Abstract
We report on a method for chemiluminescence (CL) determination of the glycoprotein tenascin-C. Carboxylated carbon nanoparticles (cCNPs) were prepared from activated carbon. Next, the cCNPs were conjugated to magnetic beads (MBs) with a diameter of ~1 μm by linking its carboxy groups to the amino groups of the MBs. The assay involves the following steps: (a) An aptamer labeled with the CL reagent N-(4-aminobutyl)-N-ethylisoluminol (ABEI) (the labeled aptamer) was adsorbed onto the surface of the carboxy-modified magnetic carbon nanoparticles to form labeled aptamer modified cCNPs-MBs. (b) On addition of sample tenascin-C, it will interact with the labeled aptamer to form a complex with the labeled aptamer. (c) This tenascin-aptamer complex is then dissociated from the surface of the particles and detected by CL whose intensity is linearly related to the concentration of tenascin-C in the 1 pM to 1 nM range. The detection limit is as low as 0.4 pM, and the RSD is 4.2 % at a 50 pM level (for n = 7). The method has been successfully applied to the determination of tenascin-C in human serum samples and holds promise as a widely applicable general platform for aptamer-based CL detection of proteins.
Figure
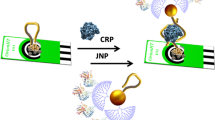
A novel method for Glycoprotein tenascin-C detection based on interaction between carboxylic carbon nanoparticles and aptamer is developed.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon-based nanomaterials, which include carbon nanotubes, fullerenes, nanofibers, graphene, nanodiamonds, carbon-based thin films and molecules, etc., have been widely studied and applied in nanotechnology, biosensing, and drug delivery, etc. [1].
Very recently, carbon nanoparticles, a new kind of carbon-based nanomaterial has been developed and researched. Carbon nanoparticles (also known as, carbon dots, carbon nanodots) are discrete nanoparticles of near spherical geometry with sizes below 10 nm [2]. Carbon nanoparticles are the newly emerged member of the nanocarbon material family [3]. But carbon nanoparticles have attracted much attention because they have many advantages, such as chemical inertness, lack of blinking, inherently low cytotoxicity, excellent biocompatibility, and amphiphilic characteristics depending on the surface capping materials [4–13]. Up to now, a few aptamer-based biosensors coupling with carbon nanoparticles have been exploited [14–17].
Tenascin-C is a large extracellular matrix protein newly expressed during tissue remodeling processes including angiogenesis, inflammation, and tumor growth. Tenascin-C is especially overexpressed in tumor tissues [18, 19]. Western blot was usually been used for Tenascin-C detection [20, 21]. Since the advantages of aptamers, such as the absence of risk, ease of production and chemical modification, and high affinity to targets, aptamers have attracted extensive attention. Kim’s group has reported a method based on QD-conjugated aptamer for expression research of Tenascin-C in cancer cell. As far as we know, there are no other reports about Tenascin-C detection [22].
This work focuses on researching and developing a novel CL method for the determination of protein. The main idea in our work is that adsorption of CL reagent labeled aptamer on carbon nanoparticles results from competition of the hydrophobic and electrostatic repulsion interactions. As a model system, the Tenascin-C was used as the interest target. In the presence of Tenascin-C, Tenascin-C aptamer preferentially binds with Tenascin-C and the Tenascin-C aptamer complex dissociates from the carbon nanoparticles. The released Tenascin-C aptamer complex was detected with CL. To the best of our knowledge, it is the first time that carbon nanoparticles were used for Tenascin-C detection with the merits of being simple and sensitive.
Experimental
Reagents and apparatus
Human Tenascin-C was purchased from EMD Millipore Corporation (Billerica, MA, USA, http://www.millipore.com/index.do). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS) and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, USA. www.sigmaaldrich.com). ABEI were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan. http://www.tcichemicals.com/). Amino groups modified magnetic beads (about 1 μm, 10 mg mL−1) and magnetic racks were obtained from BaseLine ChromTech Research Centre (Tianjin, China. http://www.qiuhuan.com/). All the reagents were analytical grade and used without further purification.
The synthetic oligonucleotide was purchased from SBS Genetech Co. Ltd. (Beijing, China. http://www.sbsbio.com/default.asp). The sequence was as following [22, 23]:
5′- PO4 3- CCTGCACTTGGCTTG GATTTCAGAAGGGAGACCC-3′
Chemiluminescence emission was detected with a FI-CL instrument (IFFM-E, Remex Analytical Instrument Co. Ltd., Xian, China. http://www.xaremex.com). Transmission electron microscopy (TEM) image was taken with a JEM-2000EX instrument (JEOL Ltd, Japan. http://www.jeol.com/). A TGL-16G centrifuge (Shanghai Anting Science Instrument Co., China. http://www.centrifuge.com.cn/) was used for centrifugation.
The Preparation of carboxylated carbon nanoparticles
Carboxylated carbon nanoparticles were prepared by adopting a procedure reported in the literature [17, 24]. In a typical procedure, 100 mg activated carbon was mixed with 20 mL 5 M HNO3 and refluxed for 12 h. After cooled down to room temperature, the solution was neutralized by NaOH. Then the solution was collected by centrifugation (16,000 g for 30 min). The collected precipitates were prepared as carboxylic carbon nanoparticles solution of the concentration of 2.0 mg mL−1. The formation of carbon nanoparticles was confirmed by TEM measurements. The diameter of the carbon nanoparticles was found to be 2 ~ 3 nm (Figure. S1, ESM).
Preparation of carboxylated carbon nanoparticles modified magnetic beads
The carboxylic carbon nanoparticles modified magnetic beads were prepared as following [25, 26]. Briefly, 100 μL suspension of aminated MBs was placed in a 1.5 mL Eppendorf tube and separated from the solution on a magnetic rack. After washing three times with 200 μL of phosphate buffer solution, 20 mg EDC and 10 mg NHS in 1.0 mL water were added. And the mixture kept at room temperature for 30 min with continuous mixing. Then the resulting solution was washed three times with 200 μL of 0.1 M phosphate buffer solution and resuspended to a final volume of 100 μL. And then 100 μL carboxylic carbon nanoparticles was added to the above MBs, and incubated at 37 °C overnight. Finally, the resulting carboxylic carbon nanoparticles modified magnetic beads (cCNPs-MBs) were washed with 200 μL of 0.1 M phosphate buffer solution for three times, and resuspended in 200 μL phosphate buffer solution and stored at 4 °C for further use.
Preparation of labeled aptamer
Labeled aptamer were prepared according to the reference with a slight modification [26, 27]. Briefly, 200 μL of a 0.1 mol L−1 imidazole solution (pH 6.8) was added to the 2 OD (about 66 μg of DNA) 5′-phosphate terminal of aptamer for the activation of the phosphate group for 30 min, then 100 μL of 0.1 mol L−1 EDC and 100 μL of 1 mmol L−1 ABEI were added. The labeling reaction was incubated at room temperature for 12 h with shaking. Finally the solution was transferred to a 5 mL centrifuge tube, and 100 μL (1/5 volumes) of 3 mol L−1 sodium acetate and 2.0 mL (4 volumes) of 100 % cold ethanol was added. The solution was chilled for 8 h at −16 °C and then centrifuged for 20 min (18,000 rpm min−1). The precipitate was washed with 200 μL of cold 70 % ethanol several times to remove any free ABEI. Finally, the resulting labeled aptamer was dissolved in 200 μL water and stored at −16 °C for futher use without dilution.
Fabrication of tenascin-C biosensor
The procedure of the fabrication of tenascin-C biosensor based on aptamer noncovalent assembly on cCNPs is illustrated in Scheme 1. Briefly, 10 μL labeled aptamer was added to 50 μL cCNPs-MBs solution. After incubate for 30 min., the mixture was separated with a magnetic rack. The precipitate was transferred to the quartz cuvette containing 50 μL tenascin-C sample sulution (containing 50 mM KCl, 2.5 mM MgCl2, 1 mM CaCl2) and incubated at 4 °C about 30 min. After separation with a magnetic rack at 4 °C, supernatant was taken for CL detection.
Flow injection-chemiluminescence detection
The flow injection system used in this work is shown in Scheme S1. The sodium hydroxide solution delivered the samples in the sample loop solution to react with the mixture of hydrogen peroxide solution and cobalt ions solution in the flow cell to produce CL. And the CL signal was detected by IFFM-E FI-CL analyzer. The CL reaction was triggered by injecting supernatant with a syringe through a septum. The peak heights of the emission curves were measured by means of a photon counting unit. The peak height of intensities was used for quantification.
Preparation of blood samples
Fresh blood was collected in clean plastic tubes; no anticoagulant was added to the blood samples allowing the blood coagulation naturally and then centrifuged at 3,500 rpm for 5 min. The supernatant part was extracted as serum with a mini sample collector. Serums were stored at −20 °C before using. The serum was properly diluted in assay buffer before using.
Results and discussion
Fabrication of biosensor and detection process
It has been validated that ssDNA can absorb strongly to carboxylic carbon nanoparticles surfaces [28]. ssDNA containing hydrophobic aromatic structures in the nucleobases can be adsorbed on the hydrophobic regions of the cCNPs surfaces through the π-stacking. Milewhile, the electrostatic repulsion between the –COOH groups of cCNPs and phosphate groups of the oligonucleotides weaks the adsorption of the nucleobases on the cCNPs surfaces. These two competitive interactions result in a total decrease in the adsorption of oligonucleotides on cCNPs. Under the conditions that the electrostatic repulsion is less than the π-stacking interaction, the oligonucleotides will be adsorbed on cCNPs surfaces (Scheme 1).
For the fabrication of the tenascin-C sensor based on the interaction between carboxylic carbon nanoparticles and ssDNA, cCNPs-MBs was first prepared with the cCNPs and MBs. Then the labeled aptamer was added into cCNPs-MBs to formed labeled aptamer modified cCNPs-MBs. Then the sample solution was added to the labeled aptamer modified cCNPs-MBs solution to make tenascin-C bind to labeled aptamer. Then the formed tenascin-C and labeled aptamer complex dissociated from the cCNPs-MBs. After separation on a magnetic rack, the supernatant was taken for CL detection.
To examine the successful adsorption of labeled aptamer to cCNPs-MBs mentioned above, we tested the CL intensities of labeled aptamer and cCNPs-MBs to investigate experimentally if the labeled aptamer was absorbed to cCNPs-MBs. The experimental results were show in Fig. 1. The cCNPs-MBs showed weak CL signal. And the labeled aptamer gave strong CL signal. After separation with a magnetic rack when labeled aptamer being added into cCNPs-MBs, the cCNPs-MBs gave strong CL signal. And the supernatant which contained labeled aptamer gave weaker CL signal than the labeled aptamer. This contributed to the decreasing of concentration of labeled aptamer in supernatant. Whereas, using ABEI to substitute labeled aptamer, at this time cCNPs-MBs produced weak CL signal. This change largely originated from adsorption of labeled aptamer on the cCNPs-MBs. Furthermore, we also studied if the labeled aptamer was absorbed onto cCNPs-MBs through the interaction between ssDNA and cCNPs. cCNPs-MBs was replaced by MBs. After treated MBs with labeled aptamer, the MBs gave no obviously CL intensity. It can be concluded that the labeled aptamer was absorbed onto cCNPs-MBs through the interaction between ssDNA and cCNPs[28].
CL responses of different solutions: cCNPs-MBs solution (a), labeled aptamer solution (b), cCNPs-MBs solution after treated with labeled aptamer (c), the supernatant of cCNPs-MBs after treated with labeled aptamer (d), cCNPs-MBs solution after treated with ABEI (e), MBs solution after treated with labeled aptamer (f) respectively
The effect of binding time of tenascin-C with labeled aptamer modified cCNPs-MBs on chemiluminescence intensity
In order to get the optimal analytical parameters, the relative experimental conditions such as binding time of tenascin-C with labeled aptamer modified cCNPs-MBs and dose of labeled aptamer were investigated.
The optimal binding time of the tenascin-C with labeled aptamer modified cCNPs-MBs was first studied. Figure 2a shows the effect of the binding time of tenascin-C with labeled aptamer modified cCNPs-MBs on CL intensity. It is apparent that CL intensity obviously increases with increasing the binding time from 5 to 30 min and then reaches a plateau in 35 min. This suggested that 35 min was enough for the tenascin-C to bind aptamer and induce the tenascin-C labeled aptamer complex displacement from the surface of cCNPs-MBs. Thus, 35 min was the binding time chosen in the following experiments.
The effect of dose of labeled aptamer on chemiluminescence intensity
The experimental results showed that the dose of labeled aptamer added into cCNPs-MBs solution greatly affected the CL response. Figure 2b shows the effect of dose of labeled aptamer on CL intensity. The cCNPs-MBs solution was keep 50 μL. It was found that the CL intensity increased with an increase in dose of labeled aptamer in the range from 2 to 15 μL, and reaches a plateau over the dose of 10 μL. The maximal CL signal was obtained at 12 μL labeled aptamer. Therefore, the dose of labeled aptamer was chosen 12 μL as the optimal concentration.
The effect of parameters of Flow injection-chemiluminescence system on chemiluminescence intensity
The parameters of FI-CL system were also investigated systematically to obtain the optimal conditions for the CL reaction. Considering both the CL intensity and the signal/background ratio, supernatant which contained tenascin-C and labeled aptamer complex was first mixed with H2O2 and then with Co2+. Furthermore, the pH values of the sodium hydroxide solution and the concentrations of H2O2, Co2+ standard solutions were also studied [29]. The optimal pH values of the sodium hydroxide solution and concentrations of H2O2, Co2+ were 11.5, 1.5 mM, 0.2 mM, respectively.
Sensitivity of the biosensor
Based on the combination of the adsorption of aptamer on cCNPs with sensitivity of the FI-CL detection method, under the optimized experimental conditions, a linear function range for tenascin-C was achieved from 1.0 × 10−12 ~ 1.0 × 10−9 g mL−1 with the equation of I CL = 3.56 C + 31.61 (I CL is the CL intensity; C is the concentration of tenascin-C, 10−12 g mL−1; n = 7, R2 = 0.9997). And the detection limit of tenascin-C is 4.0 × 10−13 g mL−1 estimated using 3σ. A series of seven repetitive measurements of 5.0 × 10−11 g mL−1 tenascin-C were used for estimating the precision, and the relative standard deviation (RSD) was 4.2 %. It was shown that the protein biosensor had good reproducibility. This method and some other detection techniques, which can sensitively detect the tenascin-C, are listed in Table 1. Comparing to other methods listed in Table 1, the current protocol is one of the most sensitive assay for the determination of tenascin-C [30−34].
Selectivity of the biosensor
To assess the specificity of the method for the detection of tenascin-C, experiments were conducted on some relative substance such as thrombin, lysozyme, IgG, BAS and ATP. Different CL signals for the detection of 1.0 × 10−7 g mL−1 thrombin, lysozyme, IgG, BAS and ATP, were recorded under the same experimental conditions according to the protocol described in Section 2.5, respectively. Thrombin, lysozyme, IgG, BAS and ATP did not induce any significant changes in the CL signal as compared to that of tenascin-C, which suggests that the developed strategy has a sufficient selectivity and tenascin-C could be detected selectively. This may be explained by the fact that the tenascin-C can bind to labeled aptamer and induce the formed the tenascin-C labeled aptamer complex to divorce from the surfaces of the cCNPs-MBs.
Determination of tenascin-C in the human serum samples
The feasibility of applying the developed method to measure tenascin-C in a complex matrix was studied. This was conducted by adding various levels of tenascin-C into human serum samples. After adding the sample into the labeled aptamer modified cCNPs-MBs, the mixtures were subjected to incubation for 30 min to ensure the full reaction between the labeled aptamer and tenascin-C. Then CL intensity of each sample of the supernatant was applied to the calibration curve of the standard tenascin-C solution to obtain the tenascin-C level of the sample. The results of recovery test were shown in Table 2. The results indicate that the developed method is highly reliable.
Conclusions
In conclusion, a new strategy for the simple and sensitive detection of protein based on protein-aptamer recognition action and absorption interaction between the ssDNA and cCNPs was developed. The detection limit of 4.0 × 10−13 g mL−1 of target protein was obtained. The resulting protein biosensor exhibited ultrasensitivity, high selectivity, and good reusability as a promise alternative technique for other bioassays. It holds great promise for protein diagnostics, microarrays, and microchips, as well as for bioanalysis in general.
References
Liu HP, Ye T, Mao CD (2007) Fluorescent carbon nanoparticles derived from candle soot. Angew Chem Int Ed 46:6473–6475
Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Georgakilas V, Giannelis EP (2008) Photoluminescent carbogenic dots. Chem Mater 20:4539–4541
Chen SM, Zheng HZ, Wang JN, Hou J, He Q, Liu HH, Xiong CQ, Kong XL, Nie ZX (2013) Carbon nanodots as a matrix for the analysis of low-molecular-weight molecules in both positive- and negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and quantification of glucose and uric acid in real samples. Anal Chem 85:6646–6652
Sun YP, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie SY (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128(24):7756–7757
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49(38):6726–6744
Wang F, Xie Z, Zhang H, Liu CY, Zhang YG (2011) Highly luminescent organosilane-functionalized carbon dots. Adv Funct Mater 21(6):1027–1031
Zhu AW, Qu Q, Shao XL, Kong B, Tian Y (2012) Carbon-dot-based dual-emission nanohybrid produces a ratiometric fluorescent sensor for in vivo imaging of cellular copper ions. Angew Chem Int Ed 51(29):7185–7189
Tao HQ, Yang K, Ma Z, Wan JM, Zhang YJ, Kang ZH, Liu Z (2012) In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 8(2):281–290
Goh EJ, Kim KS, Kim YR, Jung HS, Beack S, Kong WH, Scarcelli G, Yun SH, Hahn SK (2012) Bioimaging of hyaluronic acid derivatives using nanosized carbon dots. Biomacromol 13(8):2554–2561
Zhang Z, Hao JH, Zhang J, Zhang BL, Tang JL (2012) Protein as the source for synthesizing fluorescent carbon dots by a one-pot hydrothermal route. RSC Adv 2:8599–8601
Hu SL, Tian RX, Wu LL, Zhao Q, Yang JL, Liu J, Cao SR (2013) Chemical regulation of carbon quantum dots from synthesis to photocatalytic activity. Chem Asian J 8(5):1035–1041
Wang DM, Gao MX, Gao PF, Yang H, Huang CZ (2013) Carbon nanodots-catalyzed chemiluminescence of luminol: a singlet oxygen-induced mechanism. J Phys Chem C. doi:10.1021/jp404973b
Yang X, Feng B, He XL, Li FP, Ding YL, Fei JJ (2013) Carbon nanomaterial based electrochemical sensors for biogenic amines. Microchim Acta 180(11–12):935–956
Noguera PS, Posthuma-Trumpie GA, Tuil MV, Wal FJVD, Boer AD, Moers APHA, Amerongen AV (2011) Carbon nanoparticles as detection labels in antibody microarrays. Detection of genes encoding virulence factors in shiga toxin-producing escherichia coli. Anal Chem 83(22):8531–8536
Lu W, Qin X, Liu S, Chang G, Zhang Y, Luo Y, Asiri AM, Al-Youbi AO, Sun X (2012) Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal Chem 84(12):5351–5357
Wang YH, Bao L, Liu ZH, Pang DW (2011) Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma. Anal Chem 83(21):8130–8137
Liu J, Li J, Jiang Y, Yang S, Tan W, Yang R (2011) Combination of π–π stacking and electrostatic repulsion between carboxylic carbon nanoparticles and fluorescent oligonucleotides for rapid and sensitive detection of thrombin. Chem Commun 47(40):11321–11323
Kang WJ, Chae JR, Cho YL, Lee JD, Kim S (2009) Multiplex imaging of single tumor cells using quantum-dot-conjugated aptamers. Small 5(22):2519–2522
Hicke BJ, Stephens AW, Gould T, Chang YF, Lynott CK, Heil J, Borkowski S, Hilger CS, Cook G, Warren S, Schmidt PG (2006) Tumor targeting by an aptamer. J Nucl Med 47(4):668–678
Dueck M, Riedl S, Hinz U, Tandara A, Möller P, Herfarth C, Faissner A (1999) Detection of tenascin-C isoforms in colorectal mucosa, ulcerative colitis, carcinomas and liver metastases. Int J Cancer 82(4):477–483
Nicolò M, Piccolino FC, Zardi L, Giovannini A, Mariotti C (2000) Detection of tenascin-C in surgically excised choroidal neovascular membranes. Graefes Arch Clin Exp Ophthalmol 238(2):107–111
Kang WJ, Chae JR, Cho YL, Lee JD, Kim S (2009) Multiplex imaging of single tumor cells using quantum-dot-conjugated aptamers. Small 5(22):2519–2522
Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L (2003) A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci U S A 100(23):5416–15421
Dong YQ, Zhou NN, Lin XM, Lin JP, Chi YW, Chen GN (2010) Extraction of electrochemiluminescent oxidized carbon quantum dots from activated carbon. Chem Mater 22(21):5895–5899
Gunn DL, Roszman TL (1972) Preparation of sensitive and stable erythrocytes by the carbodiimide method for the detection of primary and secondary IgM and IgG antibody. J Immunol Methods 1(4):381–389
Yang ML, Liu CZ, Qian KJ, He PG, Fang YZ (2002) Study on the electrochemiluminescence behavior of ABEI and its application in DNA hybridization analysis. Analyst 127(9):1267–1271
Hun X, Wang Z (2012) L-Argininamide biosensor based on S1 nuclease hydrolysis signal amplification. Microchim Acta 176(1–2):209–216
Jung SW, Cha M, Park JY, Jeong N, Kim G, Park C, Ihm J, Lee J (2010) Dissociation of single-strand DNA: single-walled carbon nanotube hybrids by watson—crick base-pairing. J Am Chem Soc 132(32):10964–10966
Hun X, Liu F, Mei Z, Ma LF, Wang ZP, Luo XL (2013) Signal amplified strategy based on target-induced strand release coupling leavage of nicking endonuclease for the ultrasensitive detection of ochratoxin A. Biosens Bioelectron 39:145–151
Steude A, Schmidt S, Robitzki AA, Pänke O (2011) An electrode array for electrochemical immuno-sensing using the example of impedimetric tenascin C detection. Lab Chip 11(17):2884–2892
Riedl S, Bodenmüller H, Hinz U, Holle R, Möller P, Schlag P, Herfarth C, Faissner A (1995) Significance of tenascin serum level as tumor marker in primary colorectal carcinoma. Int J Cancer 64(1):65–69
Ropers T, Kroll W, Becka M, Voelker M, Burchardt ER, Schuppan D, Gehrmann M (2000) Enzyme immunoassay for the measurement of human tenascin-C on the Bayer Immuno 1 analyzer. Clin Biochem 33(1):7–13
Kimura S, Ishida S, Matunaga K, Washizu K, Hiraiwa H, Takeuchi K, Wakabayashi T, Yoshida J, Kato K (1993) Determination of tenascin in human serum by the use of a new enzyme immunoassay. Biomed Res 14(3):203–208
Ylätupa S, Mertaniemi P, Haglund C, Partanen P (1995) Enzyme immunoassay for quantification of tenascin in biologic samples. Clin Biochem 28(3):263–268
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1310 kb)
Rights and permissions
About this article
Cite this article
Wu, Y., Nie, F. & Xia, D. Chemiluminescence assay for the glycoprotein tenascin-C based on aptamer-modified carboxylated magnetic carbon nanoparticles. Microchim Acta 182, 227–232 (2015). https://doi.org/10.1007/s00604-014-1319-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1319-1