of the MWNTs@Fe3O4-MIPs. All tests were carried out in triplicate.
Adsorption
The adsorption kinetics of the MWNTs@Fe3O4-MIPs toward BSA was investigated by varying the adsorption time from 10 to 80 min. Ten milligrams of the MWNTs@Fe3O4-MIPs were suspended in 10 mL of a 0.4 mg/mL BSA Tris-HCl buffer solution. The tube was incubated at room temperature. At different time intervals, the amount of BSA adsorbed by the MWNTs@Fe3O4-MIPs was determined by HPLC.
Ten milligrams of MWNTs@Fe3O4-MIPs or MWNTs@Fe3O4-NIPs were suspended in 5.0 mL of Tris-HCl buffer solution with different initial BSA concentrations ranging from 0.1 mg/mL to 1.0 mg/mL. After incubating for 40 min, the MWNTs@Fe3O4-MIPs or the MWNTs@Fe3O4-NIPs were isolated by an external magnetic field. Then the BSA concentration in the supernatant was measured by HPLC analysis. The amount of BSA bound by the MWNTs@Fe3O4-MIPs or the MWNTs@Fe3O4-NIPs was calculated by subtracting the amount of free BSA in the supernatant from the amount of BSA initially added.
Selectivity of the MWNTs@Fe3O4-MIPs
Competitive adsorption was investigated with a protein mixture containing 0.5 mg/mL BSA and 0.5 mg/mL BHb, HSA, Lyz, and OB in Tris-HCl buffer solution. Ten milliliters of the mixed protein solution and 10.0 mg of MWNTs@Fe3O4-MIPs or MWNTs@Fe3O4-NIPs were suspended in 5.0 mL of Tris-HCl buffer solution. After incubating for 40 min, the MWNTs@Fe3O4-MIPs or the MWNTs@Fe3O4-NIPs were isolated using an external magnetic field. The protein concentrations in the supernatant were measured by HPLC analysis.
The specific recognition properties of the MWNTs@Fe3O4-MIPs were evaluated by determining the imprinting factor (α), which is defined as follows [18]:
where Q(A) and Q(B) are the capacities of the MWNTs@Fe3O4-MIPs and the MWNTs@Fe3O4-NIPs, respectively, to adsorb the template protein or the competitive proteins.
The selectivity factor (β) is defined as follows [18]:
where α 1 is the imprinting factor with respect to the template protein and α 2 is the imprinting factor with respect to the competitive proteins.
Chromatographic measurements
HPLC measurements were carried out with a Shimadzu LC-2010AHT solution system (Kyoto, Japan). The HPLC conditions employed in this study were as follows: mobile phase, 0.3 mol/L NaCl solution; stainless steel column (100 mm × 4.6 mm i.d.); 10 μL of 1.0 mg/mL analytes in 10 mmol/L Tris-HCl buffer solution were injected for analysis; flow rate, 0.5 mL/min; room temperature; UV detection wavelength, 280 nm.
Real sample analysis
The MWNTs@Fe3O4-MIPs were applied to separate BSA from a real sample of bovine calf serum. The sample was processed as described by Hua et al. [19]. The serum was diluted tenfold with Tris-HCl buffer solution (10 mmol/L, pH 7.0) containing 1.0 mmol/L NaCl. First, the MWNTs@Fe3O4-MIPs were treated with Tris-HCl buffer solution (10.0 mmol/L, pH 7.0) containing 3.0 mmol/L NaCl to wash out the non-specifically adsorbed protein. Then 5.0 mmol/L NaCl were employed to elute the specifically adsorbed protein. The eluates were desalted and concentrated tenfold using an ultrafiltration membrane. Finally, 10 μL of the sample were injected for HPLC analysis.
Results and discussion
Preparation of magnetic molecularly imprinted nanoparticles
The synthesis of the MWNTs@Fe3O4-MIPs via a multi-step procedure is illustrated in Fig. 1, which involves the synthesis of magnetic MWNTs@Fe3O4, PANI deposition, MIPs functionalization, and extraction of the BSA template. Fe3O4 was synthesized in the manner reported by Jia et al. [15]. Since the MWNTs@Fe3O4-MIPs are very susceptible to a magnetic field, they can be separated from a suspension easily and quickly (see the Electronic supplementary material, ESM, Fig. S1). Generally, the treatment of MWNTs with nitric acid creates a considerable number of carboxyl groups on the surfaces of the MWNTs, which then become negatively charged. Thus, the positive metal ions in the system attach to and interact with the carboxyl groups via electrostatic attraction, and serve as nucleation precursors. In our case, ferric ions in the solution attached to some particular positions on the MWNTs with high carboxyl densities, and were then reduced in situ to Fe3O4 during subsequent hydrothermal treatment [20]. The nanocomposites were then decorated with PANI via a self-assembly process. In order to prevent the destruction of the nanostructure of the MWNTs@Fe3O4, FeCl3 was used as the oxidant. Phenyl groups of PANI were then introduced onto the surfaces of the MWNTs@Fe3O4, which are known to interact with acrylate via covalent bonding [21]. Thus, phenyl groups grafted on the surfaces of the MWNTs were able to copolymerize directly with the functional monomer and crosslinker. After polymerization, the MWNTs@Fe3O4-MIPs were washed with Tris-HCl buffer (pH 7.0) containing 0.5 mol/L NaCl to remove the template protein. Almost all of the template protein was extracted in the first washing cycle; only a small amount was retrieved in the next two cycles. This neutrality of the solution helped the protein to retain its activity, which made the recovery and reuse of the template protein possible [19].
Generally, the molar ratio of the functional monomer to the template is a very important influence on the specific affinity of the MIPs and the number of recognition sites. High ratios of the functional monomer to the template result in high nonspecific affinity, while low ratios lead to fewer template and functional monomer complexes due to an insufficient number of functional groups [22]. As shown in Fig. 2, Q increases with the amount of functional monomer or crosslinker, due to the increase in the number of recognition cavities in the MWNTs@Fe3O4-MIPs. However, MWNTs@Fe3O4-MIPs agglomeration occurs when excessive amounts of monomer and crosslinker are used at the preparation stage [23]. Studies showed when the molar ratio was more than 1:5:20, MWNTs@Fe3O4-MIPs agglomeration occurs. Thus, an adsorption experiment was not carried out due to the agglomeration of MWNTs@Fe3O4-MIPs. The results revealed that the optimum molar ratio of template molecule to functional monomer to crosslinkers was 1:4:20, so that ratio was used in the remainder of this work.
Characterization of magnetic molecularly imprinted nanoparticles
Typical SEM images for the crude MWNTs, MWNTs@Fe3O4, and MWNTs@Fe3O4-MIPs are shown in Fig. 3. Figure 3a indicates that the crude MWNTs were tubular and had diameters of 10–20 nm. Figure 3b shows that Fe3O4 nanoparticles with diameters of 50–60 nm were homogeneously deposited onto the surfaces of the MWNTs. However, the Fe3O4 nanoparticles were only deposited onto the MWNTs at certain specific positions, leading to necklace-like nanostructures. This meant that the magnetic MWNTs@Fe3O4-MIPs exhibited a fast, sensitive magnetic response. Figure 3c shows that the Fe3O4 nanoparticles were coated with a layer of MIPs, as the diameters of the imprinted polymers have clearly increased to 75–90 nm at the same magnification as in Fig. 3b. Thus, the MIPs layer had an average thickness of 25–30 nm.
FT-IR spectra were obtained in order to characterize the chemical structures of crude MWNTs, MWNTs@Fe3O4, MWNTs@Fe3O4/PANI, and MWNTs@Fe3O4-MIPs. These spectra are shown in Fig. 4. Compared with Fig. 4a, a sharp and strong Fe–O stretching peak at 586 cm−1 was observed in Fig. 4b, which indicated that the Fe3O4 nanoparticles were successfully deposited on the surfaces of the MWNTs. The characteristic peaks at 3428 cm−1 (N–H stretching peak), 1493 cm−1 (benzenoid ring), 1304 cm−1 (C–N stretching peak for a secondary aromatic amine) seen in Fig. 4c were similar to observed for the PANI sample [24], which demonstrated that PANI was successfully introduced onto the surface of MWNTs@Fe3O4. In Fig. 4d, the peak at 1720 cm−1 can be ascribed to a stretching vibration of COOH from MAA. The obvious stretching vibration peaks from C–O (at 1720 cm−1) and C–O–C (at 1020 cm−1) indicate the existence of EGDMA [25]. These vibrations confirmed that the MIPs were grafted onto the MWNTs@Fe3O4 successfully.
Effect of the pH on the rebinding of protein
The effect of the pH on the adsorption of the BSA template was studied for the MWNTs@Fe3O4-MIPs and the MWNTs@Fe3O4-NIPs in the presence of a 0.4 mg/mL BSA solution. Their capacities to adsorb the template protein at different pH values were measured, and the results are shown in Fig. 5. It was found that the capacity of the MWNTs@Fe3O4-MIPs to adsorb BSA depended significantly on the pH value. The adsorption capacity was greatest when the pH value was between 4.7 and 5.0. This is because the isoelectric point (pI) of BSA is about 4.7, so when the system’s pH is close to 4.7, the BSA template is neutral and the hydrophobic interaction between BSA and the MWNTs@Fe3O4-MIPs is maximized [26]. Therefore, an incubation buffer of pH 4.7 was adopted for subsequent selective adsorption experiments.
Characterizing the adsorption of MWNTs@Fe3O4-MIPs
The kinetics of protein rebinding was investigated by varying the adsorption time from 10 to 80 min and keeping the initial concentration of BSA constant at 0.4 mg/mL. The results are presented in Fig. 6. The kinetic curve observed is typical of most rebinding processes, and reveals rapid dynamic adsorption of the BSA onto the magnetic MIPs. During the first 40 min, the adsorption capacity increased with adsorption time, and after that the adsorption capacity remained constant over time. These results show that the adsorption takes about 40 min to equilibrate. Compared to previous studies [13, 27, 28], the MWNTs@Fe3O4-MIPs produced in this work take a shorter time to reach adsorption equilibrium. Thus, it is believed that the MWNTs@Fe3O4-MIPs possess the property of good mass transport.
The adsorption capacity is an important factor when evaluating the MWNTs@Fe3O4-MIPs. A series of solutions with different BSA concentrations were investigated. As shown in Fig. 7, the capacity of the MWNTs@Fe3O4-MIPs to adsorb the template increased with increasing initial BSA concentration. The static BSA adsorption capacities of the MWNTs@Fe3O4-MIPs and MWNTs@Fe3O4-NIPs were 52.8 mg/g and 14.0 mg/g, respectively. Obviously, the static adsorption capacity of the MWNTs@Fe3O4-MIPs was much larger than that of the MWNTs@Fe3O4-NIPs. This demonstrates that the magnetic MWNTs@Fe3O4-MIPs are capable of specifically adsorbing the template molecule, because the imprinted cavities complement the size and shape of the template protein. However, the MWNTs@Fe3O4-NIPs cannot form specific BSA recognition sites in the absence of the template molecule.
Selectivity of the MWNTs@Fe3O4-MIPs
The selectivity of the MWNTs@Fe3O4-MIPs toward the template and towards other proteins was tested. Four types of proteins (BHb, HSA, Lyz, and OB) were selected to investigate the selectivity of the imprinted polymers. The adsorption capacities of the MWNTs@Fe3O4-MIPs and MWNTs@Fe3O4-NIPs toward these proteins were determined using the equilibrium adsorption method. The selected proteins possess large differences in molecular weight and isoelectric points (pI). As shown in Fig. 8, the capacity of the MWNTs@Fe3O4-MIPs to adsorb BSA was more than their capacity to adsorb competitive proteins. Moreover, except in the case of Lyz, the capacities of the magnetic MWNTs@Fe3O4-MIPs and MWNTs@Fe3O4-NIPs to adsorb other proteins did not differ significantly. Lyz, with its smaller molecular weight, diffused more easily into the imprinted cavities and caused some nonspecific adsorption. The larger proteins (BHb, HSA, and OB) were easier to exclude from the binding cavities due to steric effects, which led to low capacities to adsorb these proteins. This is because the cavities in the MWNTs@Fe3O4-MIPs were created by BSA, so access to the imprinted sites was hindered for polymer chains of proteins larger than BSA.
Additionally, the imprinting factor (α) and the selectivity factor (β) were used to evaluate the specific recognition properties of the magnetic molecularly imprinted polymers, and the results are listed in Table 1. The imprinting factor (α) for the template protein BSA is 4.20, which is greater than those for the competitive proteins BHb (1.09), HSA (1.08), Lyz (1.38), and OB (1.06). The β values of the competitive proteins were 3.85 (BHb), 3.88 (HSA), 3.04 (Lyz), and 3.23 (OB), indicating that the adsorption of each of the competitive proteins by MWNTs@Fe3O4-MIPs was low. Thus, we can infer that the recognition mechanism of the MWNTs@Fe3O4-MIPs depends on shape memory effects.
Separation of proteins on a MWNTs@Fe3O4-MIPs column
To evaluate the ability of a MWNTs@Fe3O4-MIPs column to separate out the template protein, 100 mg of MWNTs@Fe3O4-MIPs or MWNTs@Fe3O4-NIPs were packed into a stainless steel column (100 mm × 4.6 mm i.d.) coupled to an HPLC and analyzed. Among the competitive proteins considered in this work, BHb has a molecular weight and an isoelectric point that is closest to those of BSA, so BHb was selected as the competitive protein to use to evaluate the separation ability of the MWNTs@Fe3O4-MIPs column. Figure 9 shows separation chromatograms of BHb and BSA obtained with MWNTs@Fe3O4-MIPs and MWNTs@Fe3O4-NIPs columns. The MWNTs@Fe3O4-MIPs exhibited excellent ability to separate BSA from the competitive protein BHb, and near-baseline separation was achieved. These results indicate that the imprinting process for the MWNTs@Fe3O4-MIPs results in the formation of recognition sites that are complementary in shape and size with respect to the BSA template. In contrast, nonspecific adsorption was the dominant effect in the MWNTs@Fe3O4-NIPs column due to the lack of an imprinting process. Thus, the MWNTs@Fe3O4-NIPs column did not display any ability to recognize the template protein, and baseline separation was not achieved.
Chromatograms for the separation of competitive protein and BSA on the MWNTs@Fe3O4-MIPs column (a) and the MWNTs@Fe3O4-NIPs column (b). Experimental conditions: mobile phase, 0.3 mol/L NaCl solution; flow rate, 0.5 mL/min; column pressure, 10 MPa; stainless steel column, 100 mm × 4.6 mm i.d.; detection wavelength, 280 nm; C BSA = C BHb = 1.0 mg/mL
Real sample analysis
To further validate the feasibility of the application of this method to the analysis of real samples, the MWNTs@Fe3O4-MIPs column was applied to separate BSA spiked at four different levels into bovine calf serum. In our experiment, spiking was performed by adding a microvolume of solution containing four different concentrations of BSA to each sample. The samples were extracted according to the procedure described in the “Materials and methods” section. The recoveries were calculated and are summarized in Table 2. As shown in Table 2, the recoveries ranged from 92.0% to 97.3%, suggesting that this method can feasibly be applied to the separation of BSA from bovine calf serum.
Conclusions
In this paper, magnetic MWNTs@Fe3O4-MIPs were successfully synthesized with a novel surface molecular imprinting technique using carbon nanotube as the support matrix. The MWNTs@Fe3O4-MIPs were evaluated by scanning electron microscopy and Fourier transform infrared spectroscopy. The results suggested that the MIPs were successfully immobilized on the surfaces of the MWNTs. The magnetic MWNTs@Fe3O4-MIPs can be easily dispersed and retrieved through the application of a magnetic field. As the BSA-imprinted sites are located at or close to the surface, the magnetic MIPs exhibited fast adsorption dynamics as well as excellent adsorption specificity and recognition capacity towards BSA. Furthermore, the MWNTs@Fe3O4-MIPs were successfully employed as an HPLC stationary phase to separate the template BSA from a binary protein solution. The results of a real sample analysis suggested that the MWNTs@Fe3O4-MIPs column is a feasible technique for selectively separating BSA from bovine calf serum. Due to their easy preparation and its high selectivity and binding capacity, the MWNTs@Fe3O4-MIPs appear to be a highly promising candidate for a novel stationary phase to use in biochemical separations.
References
Tamayo FG, Turiel E, Martin-Esteban A (2007) Molecularly imprinted polymers for solid-phase extraction and solid-phase microextraction: recent developments and future trends. J Chromatogr A 1152:32–40
Long Y, Xing XC, Han RF, Sun Y, Wang Y, Zhao Z, Mi HF (2008) Two-step purification of low-content cellular protein using protein-imprinted polymers. Anal Biochem 380:268–275
Baggiani C, Baravalle P, Giraudi G, Tozzi CJ (2007) Molecularly imprinted solid-phase extraction method for the high-performance liquid chromatographic analysis of fungicide pyrimethanil in wine. J Chromatogr A 1141:158–164
Sontimuang C, Suedee R, Dickert F (2011) Interdigitated capacitive biosensor based on molecularly imprinted polymer for rapid detection of Hev b1 latex allergen. Anal Biochem 410:224–233
Pasetto P, Maddock SC, Resmini M (2005) Synthesis and characterisation of molecularly imprinted catalytic microgels for carbonate hydrolysis. Anal Chim Acta 542:66–75
Vlatakis G, Andersson LI, Muller R, Mosbach K (1993) Drug assay using antibody mimics made by molecular imprinting. Nature 361:645–647
Wulff G (1995) Molecular imprinting in cross-linked materials with the aid of molecular templates-a way towards artificial antibodies. Chem Int Ed Engl 34:1812–1832
Gao DM, Zhang ZP, Wu MH, Xie C, Guan GJ, Wang DP (2007) A surface functional monomer-directing strategy for highly dense imprinting of TNT at surface of silica nanoparticles. J Am Chem Soc 129:7859–7866
Niu J, Liu ZH, Fu L, Shi F, Ma HW, Ozaki Y, Zhang X (2008) Surface-imprinted nanostructured layer-by-layer film for molecular recognition of theophylline derivatives. Langmuir 24:11988–11994
Zhai CX, Lu Q, Chen XM, Peng Y, Chen L, Du SH (2009) Molecularly imprinted layer-coated silica nanoparticles toward highly selective separation of active diosgenin from Dioscorea nipponica Makino. J Chromatogr A 1216:2254–2262
Xie CG, Liu BH, Wang ZY, Gao DM, Guan GJ, Zhang ZP (2008) Molecular imprinting at walls of silica nanotubes for TNT recognition. Anal Chem 80:437–443
Li HB, Li YL, Cheng J (2010) Molecularly imprinted silica nanospheres embedded CdSe quantum dots for highly selective and sensitive optosensing of pyrethroids. Chem Mater 22:2451–2457
Tan CJ, Chua HG, Ker KH, Tong YW (2008) Preparation of bovine serum albumin surface-imprinted submicrometer particles with magnetic susceptibility through core–shell miniemulsion polymerization. Anal Chem 80:683–692
Li Y, Yin XF, Chen FR, Yang HH, Zhuang ZX, Wang XR (2006) Synthesis of magnetic molecularly imprinted polymer nanowires using a nanoporous alumina template. Macromolecules 39:4497–4499
Jia BP, Gao L, Sun J (2007) Self-assembly of magnetite beads along multiwalled carbon nanotubes via a simple hydrothermal process. Carbon 45:1476–1481
De Araújo ACV Jr, Alves S, Azevedo WM (2008) A new synthesis route to prepare polyaniline (PANI) nanotubes containing magnetic nanoparticles. Adv Sci Technol 54:325–330
Kong LR, Lu XF, Zhang WJ (2008) Facile synthesis of multifunctional multiwalled carbon nanotubes/Fe3O4 nanoparticles/polyaniline composite nanotubes. J Solid State Chem 181:628–636
Zhang W, Qin L, He XW, Li WY, Zhang YK (2009) Novel surface modified molecularly imprinted polymer using acryloyl-β-cyclodextrin and acrylamide as monomers for selective recognition of lysozyme in aqueous solution. J Chromatogr A 1216:4560–4567
Hua ZD, Chen ZY, Li YZ, Zhao MP (2008) Thermosensitive and salt-sensitive molecularly imprinted hydrogel for bovine serum albumin. Langmuir 24:5773–5780
Zhao LP, Gao L (2004) Coating of multi-walled carbon nanotubes with thick layers of tin(IV) oxide. Carbon 42:1858–1861
Kan X, Zhao Y, Geng Z, Wang Z, Zhu JJ (2008) Composites of multiwalled carbon nanotubes and molecularly imprinted polymers for dopamine recognition. J Phys Chem C 112:4849–4854
Rachkov A, Minoura N (2000) Recognition of oxytocin and oxytocin-related peptides in aqueous media using a molecularly imprinted polymer synthesized by the epitope approach. J Chromatogr A 889:111–118
Zhang MS, Huang JR, Yu P, Chen X (2010) Preparation and characteristics of protein molecularly imprinted membranes on the surface of multiwalled carbon nanotubes. Talanta 81:162–166
Lu XF, Yu YH, Chen L, Mao HP, Zhang WJ, Wei Y (2004) Preparation and characterization of polyaniline microwires containing CdS nanoparticles. Chem Commun 13:1522–1523
Liu HM, Liu CH, Yang XJ, Zeng SJ, Xiong YQ, Xu WJ (2008) Uniformly sized-β-cyclodextrin molecularly imprinted microspheres prepared by a novel surface imprinting technique for ursolic acid. Anal Chim Acta 628:87–94
Tang PP, Cai JB, Su QD (2010) Synthesis and adsorption study of BSA surface imprinted polymer on CdS quantum dots. Chinese J Chem Phys 23:195–200
Piletsky SA, Piletska EV, Bossi A, Karim K, Lowe P, Turner APF (2001) Substitution of antibodies and receptors with molecularly imprinted polymers in enzyme-linked and fluorescent assays. Biosens Bioelectron 16:701–707
Bossi A, Piletsky SA, Piletska EV, Righetti PG, Turner APF (2001) Surface-grafted molecularly imprinted polymers for protein recognition. Anal Chem 73:5281–5286
Acknowledgment
This project was supported by the National Natural Science Foundation of China (no. 21005030), the Research Foundation of Education Bureau of Hunan Province, China (No.10A099), and the Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplemental material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 123 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Yang, X., Chen, X. et al. Novel magnetic bovine serum albumin imprinted polymers with a matrix of carbon nanotubes, and their application to protein separation. Anal Bioanal Chem 401, 2855–2863 (2011). https://doi.org/10.1007/s00216-011-5373-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5373-9