Abstract
We have prepared molecularly imprinted beads with molecular recognition capability for target molecules containing the penicillanic acid substructure. They were prepared by (a) grafting mesoporous silica beads with 6-aminopenicillanic acid as the mimic template, (b) filling the pores with a polymerized mixture of methacrylic acid and trimethylolpropane trimethacrylate, and (c) removing the silica support with ammonium fluoride. The resulting imprinted beads showed good molecular recognition capability for various penicillanic species, while antibiotics such as cephalosporins or chloramphenicol were poorly recognized. The imprinted beads were used to extract penicillin V, nafcillin, oxacillin, cloxacillin and dicloxacillin from skimmed and deproteinized milk in the concentration range of 5–100 μg·L−1. The extracts were then analyzed by micellar electrokinetic chromatography by applying reverse polarity staking as an in-capillary preconcentration step, and this resulted in a fast and affordable method within the MRL levels, characterized by minimal pretreatment steps and recoveries of 64–90 %.

Penicillanic acid-imprinted beads prepared in preformed porous silica by an imprinting & etching approach show selectivity towards β-lactams antibiotics. Molecularly imprinted solid phase extraction/micellar electrokinetic chromatography coupled with in-capillary preconcentration resulted in a fast and affordable method for penicillins in milk at MRL levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing awareness of food safety by the consumers leads public control agencies and food industry to ensure high levels of control on food for human consumption. Between anthropogenic contaminants, antibiotics are of particular concern, as the development of antibiotic resistance, for long time attributed to their overuse in human medicine, has been recently related also to their use in animal breeding with possible contaminations of foodstuffs [1]. Thus, the detection of low levels of antibiotics in food is of great relevance.
Among antibiotics, penicillins are frequently used in both permitted and clandestine veterinary practices. In fact, since the fifties, these antibiotics have been used in livestock farming as prophylactic and therapeutic agents and also as feed additives for growth promotion [2]. Thus, penicillins residues are frequently monitored in a wide range of sample matrices representative of foodstuffs, like muscle, kidney, liver, fat, milk, eggs, or honey.
High performance liquid chromatography coupled to mass spectrometry (HPLC/MS) is at the moment the top analytical methodology for simultaneous, unambiguous identification and quantification of these residues [3, 4], while an emerging trend for multi-residue determination is the use of accurate mass full-scan techniques that allows simultaneous determination of hundreds of different compounds in complex matrices in a single analytical run [5, 6]. Notwithstanding the excellent performance of these techniques, there are still limitations in the majority of the HPLC/MS methods, mainly due to sample preparation procedures and to the presence of matrix effects.
The clean-up of the samples plays indeed a key role in determining the detection capability of the instrumental techniques because of its ability to reduce matrix interferences [7]. Several strategies can be used for the elimination of matrix effects in LC/MS methods, and the use of immunoaffinity-based solid phase extraction systems results to be one of the best performing strategies [8, 9]. Immunoaffinity columns, however, are expensive because of the costs associated with the antibody production and their use is limited to one analysis as a consequence of the antibody instability to elution conditions. To overcome all these problems, the use of molecularly imprinted polymers (MIPs) in the so-called molecularly imprinted solid phase extraction (MISPE) is a good alternative to antibodies [10].
MIPs are frequently synthesized by using the target molecule as the template, and one of the main drawbacks in this technology is represented by the residual template leakage due to the presence in the polymeric matrix of minute amounts of the template molecule. It can bleed during the extraction process, thus contaminating the sample extract and affecting the analytical result. To overcome this drawback, a structural analogue of the analytical target can be used as a template in the so called “mimic template approach”. At the present, notwithstanding several imprinted polymers with molecular recognition properties towards one or more penicillins have been reported in literature [11–22] and some of them have been yet used to set up MISPE methods for clinical [18], environmental [16, 21] and food samples [19, 22], very few attention has been paid to the use of mimic templates.
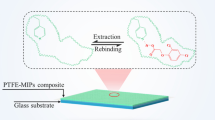
In this paper we present the development of a MISPE technique with selective molecular recognition properties towards structurally related penicillins obtained by using a mimic template in the so-called “fragmental template approach” [23]. The polymer was prepared in silica beads according with Yilmaz et al. [24], consisting in covalently immobilizing the template mimic in the pores of silica beads through a spacer arm, filling the pores of beads with an imprinting mixture, polymerizing it and dissolving the silica support, leaving porous imprinted beads that are the “negative image” of the silica beads. The use of 6-aminopenicillanic acid — a structure common to all the penicillins — as template molecule solved the need for a mimic template able to represent an entire class of target analytes.
Experimental
Reagents and materials
6-aminopenicillanic acid (APA), 3-aminopropyltrimethoxysilane (APSMS), amoxicillin trihydrate, ampicillin, carbenicillin, cefalexin, cefazolin, ceftiofur, chloramphenicol, ciprofloxacin, cloxacillin, dicloxacillin, N,N’-diisopropylcarbodiimide (DIPCD),hexamethyldisilazane (HMDS), N-hydroxysuccinimide (NHS), methacrylic acid (MAA), moxifloxacin, nafcillin, oxacillin, penicillin G, penicillin V, piperacillin, succinic anhydride, ticarcillin, trimethylolpropane trimethacrylate (TRIM) were from Sigma–Aldrich–Fluka (Milan, Italy, www.sigmaaldrich.com). Acetone, acetonitrile, ammonium hydrogen fluoride, 2,2′-azo-bis-(2-methylpropionitrile) (AIBN), 4-(N,N-dimethylamino)pyridine (DMAP), N,N-dimethylformamide (DMF), ethanolamine, methanol, pyridine, spherical porous silica beads (PharmPrep 60CC, 25–40 μm mean diameter, mean pore size 6 nm, specific pore volume: 0.80 mL·g, specific surface area: 500 m2·g−1), toluene, were from VWR International (Milan, Italy, it.vwr.com). All the solvents were of HPLC quality, other chemicals were of analytical grade. Polymerization inhibitors in vinyl monomers were removed by clean-up on activated alumina columns.
Ultrapure water was obtained by reverse osmosis with a Purelab Prima System from Elga (Bucks, UK). All the buffers were prepared by dissolving the corresponding salt in ultrapure water; pH values were adjusted with sodium hydroxide or hydrogen chloride 1 M and the solutions filtered through a 0.45 μm filter before use. Stock solutions of antibiotics were prepared by dissolving 25.0 mg of substance in 10.0 mL of ultrapure water (DMSO for amoxicillin trihydrate) and stored in the dark at −20 °C.
The high-performance liquid chromatography apparatus (L-6200 constant-flow binary pump, L-4250 UV–Vis detector, Rheodyne 7100 six-port injection valve provided with 5 μl injection loop and a D7000 data acquisition system) was from Merck-Hitachi (Darmstadt, Germany). Capillary electrophoresis was performed using an Agilent CE system (Agilent Technologies, CA, USA, www.agilent.com) equipped with a diode-array detector. Data acquisition and signal processing were performed by using the Agilent ChemStation, Agilent Technologies). The fused silica capillaries from Polymicro Technologies, Optronis GmbH, Kehl, Germany, (www.explorethecapabilities.com) were 64 cm in length (56 cm to the detector) with an I.D. of 50 μm.
Grafting of mimic template onto silica beads
In a 500-mL round-bottomed flask, 10.0 g of silica beads were suspended in 100 mL of 6 M hydrogen chloride aqueous solution under sonication. The homogenous dispersion was refluxed overnight, diluted with 400 mL of cold ultrapure water and filtered on a nylon membrane (0.22 μm nominal porosity). The beads were washed with ultrapure water till neutrality, transferred in a 500 mL round-bottomed flask and dried at 105 °C overnight.
The dried beads were transferred in a 250-mL round-bottomed flask and dispersed in 100 mL of toluene, removing water by azeotropic distillation. Then, the flask was cooled to room temperature, 1.75 mL (10 mmoles) of APSMS were added. The homogenous dispersion was refluxed overnight under gentle agitation. The aminosilanized beads were filtered on a nylon membrane (0.22 μm nominal porosity), washed with toluene, transferred in a 250-mL round-bottomed flask and dispersed again in 100 mL of toluene, removing water by azeotropic distillation. Then, the flask was cooled to room temperature, 1.67 mL (8 mmoles) of HMDS was added to the beads dispersion and the mixture again let to react overnight under reflux. The beads were filtered on a nylon membrane, washed with toluene and transferred in a 100-mL round-bottomed flask.
The end-capped beads were suspended in 50 mL of anhydrous pyridine containing a catalytical amount (~5 mg) of DMAP and 1.0 g (10 mmoles) of succinic anhydride. The reaction mixture was heated overnight at 60 °C under gentle agitation. The succinylated beads were separated from the reaction mixture by filtration on a nylon membrane, washed with acetone, dried under vacuum and stored in a desiccator at room temperature.
The succinylated beads (3 g) were suspended in 15 mL of ice-cold anhydrous DMF. Then, 310 mg (2.7 mmoles) of NHS and 419 μl (2.7 mmoles) of (DIPCD) were rapidly added and the reaction mixture was gently stirred to 4 °C for 1 h. The activated beads were separated from the reaction mixture by filtration on a nylon membrane, washed with 3 × 15 mL of anhydrous DMF and transferred in a 50-mL flask containing 30 mL of a freshly prepared solution of APA (58 mg, 0.27 mmoles) in 1/1 DMF – 0.15 M bicarbonate buffer, pH 8.5. The reaction mixture was gently stirred at room temperature overnight and filtered on a nylon membrane. The grafted beads were washed with 3 × 15 mL of DMF, 3 × 15 mL of ultrapure water, dried with 15 mL of anhydrous acetone and stored in the dark at −15 °C.
Silica beads for the preparation of the non-imprinted polymer (NIP) were treated with the same protocol, substituting APA with freshly distilled ethanolamine.
Solid phase titration
The amount of carboxyl groups available after the hemisuccination step was measured by acid–base return titration. Hemisuccinated silica beads (about 100 mg) were carefully weighted in a stoppered 25 mL-Erlenmeyer flask. Then, 10.0 mL of freshly titrated 0.100 N aqueous sodium hydroxide were added and the suspension gently stirred in a nitrogen atmosphere for 1 h. After settled the suspension, 5.00 mL of solution were sampled, diluted with 5.00 mL of ultrapure water and rapidly titrated under nitrogen with freshly prepared 0.100 N aqueous hydrogen chloride. All the titrations were repeated three times.
Synthesis of molecularly imprinted beads
A prepolymerization mixture was prepared dissolving AIBN (1 % of the vinyl groups present in the final mixture) in dried acetonitrile (10 % of the resulting total volume) adding MAA and TRIM in molar ratio 2/3, and purging with a gentle stream of nitrogen under sonication in a water bath for 5 min. In order to obtain discrete silica–polymer composite beads and to prevent particle agglomeration the amount of prepolymerization mixture added to the silica beads was slightly lower (about 5 %) than the nominal pore volume of the silica. Thus, in 10 mL thick wall glass vials maintained under continuous sonication to remove any entrapped air bubbles, an adequate amount of the prepolymerization mixture were slowly added to 3 g of silica beads. Then, the mixture was homogenized with a steel spatula obtaining a free flowing powder, sparged with nitrogen, sealed and allowed to polymerize in a water bath at 65 °C for 3 days. After polymerization was completed, the silica–polymer composite were transferred into a 50 mL screw-capped polypropylene tube and 10 mL of acetone were added to increase the wettability of the beads. Then 20 mL of 3 M ammonium hydrogen fluoride aqueous solution was added. Suspension was gently stirred overnight to completely dissolve the silica matrix of the composite. After dissolution of the silica, the suspension of imprinted polymeric beads was diluted with 100 mL deionised water, filtered on a polycarbonate filter funnel equipped with a nylon membrane and washed extensively with ultrapure water till neutrality.
Liquid chromatography
Adequate amount of polymer was suspended in a 1/1 methanol–water mixture, and the slurry was packed in a 3.9 × 100 mm stainless-steel HPLC column by eluting it with 1/1 methanol–water mobile phase at constant pressure of 15 MPa. The packed column was washed at 0.5 mL·min−1 with 9/1 methanol–acetic acid until a stable baseline was reached (254 nm).
The column was equilibrated at a flow rate of 1 mL·min−1 with 50 mL of acetonitrile; then, 5 μL of stock solution of antibiotic diluted to 50 μg·mL−1 with acetonitrile were injected and eluted at 1 mL·min−1, and the absorbance was recorded at 240 nm. Each elution was repeated three times to assure chromatogram reproducibility. Column void volume was measured by eluting 5 μL of acetone 0.1 % in acetonitrile, and the absorbance was recorded at 260 nm.
The capacity factor (k) was calculated as (t–t0)/t0, where t is the retention time of the analyte and t0 is the retention time corresponding to the column void volume. The imprinting factor (IF) is defined as an index of the imprinting efficacy respect to a NIP prepared in the same conditions. It was calculated as kMIP/kNIP, where kMIP is the capacity factor of the antibiotics eluted on the MIP column and kNIP is the capacity factor of the antibiotics eluted on the NIP. The specific selectivity factor (SSF) is defined as an index of polymer selectivity toward antibiotics due to the presence of imprinted binding sites, thus neglecting any effect due to the bulk of the polymer. It was calculated as \( {{{\mathrm{I}{{\mathrm{F}}_{\mathrm{analyte}}}}} \left/ {{\mathrm{I}{{\mathrm{F}}_{\mathrm{APA}}}}} \right.} \) [25].
MISPE of milk samples
Polymeric beads (100 mg) were suspended in methanol, sonicated in a water-bath for 10 min and packed in 3-mL empty polypropylene SPE cartridges provided with frits to secure the packing and outlet stopcocks. The columns were connected to a vacuum manifold, washed extensively with glacial acetic acid, 1/1 methanol–acetic acid, methanol, and then dried under vacuum. Immediately before any use, the cartridges were activated with 3 × 1 mL of acetone and 3 × 1 mL of 0.5 M citrate buffer, pH 4.8.
Bovine skimmed milk samples purchased from a local supermarket were spiked with 5 penicillins (penicillin V, nafcillin, oxacillin, cloxacillin and dicloxacillin) to a final concentration of 5, 25 and 100 μg·L−1. Then, 2 mL of sample was treated with 2 mL of 1 M citrate buffer, pH 4.8, homogenized with a vortex for 1 min, centrifuged at 5000g for 30 min and filtered on a 0.22 μm polypropylene membrane. One mL of the filtrate was loaded on the MISPE cartridge applying a vacuum to facilitate the passage of the sample through the cartridge bed. After sample loading, air was passed through the column for 5 min, the cartridge was washed with 3 × 1 mL of 7/3 water–methanol, dried again with a stream of air and β-lactams recovered by elution with 2 × 1 mL of acetone. The organic extract was evaporated to dryness using a gentle stream of nitrogen at room temperature and preserved in the dark at −20 °C. The evaporated extract was dissolved under sonication in 200 μl of ultrapure water immediately before the electrophoretic run to provide a low-conductivity analyte matrix suitable for sample stacking.
Capillary electrophoresis
The capillary was rinsed with potassium hydroxide 1 M for 5 min, ultrapure water for 10 min, and finally with the background solution (0.2 M TRIS-borate buffer, 30 mM SDS, pH 8) for 10 min. The sample was loaded by hydrodynamic injection at a pressure of 0.2 MPa for 20 s so that the whole capillary was almost full. Reverse electrode polarity stacking was produced by applying a negative voltage till 95 % of the maximum current was reached. Then, a positive voltage of 15 kV was applied to separate the analytes, monitoring the absorbance at 205 nm. Reference standard in the background solution of penicillins of concentration 5, 10, 25, 50, 100, 250 and 500 μg·L−1 were analysed three times consecutively and peak areas were plotted against concentration. A calibration plot was drawn using a weighted linear regression (weight = 1/conc.).
Results and discussion
Synthesis of molecularly imprinted beads
The molecular imprinting approach used in this work is based on the oriented immobilization of a template onto the pore surface of sacrificial silica beads. After the polymerization this support is removed by etching with an aqueous solution of ammonium fluoride, leaving polymeric beads with the imprinted binding sites localized onto the internal surface of the newly formed mesopores. We have recently reported experimental insights on the relation between the porosity of sacrificial silica beads and the binding properties of the resulting imprinted beads [26]. On these basis, we decided to use mesoporous silica beads (mean pore size: 6 nm) instead of macroporous materials to obtain a gain in terms of available pore surface, thus, increasing the amount of template grafted onto the pores and, consequently, the binding capacity of the resulting imprinted polymer. The amount of carboxyl groups present in the pores of the silica beads after the hemisuccination step was estimated by acid–base titration, resulting in 0.87 mmoles·g−1, a value corresponding to about 1 carboxyl group·nm−2, which is the 20 % of the generally accepted silanol surface density in fully hydroxilated silica [27]. This is a value potentially sufficient to assure the grafting of large amounts of 6-aminopenicillanic acid. Anyway, preliminary experiments revealed that no more than 50–100 μmoles·g−1 of template could be successfully grafted onto the silica beads, irrespectively of the amount introduced in the grafting mixture. Thus, a more limited amount of mimic template, corresponding to 90 μmoles·g−1, was used to graft the silica beads.
The use of 6-aminopenicillanic acid solves the need for a mimic template able to induce selectivity towards a broad class of targets in an imprinted polymer. In fact, this molecule shows the [3.2.0]bicyclic structure represented by the β-lactam ring fused with a five-membered thiazolidine ring and unique to all the penicillins. The amine in position 6 makes possible to covalently link this structure to the silica surface through a bis-succinamido spacer arm, thus preserving the amido structure in position 6 typical of all the penicillins. The presence of this spacer arm is intended to provide a further effect of distancing the mimic template from the surface of silica, generating space to host β-lactam substituents typically present in the considered antibiotics.
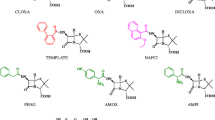
From Table 1, it is possible to see that the template grafting design here described is successful. In fact, of 17 antibiotics screened for the binding with the imprinted beads, penicillin G, penicillin V, oxacillin, cloxacillin, dicloxacillin and nafcillin show a specific selectivity factor (SSF>1) compatible with a good molecular recognition by the imprinted polymer, while the others are poorly recognized (SSF<1). Considering the structural formulas reported in Fig. 1, it is clear that binding selectivity is controlled by the nature of the side arm in position 6. Considering penicillins, the presence of strongly polar substituents as amino (ampicillin, amoxycillin, cefalexin), carboxy groups (carbenicillin, ticarcillin) or amido (piperacillin) represents an obstacle to the molecular recognition by the imprinted polymer, while hydrophobic side arms are much better recognized. Moreover, the poor recognition of piperacillin — a penicillin characterized by a very large side arm — shows that steric factors cannot be ruled out.
As regards the other antibiotics considered in study, it seems that they are poorly recognized by the imprinted polymer as effect of the different chemical nature of these molecules. Anyway, it is quite difficult to ascribe to the imprinted polymer an ability to well discriminate between the [3.2.0]bicyclic structure characteristic of penicillins from the slightly enlarged [4.2.0]bicyclic structure characteristic of cephalosporins, as the presence of a polar phenylglycinamido side arm in position 7 for cefalexin, and of a bulky substituent in position 3 for ceftiofur and cefazolin makes the interpretation of the experimental data ambiguous.
MISPE of milk samples
As effect of the molecular recognition properties of the imprinted beads, satisfactory sample clean-up was achieved by the MISPE protocol when performed on skimmed and deproteinized milk. An example of successful clean-up is reported in Fig. 2, where milk samples spiked at different levels (5, 25 and 100 μg·L−1) with a mixture of penicillin V, nafcillin, oxacillin, cloxacillin and dicloxacillin were, cleaned by MISPE and subsequently separated by MECK. While detection of penicillins is unfeasible when the milk sample is directly separated without any extraction procedure, the same samples analysed after a MISPE step show very clean traces, where peaks corresponding to penicillins can be easily detected and, as a consequence, quantified. The recovery of the MISPE extraction was determined by comparing the detector response of extracted milk with that of directly loaded standards prepared in background solution. Recovery rates, reported in Table 2, were determined at three concentration levels (5, 25 and 100 μg·L−1). They came out at between 64 and 90 %. An analysis of variance performed comparing the recovery rate obtained at the different concentration levels for each of the analysed penicillins showed a relevant statistical difference between groups of measures, where the 5 μg·L−1 level came out different (underestimated) compared to the 25 and 100 μg·L−1 levels for all the penicillins considered. Thus, the extraction protocol performed quite well, with good recovery rates for all the considered analytes at 25 and 100 μg·L−1 levels and a reduced, even if acceptable, recovery rate at the lower level of 5 μg·L−1.
MECK of milk samples spiked at different levels (a, b, c: 5, 25 and 100 μg·L−1) with a mixture of penicillin V (PENV), nafcillin (NAF), oxacillin (OXA), cloxacillin (CLO) and dicloxacillin (DIC) and cleaned by MISPE. Experimental conditions as reported in the Experimental section
Conclusions
In this work, we show that it is possible to obtain a class-selective molecularly imprinted polymer through the use of a template mimic characterized by a molecular structure common to all analytes of the class itself. The mimic is covalently anchored on the inner surface of the pores of silica microspheres in order to obtain a polymer with binding sites capable of recognizing only the structure of the template, common to all analytes, masking other structures that uniquely characterize each of the analytes. Moreover, the use of commercially available porous silica makes easy to prepare spherical beads with controlled diameter and porosity without resorting to complex polymerization protocols. The validity of this approach is shown by the successful setup of a MISPE-CE format for the determination of penicillins in skimmed milk. The method was tested at three concentration levels between 5 and 100 μg·L−1, showing it to be fast and affordable, with minimum pre-treatment of the milk samples, fairly precise and with good recovery levels of 64–90 %.
References
Viola C, De Vincent SJ (2006) Overview of issues pertaining to the manufacture, distribution, and use of antimicrobials in animals and other information relevant to animal antimicrobial use data collection in the United States. Prev Vet Med 73:111–131
Kools SAE, Moltmann JF, Knacker T (2008) Estimating the use of veterinary medicines in the European Union. Regul Toxicol Pharmacol 50:59–65
Blasco C, Torres CM, Picó Y (2007) Progress in a antibacterials analysis of residual in food. Trends Anal Chem 26:895–913
Stolker AAM, Zuidema T, Nielen MWF (2007) Residue analysis of veterinary drugs and growth-promoting agents. Trends Anal Chem 26:967–979
Samanidou V, Nisyriou S (2008) Multi-residue methods for confirmatory determination of antibiotics in milk. J Sep Sci 31:2068–2090
Le Bizec B, Pinel G, Antignac JP (2009) Options for veterinary drug analysis using mass spectrometry. J Chromatogr A 1216:8016–8034
Bogialli S, Di Corcia A (2009) Recent applications of liquid chromatography–mass spectrometry to residue analysis of antimicrobials in food of animal origin. Anal Bioanal Chem 395:947–966
Kinsella B, O’Mahony J, Malone E, Moloney M, Cantwell H, Furey A, Danaher M (2009) Current trends in sample preparation for growth promoter and veterinary drug residue analysis. J Chromatogr A 1216:7977–8015
Marazuela MD, Bogialli S (2009) A review of novel strategies of sample preparation for the determination of antibacterial residues in foodstuffs using liquid chromatography-based analytical methods. Anal Chim Acta 645:5–17
Baggiani C, Giovannoli C, Baravalle P, Anfossi L (2007) Solid phase extraction of food contaminants using molecular imprinted polymers. Anal Chim Acta 591:29–39
Skudar K, Brüggemann O, Wittelsberger A, Ramström O (1999) Selective recognition and separation of β-lactam antibiotics using molecularly imprinted polymers. Anal Commun 36:327–331
Lübke C, Lübke M, Whitcombe MJ, Vulfson EN (2000) Imprinted polymers prepared with stoichiometric template-monomer complexes: efficient binding of ampicillin from aqueous solutions. Macromolecules 33:5098–5105
Cederfur J, Pei Y, Zihui M, Kempe M (2003) Synthesis and screening of a molecularly imprinted polymer library targeted for penicillin G. J Comb Chem 5:67–72
Benito-Pena E, Moreno-Bondi MC, Aparicio S, Orellana G, Cederfur J, Kempe M (2006) Molecular engineering of fluorescent penicillins for molecularly imprinted polymer assays. Anal Chem 78:2019–2027
Urraca JL, Hall AJ, Moreno-Bondi MC, Sellergren B (2006) A stoichiometric molecularly imprinted polymer for the class-selective recognition of antibiotics in aqueous media. Angew Chem Int Ed 45:5158–5161
Urraca JL, Hall AJ, Moreno-Bondi MC, Sellergren B (2007) Direct extraction of penicillin G and derivatives from aqueous samples using a stoichiometrically imprinted polymer. Anal Chem 79:695–701
Zhang J, Wang H, Liu W, Bai L, Ma N, Lu J (2008) Synthesis of molecularly imprinted polymer for sensitive penicillin determination in milk. Anal Lett 41:3411–3419
Beltran A, Marcè RM, Cormack PAG, Sherrington DC, Borrull F (2008) Selective solid-phase extraction of amoxicillin and cephalexin from urine samples using a molecularly imprinted polymer. J Sep Sci 31:2868–2874
Kempe H, Kempe M (2010) Influence of salt ions on binding to molecularly imprinted polymers. Anal Bioanal Chem 396:1599–1606
Kempe H, Kempe M (2010) QSRR analysis of β-lactam antibiotics on a penicillin G targeted MIP stationary phase. Anal Bioanal Chem 398:3087–3096
Yin J, Meng Z, Du M, Liu C, Song M, Wang H (2010) Pseudo-template molecularly imprinted polymer for selective screening of trace β-lactam antibiotics in river and tap water. J Chromatogr A 1217:5420–5426
Zhang X, Chen L, Xu Y, Wang H, Zeng Q, Zhao Q, Ren N, Ding L (2010) Determination of β-lactam antibiotics in milk based on magnetic molecularly imprinted polymer extraction coupled with liquid chromatography–tandem mass spectrometry. J Chromatogr B 878:3421–3426
Kubo T, Hosoya K, Watabe Y, Tanaka N, Sano T, Kaya K (2004) Recognition of hepatotoxic homologues of microcystin using a combination of selective adsorption media. J Sep Sci 27:316–324
Yilmaz E, Haupt K, Mosbach K (2000) The use of immobilized templates. A new approach in molecular imprinting. Angew Chem Int Ed Engl 39:2115–2118
Cheong SH, McNiven S, Rachkov A, Levi R, Yano K, Karube I (1997) Testosterone receptor binding mimic constructed using molecular imprinting. Macromolecules 30:1317–1322
Baggiani C, Baravalle P, Giovannoli C, Anfossi L, Passini C, Giraudi G (2011) Binding behaviour of molecularly imprinted polymers prepared by a hierarchical approach in mesoporous silica beads of varying porosity. J Chromatogr A 1218:1828–1834
Nawrocki J (1997) The silanol group and its role in liquid chromatography. J Chromatogr A 779:29–71
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 104 kb)
Rights and permissions
About this article
Cite this article
Giovannoli, C., Anfossi, L., Biagioli, F. et al. Solid phase extraction of penicillins from milk by using sacrificial silica beads as a support for a molecular imprint. Microchim Acta 180, 1371–1377 (2013). https://doi.org/10.1007/s00604-013-0980-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-0980-0