Abstract
We describe a silver(I)-selective carbon paste electrode modified with multi-walled carbon nanotubes and a silver-chelating Schiff base, and its electrochemical response to Ag(I). Effects of reduction potential and time, accumulation time, pH of the solution and the stripping medium were studied by differential pulse anodic stripping voltammetry and optimized. The findings resulted in a method for the determination of silver over a linear response range (from 0.5 to 235 ng mL−1) and with a detection limit as low as 0.08 ng mL−1. The sensor displays good repeatability (with the RSD of ± 2.75 % for 7 replicates) and was applied to the determination of Ag(I) in water samples and X-ray photographic films.

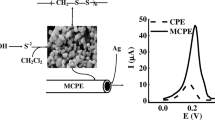
Open circuit accumulation of Ag(I) onto a surface of EHPO-MCPE and determination by Differential pulse anodic stripping voltammetry
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace metal pollution in environmental sources like water, air and soil has affected the life on earth [1, 2]. The contamination of the environment with toxic heavy metals is a major environmental problem. Their removal is a great challenge [3, 4].
The information about the interaction of silver with essential nutrients, especially selenium, copper and vitamins E and B12, has focused attention on its potential toxicity [5]. The toxicity of silver ranges through several orders of magnitude depends on the silver forms. While the ionic form of silver has been shown to be toxic to a variety of aquatic organisms, forms of silver other than ionic silver are significantly less toxic [6].
Silver is one of the industrially important elements. The widespread use of silver compounds and silver-containing procedures in industry, medicine, jewelry, cloud seeding and in the disinfection of drinking water has resulted in an increasing silver content of environmental samples. It is used for the preparation of corrosion-resistance alloys and its compounds are extensively used in the processing of foods, drugs, beverages and in filters and other equipments to purify water. Consequently, determination of trace amounts of silver is important for many areas of chemical analysis and simple and highly sensitive methods are needed to monitor the Ag levels in samples at ever decreasing concentrations. Trace and ultra-trace amount of silver ions in environmental samples is a stimulant for applying preconcentration techniques prior to its determination [7]. The most widely used techniques for separation and preconcentration of trace amount of silver ions are coprecipitation [8], solid-phase extraction (SPE) [9], liquid–liquid extraction (LLE) [10] and cloud point extraction (CPE) [11]. However, most of these procedures are time-consuming and may cause sample contamination. Several atomic spectrometric techniques such as atomic absorption spectrometry [12], inductively coupled plasma atomic emission spectrometry (ICP-AES) [13] and inductively coupled plasma mass spectrometry (ICP-MS) [14] have been used for the determination of silver in different environmental samples. Two limitations in these techniques are lower levels of analyte ions than the quantitation limits of the technique and the interferic effects of the main components of the samples [15].
Stripping voltammetric analysis has been known as one of the most sensitive techniques and is widely used for the trace heavy metal analysis in various samples, because of its ability to preconcentrate the analytes on the electrode surface [16] and to allow automatic in situ speciation measurements, with no or minimum sample change. Basically, anodic stripping voltammetry (ASV) method consisted of two steps, including preconcentration or deposition of the analyte at the electrode surface by reduction method, and stripping the preconcentrated analyte at the electrode surface by oxidation method. Modified carbon paste electrodes (MCPEs) have several advantages such as non-toxic, low background current, wide range of used potential, rapid renewal and easy fabrication [17]. Nowadays, carbon nanotubes (CNTs) have also been used in carbon paste electrodes [18–20]. CNTs have very interesting properties, such as ordered structure with high aspect ratio, ultra-light weight, high mechanical strength, high electrical conductivity, high thermal conductivity and high surface area [21–23]. Applying the CNT in carbon paste electrodes, increases the surface area, conductivity, current and finally improves the method sensitivity. A number of articles discuss about the determination of silver by stripping analysis at modified carbon paste electrodes. There are carbon paste electrodes modified by p-isopropylcalix[6]arene [24], NABHE [6], 4-(2-pyridylazo)-resorcinol [25], polythiophene [26], dl-dithiothreitol [27], 3-amino-2-mercapto quinazolin-4(3H)-one [28] and N,N′-diphenyl oxamide [29].
We describe here a simple and effective carbon paste electrode modified with CNT and (E)-4-(2-hydroxyethylimino)pentan-2-one (EHPO) (Fig. 1) was developed and the sensor was applied to the determination of Ag(I) by using differential pulse anodic stripping voltammetry (DPASV). The modifier, (E)-4-(2-hydroxyethylimino)pentan-2-one, can preconcentrate Ag(I) from aqueous solution to the surface of the modified electrode and greatly increase the sensitivity of determination. In addition, the EHPO-MCPE exhibited several advantages such as simple preparation, reproducibility of surface renewal by simple polishing, good stability and excellent ability to determine ultra-trace amounts of silver in X-ray photographic films and water samples.
Experimental
Materials and reagents
Highly pure graphite powder and multi-walled carbon nanotubes (MWCNTs) with 3–20 nm diameters, core diameter: 1–10 nm, SBET: 350 m2 g−1 and 95 % purity were purchased from Merck (Darmstadt, Germany). Silver nitrate solutions were prepared in deionized water and their concentrations were checked by an atomic absorption spectrometer (PerkinElmer model 2380). The silver solutions were placed in the dark and protected against light in amber bottles. The stripping solution for silver(I) was a HCl solution (0.1 mol L−1). Phosphate buffer solution was prepared by dissolving 1.2 g of NaH2PO4 in 90 mL of distilled water, titration to pH 7 with NaOH solution and make up volume to 100 mL with distilled water. Highly pure nitrogen was used for deaeration. The chelating agent, (E)-4-(2-hydroxyethylimino)pentan-2-one was synthesized and purified as described elsewhere [30].
Apparatus
Voltammetric experiments were performed using a Metrohm electroanalyzer (Model 757 VA Computrace, Switzerland, www.metrohm.com). The measurements were recorded using VA computrace version 2.0 (Metrohm, Herisau, Switzerland) run under Windows 98 operating system. All voltammograms were recorded with a three electrode system consisting of an Ag/AgCl electrode as the reference electrode, a platinum wire as the auxiliary electrode and the CPEs (modified or unmodified) as the working electrode. A Metrohm 827 pH meter (Switzerland, www.metrohm.com) was used for pH adjustments. All the electrochemical experiments were carried out under pure nitrogen atmosphere at room temperature. A RH B-KT/C (IKA, Staufen, Germany, www.ika.com) magnetic stirrer was employed to stir the sample solution.
Sample preparation
Three water samples, including; tap water (Kerman drinking water, Kerman, Iran), well water (Shahid Bahonar University of Kerman, Kerman, Iran) and waste water (Copper factory, Sarcheshmeh, Kerman, Iran) were selected. Then these samples were filtered to remove suspended particulate matter, their pH was adjusted to pH = 2.0 with nitric acid so as to prevent adsorption of the metallic ions onto the flask walls, stored at 4 °C in a refrigerator and the suggested method was applied to determination. For preconcentration, the pH of the samples was adjusted to 7.0 before analyzing by the described procedure.
X-ray photographic film
For X-ray photographic films analysis, the used films were washed with distilled water and were cut into small pieces after drying in an oven at 40 °C for 20 min. 20 mL of aqueous nitric acid (5 mol L−1) was added to 2.0 g of the film. The mixture was filtered and the solution was diluted to 500 mL and pH was adjusted to 7 by NaOH solution (2 mol L−1). One hundred microlitre of the solution was added to 10 mL of the buffer solution (pH 7.0) and the general procedure was applied to the resultant solution [6]. Samples were also analyzed using an independent technique, atomic absorption spectrometry (AAS).
Preparation of CPE and EHPO-MCPE
Unmodified carbon paste was prepared by hand mixing of 70 mg of reagent-grade graphite powder and 30 μL of Silicon oil with a mortar and pestle. A liquid for use as pasting agent in a carbon paste electrode should fulfill certain conditions. They should be sufficiently chemically inert, insulating, non-volatile, water immiscible and forming paste mixtures of fine consistency. However, there also are some less favorable characteristics of pastes made from paraffin oils or Nujol; for instance, their vulnerability in media with organic solvents. In this respect, better experience has been gained with silicon oil-based carbon pastes that also represent quite common type of pasting liquid [31]. Modified carbon paste was prepared by mixing 30 μL of silicon oil with 60 mg of high purity graphite powder, 5 mg chelating agent and 5 mg nanotube (for increasing the current and sensitivity) and following hand mixing in a mortar and pestle. A fresh electrode surface was obtained by squeezing out a small amount of paste into the end of a glass tube (ca. 3.0 mm i.d. and 10 cm long), scrapping off the excess against a conventional paper and polishing the electrode on a smooth paper to obtain a shiny appearance. The electrical connection was made with a copper wire.
General procedure
The electrochemical method is based on open circuit accumulation of silver ions onto a surface of modified carbon paste electrode. The analysis of Ag(I) using DPASV was carried out using the following steps: (a) EHPO-MCPE was immersed in a 20 mL of sample solution (pH 7) containing a known amount of Ag(I) and the solution was stirred for 12 min. (b) The EHPO-MCPE, the reference and counter electrodes were immersed into electrochemical cell and medium was replaced with 0.1 mol L−1 HCl solution as electrolyte, where the accumulated Ag(I) was reduced for 20 s in −0.7 V. (c) the differential pulse waveform, was recorded from −0.2 to +0.2 V to electrochemically strip the Ag0 back into Ag+ (with 20 mV s−1 scan rate, 100 mV pulse amplitude and 5 ms pulse period). The resulting oxidation peak constitutes the analytical signal. All the measurements were carried out at room temperature (∼25 °C).
Results and discussion
Voltammetric behavior of silver(I) at EHPO-MCPE
The ability of the EHPO-MCPE to preconcentrate Ag(I) was investigated. Figure 2 shows the differential pulse stripping voltammetry of unmodified CPE (a), MCPE (with nanotube and without EHPO) (b), EHPO-CPE (without nanotube and with EHPO) (c), EHPO-MCPE (with nanotube and EHPO) (d) and EHPO-MCPE (with nanotube and EHPO, no Ag(I) in accumulation medium) (e) in 0.1 mol L−1 HCl after preconcentration in accumulation medium (100.0 ng mL−1 Ag(I)). In CPE case, a small anodic peak at about +0.095 V indicates that some adsorption of Ag(I) occurred at the CPE surface. However, this miniscale peak provided an evidence for the absence of a significant preconcentration of Ag(I) at the unmodified electrode. In contrast, when EHPO-CPE (without nanotube) was applied, a well-defined anodic stripping peak at +0.095 V appeared after accumulation in the medium containing 100.0 ng mL−1 Ag(I). This peak was due to the reoxidation of elemental silver, produced by the reduction of accumulated Ag(I) at the negative potentials. As can be seen, the anodic peak current of silver at EHPO-CPE is several times larger than that of the unmodified electrode and when EHPO-MCPE (with nanotube and EHPO) was applied, peak current showed a well increase after accumulation in the medium containing 100.0 ng mL−1 Ag(I). Only residual current was observed (Fig. 2e) when EHPO-MCPE was applied in absence of Ag(I).
DP anodic stripping voltammograms in 0.1 mol L−1 HCl after open circuit accumulation in buffer solution (pH 7): a unmodified CPE with 100.0 ng mL−1 Ag(I), b modified CPE with multi-walled carbon nanotubes (MCPE) with 100.0 ng mL−1 Ag(I), c modified CPE with EHPO (EHPO-CPE) with 100.0 ng mL−1 Ag(I), d modified CPE with multi-walled carbon nanotubes and EHPO (EHPO-MCPE) with 100.0 ng mL−1 Ag(I), e EHPO-MCPE, no Ag(I) in accumulation medium. Other conditions: 12 min accumulation time, scan rate 20 mV s−1, 20 s reduction time and −0.7 V reduction potential
Principle of the method
The performance of the newly developed EHPO-MCPE is based on the preconcentration of Ag(I) from aqueous solution onto the surface of the modified electrode by forming complexes with the modifier. The modifier acts as the ligand and the metal ions are the central atom. In the other hand, the surface concentrations of Ag(I) are much larger than those of the unmodified electrode and the sensitivity is greatly increased. Regarding the above-discussed observations and under the experimental conditions, the possible pathways for analysis cycle, from accumulation step to voltammetric scan, can be presented as follows:
Accumulation step via complex formation:
Reduction step in negative potentials:
Stripping step via positive scan:
Optimization of analytical conditions
To optimize the performance of the EHPO-MCPE, for the electrochemical determination of silver(I) in aqueous solution, the parameters influencing the response of the electrode including supporting electrolyte, pH of solution, amount of modifier, accumulation time, reduction potential and time were investigated.
pH of accumulation medium
The influence of pH on the stripping peak current and potential was studied in buffer solutions (pH range 2–9). Current-pH plot (Fig. S1, Electronic Supplementary Material, ESM) reveal that, within the pH range 4–9, the current did not vary significantly. Therefore the electrode response is independent of pH in this range (4–9) and all subsequent measurements were made in neutral pH (pH 7.0).
Stripping medium
The metals have different electrochemical behaviors in different electrolytes. The effects of some electrolytes, such as HCl, HNO3, CH3COOH and H3PO4 on stripping peak currents of silver were investigated. The results show that Ag(I) have the best electrochemical responses in HCl. When the measurements were performed in this electrolyte, the largest stripping peak current, the lowest background current and the best shape of peak were obtained. The effect of HCl concentration on the shape of voltammograms was examined over the range from 0.001 to 0.5 mol L−1, a small current was observed in 0.001 mol L−1 HCl, which gradually increased up to 0.1 mol L−1 HCl and then decreased at higher concentrations. Anodic peak also shifts to negative potentials due to the effect of H+ concentration which shown in Eq. (2). Therefore the concentration of 0.1 mol L−1 HCl was used in all the measurements.
Accumulation time
When the accumulation time shifts from 0 to 12 min, the stripping peak currents increase greatly (Fig. S2, ESM). However, further increase of accumulation time does not cause the obvious enhancing of the stripping peak currents, which is probably due to the saturation loading of the electrode surface, so the prolonged accumulation time does not cause more metal ions to be reduced on the electrode surface. Thus, the accumulation time of 12 min was chosen for all subsequent analysis.
Influence of the reduction potential
The influence of reduction potential on the anodic peak current of silver(I) was studied by varying the reduction potential from 0 to −1.4 V. When the potential was increased to −0.7 V, a well-defined peak with the highest peak current was obtained. Further increase in the reduction potential from −0.8 to −1.4 V led to decreased peak currents. This is mainly due to the cohydrogen evolution at such high potentials. Therefore, −0.7 V was employed as an optimum reduction potential for further studies.
Influence of the reduction time
The effect of the reduction time was studied for Ag concentration of 100 ng mL−1 at standard measuring conditions. The time of reduction was changed from 5 to 100 s. It was found that the silver peak current increases linearly with the reduction time up to 20 s. For further study a reduction time of 20 s was chosen.
Amount of modifier
The effect of amount of (E)-4-(2-hydroxyethylimino)pentan-2-one within the carbon paste electrode was evaluated (Fig. S3, ESM). The use of (E)-4-(2-hydroxyethylimino)pentan-2-one as modifier can greatly improve the sensitivity of determination. The peak intensity increases with the increasing of amount of modifier because the concentration of EHPO on the surface of the modified electrode increases correspondingly. At 5 % of EHPO, relative to the mass of electrode, the largest peak current was obtained. However, the continuous increase of amount of modifier causes a decrease of peak current, because excessive EHPO may result in the decrease of conductivity of the modified electrode. So the best ratio of the modifier in carbon paste composition is 5 % (w/w).
Performance characteristics
In order to obtain an analytical curve for our developed method, The differential pulse stripping voltammetric determination of a series of standard solutions of Ag(I) was performed after the optimization of the experimental parameters (Fig. 3). The best results were obtained under the following optimized conditions: 20 s reduction time, −0.7 V reduction potential, 12 min accumulation time and 20 mV s−1 scan rate in 0.1 mol L−1 HCl. Standard solutions containing different amounts of Ag(I) were prepared in pH 7 and the general procedure was applied to these solutions. Voltammograms at these concentrations are shown in Fig. 3. Under optimized conditions, the suggested method showed a typical linear response ranging from 0.5 to 235 ng mL−1. The equation of calibration graph was μA = 3.2159C(ng mL−1) + 1.9028 with R 2 = 0.9985. A detection limit of 0.08 ng mL−1 was determined using a 3sb/slope ratio where sb is the standard deviation of the mean value for current of the blank. The precision expressed as the relative standard deviation (R.S.D.) was ± 2.75 % for seven successive measurements of the same samples containing 100 ng mL−1 Ag(I). This result indicates a good repeatability in the modified electrode construction possibly due to the strong adsorption of the Ag(I) in the electrode surface.
Interference study and reproducibility
Some metal ions were tested to evaluate the possible interference with the detection of trace amount of silver. The tolerance level was defined as the maximum amount of potentially interfering ions producing an error of ±5 % on the silver current. The tolerance level of each species was tested and if interference occurred, the ratio was reduced until it ceased. EHPO-MCPE was immersed in Ag solution. The experimental results indicate that 500 times of Na+, Mn2+, Au3+, Al3+, Ca2+, Zn2+, Ba2+, Fe3+, Co2+, Cr3+, Cu2+, Mg2+ and K+ have no significant influences on the signal of 100 ng mL−1 of Ag(I). However, 300 times of Ni2+ and 250 times of Hg2+ and Pb2+ was found to reduce the responses to Ag(I). These depressions can be due to compete these ions with Ag(I) to complex with EHPO in the electrode surface. The results of this study are summarized in Table 1.
Analytical application
The EHPO-MCPE electrode was applied to the preconcentration and determination of Ag in several water samples and X-ray photographic films.
As shown in Tables 2 and 3, the measured values are in agreement with those obtained by atomic absorption spectrometry and recovery of spiked sample at 95 % confidence level is good.
Comparison of EHPO-MCPE with some previously reported
A number of analytical characteristics of the EHPO-MCPE were compared with those of the previously reported Ag(I) voltammetric sensors [6, 24–28, 32–34]. The results are summarized in Table 4 and show that several modified electrodes such as modified carbon paste electrode [6, 24, 28, 32, 34], carbon ceramic electrode [25], platinum electrode [26], gold electrode [27] and glassy carbon electrode [33] were used for the determination of silver(I). Also various techniques such as differential pulse anodic stripping voltammetry [6, 24–26, 28, 32, 33] and square wave voltammetry [27, 34] were applied for the determination of Ag(I). As can be seen in this table, the EHPO-MCPE electrode shows better analytical characteristics such as the lowest detection limit except the results reported in the following literature [34] and wider linear range except the following reported [25, 26, 28, 33, 34].
Conclusion
The anodic stripping voltammetric procedure for the silver determination was investigated. We describe here that carbon paste electrode modified with EHPO and nanotube is a suitable alternative for the analytical determination of Ag(I). This developed sensor have a good characteristics such as; wide concentration range (0.5–235 ng mL−1), very good detection limit (0.08 ng ml−1) and good selectivity coefficient for many cations. In addition, the potentiometric response of this electrode is independent of the pH of the test solution in the pH range 4.0–9.0. This modified electrode, the EHPO-MCPE, coupled with differential pulse anodic stripping voltammetry was successfully applied in tap, well and waste water samples and X-ray photographic films and the results are satisfactory. In comparison with other reported modified electrodes for Ag(I) determination, the EHPO-MCPE electrode shows better analytical characteristics such as the lowest detection limit except the results reported in the following literature [34] and wider linear range except the following reported [25, 26, 28, 33, 34]. In addition, the inherent advantages of the developed electrochemical sensor are its rapid response, simple operation, precise results, low cost and direct application to the determination of silver.
References
Koréneková B, Skalická M, Nad P, Korenek M (2007) Occurrence of selected trace elements in cattle meat. Meso 9:328–331
Tuzen M, Soylak M (2006) Evaluation of some traces heavy metal levels of drinking waters from Tokat-Black Sea region of turkey. Pol J Environ Stud 15:915–919
Uluozlu OD, Kinalioglu K, Tuzen M, Soylak M (2007) Trace Metal Levels in Lichen Samples from Roadsides in East Black Sea Region, Turkey. Biomed Environ Sci 20:203–207
da Silva EGP, Hatje V, dos Santos WNL, Costa LM, Nogueira ARA, Ferreira SLC (2008) Fast method for the determination of copper, manganese and iron in seafood samples. J Food Compos Anal 21:259–263
Environment Protection Agency (EPA) (1980) Ambient water quality criteria for silver, EPA 4405-80-071. Office of Water Regulations, Washington, DC
Hassani Nadiki H, Taher MA, Ashkenani H, Sheikhshoai I (2012) Fabrication of a new multi-walled carbon nanotube paste electrode for stripping voltammetric determination of Ag(I). Analyst 137:2431–2436
Manzoori JL, Abdolmohammad-Zadeh H, Amjadi M (2007) Ultra-trace determination of silver in water samples by electrothermal atomic absorption spectrometry after preconcentration with a ligand-less cloud point extraction methodology. J Hazard Mater 144:458–463
Peker DSK, Turkoglu O (2007) Dysprosium(III) hydroxide coprecipitation system for the separation and preconcentration of heavy metal contents of table salts and natural waters. J Hazard Mater 143:555–560
Ghaedi M, Montazerozohori M, Soylak M (2007) Solid phase extraction method for selective determination of pb(II) in water samples using 4-(4-methoxybenzylidenimine) thiophenole. J Hazard Mater 142:368–373
Wang J, Hansen EH (2002) FI/SI on-line solvent extraction/back extraction preconcentration coupled to direct injection nebulization inductively coupled plasma mass spectrometry for determination of copper and lead. J Anal At Spectrom 17:1284–1289
Chen J, Xiao S, Wu X, Fang K, Liu W (2005) Determination of lead in water samples by graphite furnace atomic absorption spectrometry after cloud point extraction. Talanta 67:992–996
Sramkova J, Kotrly S, Jakoubkova P (2000) Precision attainable in the determination of silver by flame atomic absorption spectrometry analysis of thermoelectric silver-doped tellurides. Anal Chim Acta 408:183–190
Singh RP, Pambid ER (1990) Selective separation of silver fromwaste solutions on chromium(III) hexacyanoferrate(III) ion exchanger. Analyst 115:301–304
Ndung’u K, Ranville MA, Franks RP, Flegal AR (2006) On-line determination of silver in natural waters by inductively-coupled plasma mass spectrometry: influence of organic matter. Mar Chem 98:109–120
Ahmed SA (2008) Alumina physically loaded by thiosemicarbazide for selective preconcentration of mercury (II) ion from natural water samples. J Hazard Mater 156:521–529
Lan Y, Luo H, Ren X, Wang Y, Liu Y (2012) Anodic stripping voltammetric determination of arsenic(III) using a glassy carbon electrode modified with gold-palladium bimetallic nanoparticles. Microchim Acta 178:153–161
Ashkenani H, Taher MA (2012) Determination of cadmium(II) using carbon paste electrode modified with a Cd-ion imprinted polymer. Microchim Acta 178:53–60
Pérez-López B, Merkoçi A (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1–16
Zenkel C, Albuerne J, Emmler T, Boschetti-de-Fierro A, Helbig J, Abetz V (2012) New strategies for the chemical characterization of multi-walled carbon nanotubes and their derivatives. Microchim Acta 179:41–48
Zhou J, Wang F, Zhang K, Song G, Liu J, Ye B (2012) Electrochemical sensor for Baicalein using a carbon paste electrode doped with carbon nanotubes. Microchim Acta 178:179–186
Li J, Tang S, Lu L, Zeng HC (2007) Preparation of nanocomposites of metals, metal oxides, and carbon nanotubes via self-assembly. J Am Chem Soc 129:9401–9409
Ajayan PM (1999) Nanotubes from carbon. Chem Rev 99:1787–1799
Sawatsuk T, Chindaduang A, Sae-kung C, Pratontep S, Tumcharern G (2009) Dye-sensitized solar cells based on TiO2–MWCNTs composite electrodes: Performance improvement and their mechanisms. Diamond Relat Mater 18:524–527
Raoof JB, Ojani R, Alinezhad A, Rezaie SZ (2010) Differential pulse anodic stripping voltammetry of silver(I) using p-isopropylcalix[6]arene modified carbon paste electrode. Monatsh Chem 141:279–284
Rohani T, Taher MA (2010) Preparation of a carbon ceramic electrode modified by 4-(2-pyridylazo)-resorcinol for determination of trace amounts of silver. Talanta 80:1827–1831
Zejli H, Hidalgo-Hidalgo de Cisneros JL, Naranjo-Rodriguez I, Temsamani KR (2007) Stripping voltammetry of silver ions at polythiophene-modified platinum electrodes. Talanta 71:1594–1598
Zeng B, Ding X, Pan D, Zhao F (2003) Accumulation and stripping behavior of silver ions at dl-dithiothreitol self-assembled monolayer modified gold electrodes. Talanta 59:501–507
Mohadesi AR, Taher MA (2007) Stripping voltammetric determination of silver(I) at carbon paste electrode modified with 3-amino-2-mercapto quinazolin-4(3H)-one. Talanta 71:615–619
Won MS, Yeom JS, Yoon JH, Jeong ED, Shim YB (2003) Determination of Ag(I) ion at Modified Carbon Paste Electrode Containing N, N-Diphenyl Oxamide. Bull Korean Chem Soc 24:948–952
Sánchez M, Sánchez O, Höpfl H, Eugenia Ochoa M, Castillo D, Farfán N, Rojas-Lima S (2004) New boronates prepared from 2,4-pentanedione derived ligands of the NO2 and N2O2 type-comparison to the complexes obtained from the corresponding salicylaldehide derivatives. J Organomet Chem 689:811–822
Svancara I, Vytras K, Barek J, Zima J (2001) Carbon paste electrodes in modern electroanalysis. Crit Rev Anal Chem 31:311–345
Radulescu MC, Chira A, Radulescu M, Bucur B, Bucur MP, Radu GL (2010) Determination of silver(I) by differential pulse voltammetry using a glassy carbon electrode modified with synthesized n-(2-aminoethyl)-4,4′-bipyridine. Sensors 10:11340–11351
Fei W, Changzheng X, Yanju W, Yumei G, Baoxian Y (2011) Anodic stripping voltammetric determination of silver(I) in water using a 4-tert-butyl-1 (ethoxycarbonyl methoxy) thiacalix[4]arene modified glassy carbon electrode. J Anal Chem 66:60–65
Trnkova L, Krizkova S, Adamb V, Hubalek J, Kizek R (2011) Immobilization of metallothionein to carbon paste electrode surface via anti-MT antibodies and its use for biosensing of silver. Biosens Bioelectron 26:2201–2207
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 99 kb)
Rights and permissions
About this article
Cite this article
Jahandari, S., Taher, M.A., Fazelirad, H. et al. Anodic stripping voltammetry of silver(I) using a carbon paste electrode modified with multi-walled carbon nanotubes. Microchim Acta 180, 347–354 (2013). https://doi.org/10.1007/s00604-012-0935-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0935-x