Abstract
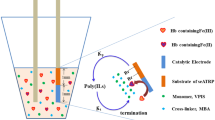
Hemoglobin (Hb)-imprinted poly(ionic liquid)s (HIPILs) were prepared on the surface of Au electrode modified with gold nanodendrites (Au/ND/HIPILs). HIPILs were synthesized with 1-vinyl-3-propyl imidazole sulfonate ionic liquids as functional monomers via electrochemically mediated atom transfer radical polymerization (eATRP) catalyzed by Hb. The Au/ND/HIPILs electrode was examined by cyclic voltammetry (CV), scanning electron microscope (SEM), and X-ray photoelectron spectroscopy (XPS). The Au/ND/HIPILs electrode was also used as an electrochemical sensor to determine Hb by differential pulse voltammetry (DPV). Under the optimal conditions, the detection range of Hb was from 1.0 × 10−14 to 1.0 × 10−4 mg/mL with a limit of detection of 5.22 × 10−15 mg/mL (S/N = 3). Compared with other methods, the sensor based on poly(ionic liquid)s had the broader linear range and lower detection limit.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molecular imprinting technology, often described as a method of making a molecular lock to match a molecular key, is a technique for the creation of molecularly imprinted polymers (MIPs) with tailor-made binding sites complementary to the template molecules in shape, size, and functional group [1]. Due to the advantages of low cost, simple preparation process, good repeatability, high stability, and easy storage [2, 3], MIPs have broad application in the field of protein separation [4] biosensing [5] and enrichment of protein [6].
The selection of functional monomer is important for the preparation of MIPs, since it can strongly interact with the template and form specific donor–receptor or antibody–antigen complexes prior to polymerization [1]. At present, methacrylic acid, acrylamide, and ethylene glycol dimethacrylate have been used as “universal” functional monomers. Many MIPs were prepared by one or two of these three monomers. However, the limited number of functional monomers used in molecular imprinting restricts the selectivity and the further applications of MIPs to some extent [1]. So it is imperative to develop novel functional monomers to prepare MIPs.
Ionic liquids (ILs) are non-volatile (vapor pressure close to zero), with wide liquid range, high conductivity, wide electrochemical window, non-flammable, and ultra-high thermal stability [7]. These unique properties allow ILs to have many applications in such as organic synthesis, catalytic reactions, electrochemistry, biomaterials, and extraction separation [8]. ILs also became a promising candidate for functional monomer in MIPs [9]. For example, Duan et al. [10] prepared MIPs using lysozyme as template and ionic liquid (4-amino-5-imidazolecarboxamide hydrochloride) as functional monomer. The sensitive detection of lysozyme at 1.0 × 10−9~8.0 × 10−8 mg/mL can be achieved. Zhu et al. [11] prepared MIPs with ionic liquid (1-vinyl-3-butylimidazolium tetrafluoroborate) as functional monomer for detecting 6-benzylaminopurine. The linear detection range is 0.5~50.0 μmol/L, and the detection limit could be improve to be 0.2 μmol/L (S/N = 3). Fan et al. [12] prepared a novel MIP using the specific ionic liquid (i.e., [COOHevim]Br), [COOHpvim]Br, [COOHavim]Br,or [COOHhvim]Br) as a functional monomer for the selective separation of synephrine from the extracts of Aurantii Fructus Immaturus in methanol–water media. The obtained polymer showed a good selectivity and high adsorption capacity for synephrine. Other molecular-imprinted poly(ionic liquid)s which were prepared by 1-ally-3-methylimidazolium chloride ([AMIM]Cl), 1-allyl-3-methyliumidazolium tetrafiuoroborate ([AMIM][BF6]), 1-(3-trimethoxysily-propyl)-3-methylimmidazolium chloride ([TMSPMIM]Cl), 1-vinyl-3-aminoformylmethyl-imidazolium chloride ([VAFMIM]Cl), 1-vinyl-3methyl imidazolium chloride ([VMIM]Cl) had also been reported to recognize bovine serum albumin(BSA) and salicylic acid [13,14,15,16].
Polymerization method is another important factor for the preparation of MIPs. Atom transfer radical polymerization (ATRP) allows the polymers with predetermined molecular weight and narrow molecular weight distribution, as well as desired composition [17] and has many applications in the preparation of MIPs [18,19,20]. However, the conventional ATRP has two main drawbacks: (1) low-cost transition metal catalysts are sensitive to air and polymerization often requires an anaerobic system; (2) the transition metal catalyst has certain toxicity to biomacromolecules such as proteins, and the treatment process for removing the catalyst is complicated. In response to above shortcomings, various improved ATRP have emerged. For example, Magenau et al. [21] reported electrochemically mediated ATRP (eATRP) in which low-valent metal was obtained by electrochemical reduction of the high-valent metal and decreased the catalyst in the polymer to only several parts per million. Silva et al. [22] replaced low-valent transition metal with Hb to conduct the ATRP when the Fe (III) in Hb was reduced to Fe (II) by using sodium ascorbate as reducing agents. Our group combined the advantages of the two methods to develop the Hb-catalyzed eATRP and used it for the preparation of Hb MIPs [23, 24]. The Hb-catalyzed eATRP needed neither transition metal catalyst nor reducing agents, which eliminates all negative effects of ATRP on the MIPs. However, up to now, only methacrylic acid, acrylamide, and N-isopropyl acrylamide were successfully used to the preparation of MIPs by Hb-catalyzed eATRP.
The design and fabrication of nanoparticles have attracted much interest, owing to their wide application in sensors, nanoelectronic devices, solar cell, and biomedical analysis [25, 26]. Gold nanoparticles, especially the nanodendrites (ND) have drawn more attention due to its availability of large surface, the stability in wide temperature ranges, size, and shape dependent morphologies and good physiochemical properties [27, 28].
Considering the advantages of Hb-catalyzed eATRP, ILs, and Au/ND, Hb-imprinted poly(ILs) were prepared on the surface of Au electrode modified with gold nanodendrites (Au/ND/HIPILs). Hb-imprinted poly(ionic liquid)s (PILs) was synthesized with 1-vinyl-3-propyl imidazole sulfone monomers via Hb-catalyzed eATRP. Cyclic voltammetry (CV), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM) were used to characterize the Au/ND/HIPILs electrode. Further research indicated that the Au/NDs/HIPILs electrode could be used as an electrochemical sensor to determine Hb by differential pulse voltammetry (DPV).
Materials and methods
Chemicals
Pt electrode (Φ = 2 mm), GCE (Φ = 3 mm), and Au electrode (Φ = 2 mm) were obtained from Chenhua Instruments Co. (Shanghai, China). 1-vinyl-3-propyl-imidazole sulfonate ionic liquids were supplied by Shanghai Cheng Jie Chemical Co. Ltd. (Shanghai, China). N, N′-methylene bis-acrylamide (MBA, cross-linker) was from Kemiou Chemical Co. (Tianjin, China). Graphite electrode (GE) was obtained from JiXing ShengAn Company (Beijing, China). Potassium ferricyanide, potassium dihydrogen phosphate, disodium phosphate, and sodium acetate were got from Sinopharm Chemical Reagent Co. Ltd. Toluidine blue, ammonium persulfate, copper sulfate, and sodium dodecyl sulfate were provided by Tianjin Komiou Chemical Reagent Co. Ltd. Sulfuric acid, chloroauric acid (HAuCl4·4H2O), and chloroplatinic acid (HPtCl6·6H2O) were purchased from Beijing Chemical Plant (Beijing, China). Bovine serum albumin (BSA, MW 66 kDa), human serum albumin (HSA, MW 69 kDa), and Hb (MW 65 kDa) were supplied by Solarbio Inc. Bovine blood sample was obtained from Wa fang dian farm in Dalian City, China. A 0.01 mol·L−1 ABS buffer solution with pH = 5 was prepared using glacial acetic acid and sodium acetate solids. The specifications for all chemicals were of analytical grade. The water used in the experiment was ultrapure water (resistivity > 18 MΩ cm).
Apparatus
CV, eATRP, and DPV were carried out with a CHI660D electrochemical workstation (Chen hua, Shanghai, China).
The CV used a gold or modified gold electrode as a working electrode, a saturated calomel electrode (SCE), and a platinum wire as a reference electrode and a counter electrode, respectively. The modified electrode was subjected to CV in PBS solution containing 5 mmol L−1 [Fe (CN)6]3−/4− + 0.1 mol L−1 KCl. CV was carried out in the potential range between − 0.2 and 0.6 V, and at a scanning rate of 100 mV s−1.
Surface characterization of the modified electrode was obtained by SEM (SU8010, Hitachi, Japan).
XPS was used to study the chemical composition of the modified electrode by using a Thermo ESCALAB 250Xi spectrometer with a monochromatic Al Ka radiation.
Preparation process of Au/ND/HIPILs
In order to improve the available surface area of the electrode, gold ND was fabricated on the clean Au electrode surface by electrodeposition, which was performed by chronoamperometry at − 0.9 V (vs. SCE) in an aqueous electrolyte containing CuSO4 ·5H2O (0.02 mol L−1) and HAuCl4·4H2O (0.8 mol L−1). The electrodeposition process was maintained for 400 s at room temperature. Then, the electrode modified with deposits was immersed in HNO3 solution (3 mol L−1) for 3 h to remove the Cu layer, thus Au/ND electrode was obtained. According to the literature [23, 29], the obtained electrode was only made of Au. Three Au/ND electrodes were prepared and characterized by CV at the same condition. As shown in Fig. 1, the CV of three Au/ND electrodes were almost the same, which shown that the Au/ND electrodes had good repeatability.
The preparation of Au/ND/HIPILs electrode was shown in Fig. 2. According to the reported method previously [24], the thiol initiator (4-mercaptophenyl 2-bromo-2-methylpropanoate, 4-HTP-Br) was immobilized on the surface of Au/ND electrode. Then, the initiator-modified electrode was inserted into a solution which contained the PBS (0.1 mol L−1, pH 7.0) solution of functional monomer (1-vinyl-3-propyl-imidazole sulfonate, 0.1 mol L−1), template molecule (Hb, 2 mg ml−1), and cross-linker (MBA, 0.1 mol L−1). In order to electrochemically reduce Hb, GCE/PTB/nPt was used as working electrode (cathode) which was selected in our previous work [19]. The SCE was used as the reference electrode, and the platinum wire was the counter electrode. When a potential (Eapp) of − 0.51 V was applied to GCE/PTB/nPt electrode, polymerization was carried out on the surface of Au/ND electrode.
Finally, Au/ND electrode modified with PILs was immersed in a mixed solution containing 10% (v/v) acetic acid and 10% (w/v) SDS for 2 h to remove the Hb template. After being washed three times with PBS solution, Au/ND electrode modified Hb-imprinted PILs (Au/ND/HIPILs) was successfully prepared.
A non-imprinted PILs electrode (Au/ND/NIPILs) was used as a compared electrode for Au/ND/HIPILs electrode. Au/ND/NIPILs was prepared by the traditional free radical polymerization method in the absence of Hb. Ammonium persulfate (0.9 mol L−1) was used as initiator to polymerize 1-vinyl-3-propyl imidazole sulfonate ionic liquids (0.1 mol L−1) and cross-linker (MBA, 0.1 mol L−1) at room temperature for 5 h on the surface of Au/ND.
Results and discussion
Characterization of Au/ND/HIPILs
Figure 3 was the CV curves of different modified electrodes in PBS solution containing 5 mmol L−1 [Fe (CN) 6]3−/4− + 0.1 mol·L−1 KCl. As expected, the curve of the bare gold electrode had a quasi-reversible characteristic peak around 0.2 V. When the bare gold electrode was modified by gold ND (Au/ND), its peak current increased, which indicated that the ND enlarged the surface of the electrode and had good electron transfer ability [23]. After the polymerization of ILs on the surface of ND (Au/ND/PILs), the CV peak current of the electrode was obviously reduced. The reason for this phenomenon may be that the poly(ILs) film acted as an inert electron and mass transfer resistance layer, which hindered the diffusion of probe ions to the electrode surface. After removing Hb, the peak current of the electrode (Au/ND/HIPILs) was significantly higher than that of without removing Hb. The reason may be that when the template molecule was removed, the imprinted holes appeared on the surface of the electrode, which made the probe ions diffuse more easily to the electrode surface, resulting in the peak current increasing [24, 30].
XPS was used to investigate the chemical composition of Au/ND/PILs. In Fig. 4, the characteristic peaks of O1s, N1s, C1s, S2p, Au4f, and Br3d were at 532.1, 401.67, 285.24, 169.82, 84.9, and 67 eV, respectively [31, 32]. There were three small (unmarked) peaks (within the range of 200~500 eV) which were characteristic peaks of Au4d3, Au4d5, and S2s. Exposure of the active Br to the surface demonstrated the successful implementation of ATRP. The inset of Fig. 4 showed the fine XPS of Fe at 716.3 eV [33]. Since the Fe element was derived from Hb, the XPS spectrum of the Fe element indicated the presence of Hb in the poly(ILs).
Figure 5 was the SEM image of the modified electrode surface [34]. It could be seen from the figure that the Au/ND (Fig. 5a), Au/ND/PILs (Fig. 5b), and Au/ND/HIPILs (Fig. 5c) electrode had similar dendritic structures. The surface of Au/ND/PILs electrode was blurred compared with that of Au/ND which was due to the formation of poly(ILs) on the gold ND, while the surface of Au/ND/HIPILs exhibited porous structure (Fig. 5c), which resulted from the presence of blotting holes in the structure of the electrode surface.
Optimization of experimental parameters
The polymerization time was studied by CV. [Fe (CN)6]3−/4− was used as the probe of CV to indicate the electron transfer ability of Au/ND/poly(ILs). As could be seen in Fig. 6a, when the time increased from 0.5 to 2.5 h, the peak current dropped sharply. As the polymerization time was further increased, the peak current changed very slowly. Based on the results, 2.5 h was selected as the optimum polymerization time (as indicated by the arrow).
The choice of Hb concentration was selected by measuring the sensitivity of the Au/ND/HIPILs electrode (slope of the working curve). Increasing the Hb concentration (from 0.5 to 2.0 mg/mL), the sensitivity of the Au/ND/HIPILs electrode improved. However, a too high concentration of Hb (2.0 to 10.0 mg/mL) resulted in a decrease in the sensitivity of the electrode. This was most likely due to the accumulation of too much protein, which led to the formation of undesirable imprinted caves and reduced the effectiveness of the Au/ND/HIPILs electrode in capturing template proteins. From the results of Fig. 6b, the concentration of Hb was optimal to be 2.0 mg/ml.
Electrochemical response of different electrodes
The electrochemical response of Au/ND, Au/ND/PILs, and Au/ND/HIPILs were compared by DPV and shown in Fig. 7. As can be seen, the Au/ND electrode had almost the same DPV curve when it was before (Fig. 7a, curve 1) or after (Fig. 7a, curve 2) rebinding with Hb (10−4 mg/mL), which showed that the Au/ND electrode had no electrochemical response toward Hb. The similar phenomenon was observed on Au/ND/PILs (Fig. 7b). As for the Au/ND/HIPILs electrode, the DPV peak current decreased greatly after it rebinding with Hb (Fig. 7c, curve 2) compared with that of before rebinding (Fig. 7c, curve 1), which showed that the Au/ND/HIPILs electrode had good electrochemical response toward Hb. This may be because the imprinted holes rebound with Hb, blocking the diffusion of probe ions to the surface of the electrode, resulting in a decrease in peak current [35].
Determination of Hb by DPV
The Au/ND/HIPILs electrode was used as an electrochemical sensor to detect Hb by DPV. The electrolyte solution and the three-electrode system were the same as the CV. The main parameters were as follows: the potential range was − 0.2~0.6 V, the potential increment was 4 mV, the amplitude is 50 mV, the first pulse width was 0.2 s, the sampling interval was 0.0167 s, the pulse period was 0.5 s, the pulse width was 0.2 s, the polarization time was 2 s, and the sensitivity was 1 × 10−4A/V. As could be seen from Fig. 8a, the DPV peak current of the Au/ND/HIPILs electrode decreased with the increasing of Hb concentrations, which was due to the increasing number of Hb bound with the imprinted holes, blocking the diffusion of probe ions to the surface of the electrode, resulting in a decrease in peak current. According to the relation of DPV current difference (response signal, ΔI) with the Hb concentration logarithm, the linear range of detecting Hb by Au/ND/HIPILs was 1.0 × 10−14~1.0 × 10−4 mg/mL. As could be seen from Fig. 8b, the linear regression equation was ΔI (μA) = 0.752 log C (mg/mL) + 13.812 with a coefficient of 0.995. From the working curve, the detection limit was 5.22 × 10−15 mg/mL (LOD, S/N = 3).
In order to further investigate, the effect of gold nanodendrites on the properties of sensor was compared between with or without nanodendrites. HIPILs were prepared on the planar Au electrode (no gold nanodendrites) using the same experimental condition. The detection results were compared between Au/HIPILs and Au/ND/HIPILs when the concentration of Hb was 10−4 mg/mL and shown in Fig. 9. The DPV current of Au/ND/HIPILs decreased after (Fig. 9a, curve 2) rebinding with Hb compared with that of before (Fig. 9a, curve 1) rebinding. The DPV response (Ibefore− Iafter) was about 14.49 μΑ. As for Au/HIPILs electrode (Fig. 9b), the DPV response was smaller than that of Au/ND/HIPILs, which showed that Au/ND/HIPILs electrode had better electrochemical response toward Hb. The prepared Au/ND/HIPILs electrodes were also compared with the performance of similar Hb sensors listed in Table 1, it could be seen that the prepared Au/ND/HIPILs electrode had lower detection limits. Especially, when the same ND and polymerization method were used, imprinted poly(ILs) had the better performance than that prepared based on poly(acrylamide).
Repeatability, reproducibility, and stability
To determine the repeatability of the Hb-imprinted sensor, 10−4 mg/mL Hb (in PBS) was analyzed five times by the same sensor repeatedly at the same day. The relative standard deviation (RSD) was 3.27%, indicating that the Au/ND/HIPILs sensor had a good repeatability. Five identical Au/ND/HIPILs sensor were prepared under the same conditions and used them to detect 10−4 mg/mL Hb (in PBS) to determine the reproducibility. The RSD was 3.26%, implying that the reproducibility of the Au/ND/HIPILs sensors was good. Typically, the prepared Au/ND/HIPILs sensor was stored at 4 °C in a refrigerator. The sensor was used to detect 10−4 mg/mL Hb (in PBS) one time every day. As shown in Fig. 10, after 5 consecutive days, the DPV response signal of the Au/ND/HIPILs sensor decreased 5% and the RSD of the 5 detection was less than 3%, which showed that the Au/ND/HIPILs sensor has good stability [39].
Selectivity and application of Au/ND/HIPILs electrodes
The selectivity of Au/ND/HIPILs electrode was carried out using lysozyme (LYZ) (MW 14.4 kDa), human serum albumin (HSA) (MW 69 kDa), and bovine serum albumin (BSA) (MW 66 kDa) as experimental interferors; the results were shown in Fig. 11. As could be seen, the response signal (ΔI) of the Au/ND/HIPILs electrode toward Hb is 9.733 μA, which is 5.51, 5.84, and 6.21 times of BSA, Lyz, and HSA, respectively. The results showed that the Au/ND/HIPILs electrode has better selectivity for the target protein (Hb), which may be due to the fact that Hb could bind well to the specific imprinted holes in the Au/ND/HIPILs electrode. The selectivity of the electrode was also evaluated by the imprinting factor (K). The calculation equation is K = ΔI(HIPILs)/ΔI(NIPILs) [37], where for protein. As shown in Fig. 11, the K values of BSA, Lyz, HSA, and Hb were calculated to be 1.15, 1.09, 1.38, and 8.85, respectively. The maximum K value of Hb indicated that Au/ND/HIPILs electrode had good selectivity for the target protein.
The actual samples were tested and analyzed by standard addition method. Firstly, the bovine blood sample was processed according to the literatures [40] and analyzed by a blood tester. Then, the actual sample was diluted and measured with Au/ND/HIPILs electrode. The results were shown in Table 2, as could be seen that the recovery obtained was from 96.5 to 104.2% and the resulting RSD was less than 4%, indicating that the prepared Au/ND/HIPILs electrode could be used for the detection and analysis of actual samples.
Conclusions
In this study, Hb was used both as catalyst and template to prepare Hb-imprinted polymer on the surface of ND modified Au electrode with ionic liquid as functional monomer. The optimum polymerization time was chosen to be 2.5 h and the optimal Hb concentration of polymerization was 2.0 mg/mL. The electrode modified with Hb-imprinted PILs (Au/ND/HIPILs) could be used as a sensor. From the experiment, the linear response range was 1.0 × 10−14~1.0 × 10−4 mg/mL, and the detection limit was 5.22 × 10−15 mg/mL (S/N = 3). Compared with other Hb sensors, Au/ND/HIPILs electrode has lower detection limits by using ionic liquid as functional monomer.
References
Chen L, Wang X, Lu W, Wu X, Li J. Molecular imprinting: perspectives and applications. Chem Soc Rev. 2016;45(8):2137–211.
Haupt K, Mosbach K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem Rev. 2000;100:2495–504.
Whitcombe MJ, Chianella I, Larcombe L, Piletsky SA, Noble J, Porter R, et al. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem Soc Rev. 2011;40(3):1547–71.
Liu Y, Wang Y, Dai Q, Zhou Y. Magnetic deep eutectic solvents molecularly imprinted polymers for the selective recognition and separation of protein. Anal Chim Acta. 2016;936:168–78.
Reddy SM, Phan QT, El-Sharif H, Govada L, Stevenson D, Chayen NE. Protein crystallization and biosensor applications of hydrogel-based molecularly imprinted polymers. Biomacromolecules. 2012;13(12):3959–65.
Cao J, Zhang X, He X, Chen L, Zhang Y. The synthesis of magnetic lysozyme-imprinted polymers by means of distillation-precipitation polymerization for selective protein enrichment. Chem Asian J. 2014;9(2):526–33.
Wilkes JS, Zaworotko MJ. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. Journal of the Chemical Society, Chemical Communications. 1992(13):965-967.
Armand M, Endres F, MacFarlane DR, Ohno H, Scrosati B. Ionic-liquid materials for the electrochemical challenges of the future. Nat Mater. 2010:129–37.
Yavir K, Marcinkowski L, Marcinkowska R, Namiesnik J, Kloskowski A. Analytical applications and physicochemical properties of ionic liquid-based hybrid materials: a review. Anal Chim Acta. 2019;1054:1–16.
Duan H, Wang X, Wang Y, Sun Y, Li J, Luo C. An ultrasensitive lysozyme chemiluminescence biosensor based on surface molecular imprinting using ionic liquid modified magnetic graphene oxide/beta-cyclodextrin as supporting material. Anal Chim Acta. 2016;918:89–96.
Zhu X, Zeng Y, Zhang Z, Yang Y, Zhai Y, Wang H, et al. A new composite of graphene and molecularly imprinted polymer based on ionic liquids as functional monomer and cross-linker for electrochemical sensing 6-benzylaminopurine. Biosens Bioelectron. 2018;108:38–45.
Fan JP, Tian ZY, Tong S, Zhang XH, Xie YL, Xu R, et al. A novel molecularly imprinted polymer of the specific ionic liquid monomer for selective separation of synephrine from methanol-water media. Food Chem. 2013;141(4):3578–85.
Ding H, Chen R, Liu M, Huang R, Du Y, Huang C, et al. Preparation and characterization of biocompatible molecularly imprinted poly(ionic liquid) films on the surface of multi-walled carbon nanotubes. RSC Adv. 2016;6(49):43526–38.
Liu M, Pi J, Wang X, Huang R, Du Y, Yu X, et al. A sol-gel derived pH-responsive bovine serum albumin molecularly imprinted poly(ionic liquids) on the surface of multiwall carbon nanotubes. Anal Chim Acta. 2016;932:29–40.
Qian L, Hu X, Guan P, Gao B, Li J, Wang C, et al. Preparation of bovine serum albumin imprinting sensitive hydrogels using ionic liquid as co-monomer and stabilizer. Talanta. 2014;121:56–64.
Wu X, Du J, Li M, Wu L, Han C, Su F. Recent advances in green reagents for molecularly imprinted polymers. RSC Adv. 2018;8(1):311–27.
Tsarevsky NV, Matyjaszewski K. “Green” atom transfer radical polymerization:from process design to preparation of well-defined environmentally friendly polymeric materials. Chem Rev. 2007;107:2270–99.
Zhang W, Feng X, Yi J, Niu Y, Xu L. A novel electrochemical biomimetic sensor based on E-MIP artificial acceptor and SI-ATRP assisted signal amplification. J Electroanal Chem. 2019;842:24–33.
Kumar S, Karfa P, Madhuri R, Sharma PK. Designing of fluorescent and magnetic imprinted polymer for rapid, selective and sensitive detection of imidacloprid via activators regenerated by the electron transfer-atom transfer radical polymerization (ARGET-ATRP) technique. J Phys Chem Solids. 2018;116:222–33.
Sun Y, Li S, Yang Y, Feng X, Wang W, Liu Y, et al. Fabrication of a thermal responsive hemoglobin (Hb) biosensor via Hb-catalyzed eATRP on the surface of ZnO nanoflowers. J Electroanal Chem. 2019;848.
Magenau AJD, Strandwitz NC, Gennaro A, Matyjaszewski K. Electrochemically mediated atom transfer radical polymerization. Science. 2011;332:81–4.
Silva TB, Spulber M, Kocik MK, Seidi F, Charan H, Rother M, et al. Hemoglobin and red blood cells catalyze atom transfer radical polymerization. Biomacromolecules. 2013;14(8):2703–12.
Sun Y, Zhang J, Li J, Zhao M, Liu Y. Preparation of protein imprinted polymers via protein-catalyzed eATRP on 3D gold nanodendrites and their application in biosensors. RSC Adv. 2017;7(45):28461–8.
Sun Y, Du H, Lan Y, Wang W, Liang Y, Feng C, et al. Preparation of hemoglobin (Hb) imprinted polymer by Hb catalyzed eATRP and its application in biosensor. Biosens Bioelectron. 2016;77:894–900.
Patra S, Roy E, Madhuri R, Sharma PK. Nanocomposite of bimetallic nanodendrite and reduced graphene oxide as a novel platform for molecular imprinting technology. Anal Chim Acta. 2016;918:77–88.
Guo S, Wang E. Synthesis and electrochemical applications of gold nanoparticles. Anal Chim Acta. 2007;598(2):181–92.
Islam S, Bakhtiar H, Aziz M, Riaz S, Aziz MSA, Naseem S, et al. Optically active phenolphthalein encapsulated gold nanodendrites for fiber optic pH sensing. Appl Surf Sci. 2019;485:323–31.
Li M, Kong Q, Bian Z, Ma C, Ge S, Zhang Y, et al. Ultrasensitive detection of lead ion sensor based on gold nanodendrites modified electrode and electrochemiluminescent quenching of quantum dots by electrocatalytic silver/zinc oxide coupled structures. Biosens Bioelectron. 2015;65:176–82.
Silverman L. Precision determination of lead in high grade copper. Anal Chem. 1948;20(10):906–9.
Yang C, Ji XF, Cao WQ, Wang J, Zhang Q, Zhong TL, et al. An ultra sensitive and selective impedance sensor based on protein-imprinted polymer. Sensors Actuators B Chem. 2019;282:818–23.
Wei X, Zhou Z, Dai J, Hao T, Li H, Xu Y, et al. Composites of surface imprinting polymer capped Mn-doped ZnS quantum dots for room-temperature phosphorescence probing of 2,4,5-trichlorophenol. J Lumin. 2014;155:298–304.
Tan L, Kang CC, Xu SY, Tang YW. Selective room temperature phosphorescence sensing of target protein using Mn-doped ZnS QDs-embedded molecularly imprinted polymer. Biosens Bioelectron. 2013;48:216–23.
Leveneur J, Waterhouse GIN, Kennedy J, Metson JB, Mitchell DRG. Nucleation and growth of Fe nanoparticles in SiO2: a TEM, XPS, and Fe L-edge XANES investigation. J Phys Chem C. 2011;115(43):20978–85.
Zhang HX, Cao AM, Hu JS, Wan LJ, Lee ST. Electrochemical sensor for detecting ultratrace nitroaromatic compounds using mesoporous SiO2-modified electrode. Anal Chem. 2006;78:1967–71.
Seo JH, Yang R, Brzezinski JZ, Walker B, Bazan GC, Nguyen T-Q. Electronic properties at gold/conjugated-polyelectrolyte interfaces. Adv Mater. 2009;21(9):1006–11.
Lee TY, Shim YB. Direct DNA hybridization detection based on the oligonucleotide-functionalized conductive polymer. Anal Chem. 2001;73:5629–32.
Luo J, Jiang S, Liu X. Electrochemical sensor for bovine hemoglobin based on a novel graphene-molecular imprinted polymers composite as recognition elemen. Sensors Actuators B Chem. 2014;203:782–9.
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001;3(4):156–64.
Bilal A. Development and Validation of Analytical Method for Qualitative and Quantitative Determination of Glibenclamide in Different Brands of Tablet Dosage form Using UV-Visible Spectroscopy. Journal of Molecular and Genetic Medicine. 2013;07(03):354-358.
Wang Z, Li F, Xia J, Xia L, Zhang F, Bi S, et al. An ionic liquid-modified graphene based molecular imprinting electrochemical sensor for sensitive detection of bovine hemoglobin. Biosens Bioelectron. 2014;61:391–6.
Funding
Authors received support from the National Natural Science Foundation of China (No. 21304041), the High Level Talent Innovation Support Project from Dalian (No. 2016RQ047), the Science Research Project of Education Department of Liaoning Province (No. LQ2019022), and the Innovative Training Program of college students in Liaoning Normal University (No. 201910165215).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts to interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Y., Feng, X., Hu, J. et al. Preparation of hemoglobin (Hb)-imprinted poly(ionic liquid)s via Hb-catalyzed eATRP on gold nanodendrites. Anal Bioanal Chem 412, 983–991 (2020). https://doi.org/10.1007/s00216-019-02324-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02324-w