Abstract
We have developed a solvent-free and sensitive method for the identification and quantification of methamphetamine (MAMP), amphetamine (AMP) and ecstasy (MDMA) in human urine. It is based on the use of an inside-needle adsorption trap (INAT) and a molecularly imprinted polymer (MIP). The MAMP-MIP layer was coated on the internal surface of a hollow stainless steel needle, which was oxidized and silylated. It was used as the extraction needle. A model solution containing the drugs was slowly passed through the extraction needle. After adsorption of the analytes, the needle was directly transferred to the injector of a gas chromatograph, where the analytes were thermally desorbed, separated by GC, and detected with a flame ionization detector. The method does not require an extraction solvent, is fast and simple. The linear range of the calibration graphs are rather wide, and the limit of detection and the limit of quantification (LOQ) for MAMP are 12 and 40 ng mL−1, respectively. The relative standard deviations (RSD%) for six repeated experiments (at 500 ng mL−1 of MAMP) is 4.9 %. The relative recoveries obtained for MAMP in spiked human urine samples are in the range of 81–93 %.

Typical chromatograms corresponding to the extraction of MAMP, AMP and MDMA in the optimum condition from human urine sample. Lower chromatogram (I) belong to non-spiked samples after extraction using MAMP-MIP coated needle and the other chromatograms, (II) and (III), are related to spiked samples with MAMP, AMP and MDMA (each 0.5 μg mL−1) and extraction using the NIP-coated needle and MAMP-MIP coated needle, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abuse of amphetamine-type stimulants (ATS) has increased continuously throughout all over the world and has become a global problem in recent years [1]. Amphetamine (AMP) and methamphetamine (MAMP) are some of the most abused ATS worldwide [2]. They are synthetic drugs used to treat mild depression, obesity, narcolepsy and certain behavioral problems in children [3]. They are also powerful central nervous stimulants capable of producing a euphoric static similar to cocaine [4]. 3,4-methylenedioxymethamphetamine (MDMA or ecstasy) is classified as illicit substance in most countries and its recreational use is prevalent, mainly among youth, despite warnings of irreversible damage to the central nervous system [5]. In order to analyse these drugs, urine sample has always been involved since it can reflect the consumption or exposure during the preceding 1–4 days [6].
Many methods have been reported to assess these drugs in biological samples using gas chromatography (GC) [7–9], high-performance liquid chromatography (HPLC) [10, 11], GC/mass spectrometry (MS) [12, 13], capillary electrophoresis (CE) [14], and CE–MS [15]. Most of these methodologies employ extraction methods, such as liquid–liquid extraction (LLE) and solid phase extraction (SPE) in order to remove impurities contained in matrix samples. However, most of these pretreatment methods involve multi-steps, which are time consuming, and using large amounts of organic solvents in the conditioning and elution steps, which may in turn, result in the loss of analytes. The large amount of organic solvent used in the extraction procedures can also cause health and environmental problems. In recent years, different microextraction techniques such as solid–phase microextraction (SPME) [16, 17] and liquid–phase microextraction (LPME) [18, 19] have been introduced as alternatives to LLE and SPE.
Solid phase microextraction (SPME) is a powerful, simple, fast and solvent-free extraction method which solves a number of problems confronting the analyst during sampling and introducing the sample into analytical instruments. It is based on equilibrium of analyte concentration between the sample and the solid-phase fiber coating. Many methods for the determination of amphetamines using SPME were reported [20–22]. In recent years the inside-needle capillary adsorption trap (INCAT) technique has been developed as a solvent-free sample preparation approach and as an alternative extraction method derived from SPME. This technique can be executed in two modes: in the first mode, which is a solid-phase dynamic extraction (SPDE), the non-selective sorbent is usually coated on the internal surface of a needle [23, 24]; the other mode involves packing lumen of the needle with commercially available sorbents or chemically synthesized polymers [25, 26].
Molecular imprinting is a technology which aims to produce polymers programmed to recognize a target or a class of target molecules which have been applied as useful materials in many fields [27, 28]. We have previously reported some monolithic SPME fibers based on MIP which can be coupled with GC or GC/MS for selective extraction of different compounds from various samples [21, 29, 30]. In the previous work, we coated a selective sorbent layer (atrazine-MIP) on the inner surface of the oxidized and silylated stainless steel extraction needle through chemical bonding and used to SPDE of triazine herbicides from aqueous samples [31]. The aim of this study is to develop INAT method based on MIP using MAMP as template, and its application for SPDE of MAMP, AMP and MDMA from urine samples and analyses by GC-FID.
Experimental
Reagents and chemicals
Methacrylic acid (MAA), ethylene glycol dimethacrylate (EGDMA), 2,2′-azobis-isobutyronitrile (AIBN), acetonitrile, methanol, chloroform, sodium nitrite and carbonate were purchased from Merck (Darmstadt, Germany, www.merck.com). 3-(trimethoxysilyl) propylmethacrylate (TMPM) was purchased from Sigma–Aldrich (St. Louis, MO, USA, www.sigma-aldrich.com). MAMP, AMP, MDMA and codeine were provided from the Research Center of Antinarcotics Police (Tehran, Iran). Ibuprofen and Chlorpheniramine maleate were provided by the Dana Pharmacy Company (Tabriz, Iran, www.zakariapharm.com). Stainless steel tube (o.d. 1.25 mm, i.d. 1.15 mm) was obtained from the Iran Needle Corporation (Tehran, Iran, www.iranneedle.com). Stock solution of the target analytes was prepared in methanol at a concentration of 1000 mg L−1 for each drug. This solution was injected into the separation system each day for quality control and the obtained peak areas were used in the calculation of response factors of the detector. Model solutions (500 ng mL−1) were prepared daily by appropriate dilutions of the stock solution of the drugs with deionized water. Drug-free urine samples were collected from healthy volunteers and subsequently used for method development.
Instrumentation
Monitoring of the analytes was performed using a GC (Shimadzu 2014, Kyoto, Japan, www.shimadzu.com) equipped with a flame ionization detector (FID) and a hydrogen generator (Shimadzu-OPGU 1500S, Kyoto, Japan). Hydrogen generator was used to generate hydrogen gas for FID at a flow rate of 30 mL min−1. Helium (99.999 %, Gulf Cryo, United Arabic Emirates) was used as the carrier gas after passing from a molecular sieve and an oxygen trap. The linear velocity of the gas and total flow were 36 cm s−1 and 51.4 mL min−1, respectively. Gas chromatographic separations were carried out on a BPX5 capillary column, coated with a 0.25 μm film thickness (30 m × 0.25 mm i.d.) (SGE Analytical Science, Forte, Australia, www.sge.com). An ultrasonic bath (Grant, Cambridge, UK, www.grantinstruments.com) was used for stirring the pre-polymer solution. A laboratory-made sampling device consisting of a PTF valve at the top side to control the flow rate and MAMP-MIP coated stainless steel tube on the down side as an extraction device was designed and fabricated. The column temperature was programmed from 30 °C (3 min) rising at 20 °C min−1 to 130 °C, rising at 10 °C min−1 to 200 °C, rising at 20 °C min−1 to 260 °C (2 min).
Treatment of stainless steel tubes
The tubes were treated by a method similar to reported previously [31]: In brief, they were cut into pieces each 6 cm in length and were immersed in a dilute commercial laundry detergent solution for 30 min at 70 °C. Afterwards, they were washed and rinsed with distilled water and acetone, consecutively followed by an aqueous solution containing 50 g·L−1 sodium nitrite and 10 g L−1 sodium carbonate for 30 min at 60 °C. The tube pieces were again rinsed in water and dried in a furnace at 150 °C for 15 min. They were silylated for 2 h by being immersed into a 10 % (v/v) TMPM solution in acetone at room temperature. Lastly they were washed with methanol and dried in a stream of helium.
Coating of the internal oxidized and silylated surface of tubes with MIP and non-imprinted polymer (NIP) layers
1.7 mmol template (MAMP) in 30 mL porogen (acetonitrile) was dissolved and 30 mmol of functional monomer (MAA) was added. After stirring ultrasonically the resulting mixture for 5 min, 120 mmol cross-linker (EGDMA) and 50 mg initiator (AIBN) were added to the solution. The solution was bubbled with nitrogen for 10 min and used as pre-polymer solution. One end of the treated tubes was sealed by a septum and to prevent blocking inner surface of the tube during polymerization, a fine and inflexible steel wire (10 cm × 0.2 mm o.d.) was inserted through the needle via middle of the septum. After filling the tube with prepared pre-polymer solution by means of a plastic syringe, the other end of it was also sealed with another septum. The tube was rotated at 100 rpm using a home-made electric motor setup and polymerization was carried out at 55 °C for 12 h in an oven. To prevent the wire from adhering to the tubes, it was manually moved every 30 min during the polymerization process. After completion of the polymerization, the wire was completely withdrawn. Coating with NIP-layer was carried out with exactly the same method described above except that MAMP was not included in the polymerization process. The MIP and NIP coated tubes were washed for several times with excess amount of a mixture containing methanol/acetic acid/doubly-distilled water (4:1:1, v/v/v) until template and non-reacted compounds were removed as much as possible and dried for 1 h in vacuum. The tubes were then conditioned by heating in a carbolite furnace for 15 min at 220 °C and in the presence of water vapor. Before extraction, they were mounted on the tip of a glass syringe and further conditioned at 250 °C for 10 min in a GC injection port under helium flow and then mounted on the bottom of the sampling device and used as extraction needles.
Sampling procedure
12 mL of the model solution containing 4 % (w/v) NaCl with pH 8 (adjusted by phosphate buffer, 50 mM) was passed through the needle at a flow rate of 0.4 mL min−1. The extraction needle was washed with 2 mL distilled water to remove the matrix interferences and then removed from the sampling device. It was mounted on a 5 mL glass syringe and was inserted into GC injection port. The analytes were thermally desorbed at 280 °C for 3 min. To prevent any peak splitting, the syringe plunger was repeatedly moved to and fro and the column temperature was kept at 30 °C at the beginning of the chromatographic run.
Real sample
Drug-free urine samples were collected from healthy volunteers and spiked with AMP, MAMP and MDMA (0.5, 1 and 1.5 μg mL−1 of each drug). Non-spiked and spiked samples were subjected to the extraction by the fabricated needles.
Results and discussion
Investigation and optimization of the coating procedure
To obtain a high strength and extractive MIP-coating, the optimization of the coating procedure is important. The parameters being effective on the coating process, namely nature and level of porogen, template-to-MIP components ratio, polymerization time and temperature were investigated and optimized.
Optimization of the nature and level of porogen
Polymerization was carried out in the most frequently used porogens, i.e., chloroform, methanol and acetonitrile (with various volumes) in the presence of constant amounts of functional monomer (30 mmol) and cross- linker (120 mmol). The fabricated extraction needles were used for the extraction of the analytes from the model solutions. The results (Fig. S1 in Electronic Supplementary Material; ESM) revealed that 30 mL of acetonitrile was the desired porogen.
Optimization of template to MIP components ratio at various temperatures
For this purpose, various types of extraction needles were fabricated in the presence of different amounts of MAMP (1.5–2.1 mmol) during 10 h at various polymerization temperatures. The results (Fig. S2 in ESM) revealed that 1.7 mmol was the optimum amount of template.
Optimization of polymerization time
To investigate the effect of polymerization time on the coating characteristics, curing of pre-polymer solution on the inner surface of the treated extraction needles was investigated at different polymerization time durations (6–24 h). The results showed that for 12 h of polymerization, peak areas reached a plateau and no considerable difference in the extraction amounts was observed beyond that, therefore 12 h was selected as the optimum polymerization time.
Evaluation and optimization of the effective variables on the extraction performance
Effect of pH
The pH value of the sample solution plays an important role in the extraction procedure, because the pH value of solution determines the existing states of the analytes and acidic or alkaline functional groups of MIP, and thus the extraction efficiency of the targeted compounds can be significantly influenced by pH. To investigate this effect, different pH values of working solution ranging from 2 to 13 were studied. The experimental results are shown in Fig. 1. It was clear from Fig. 1 that the extraction efficiencies at pH < 7 or pH > 9 were lower than those at the pH 7–9. In addition to the attraction based on the size and shape compatibility of the analytes molecules and MIP cavities, other interactions such as hydrogen bonding and ionic interactions also participated in adsorption of the analytes on the MIP-coated layer. At the lower pH values, the extraction was poor, because the analytes and MAA were in their protonated forms. Therefore, carboxylic groups of MAA could probably be interacted only with hydronium ions rather than with the hydrogen atom of the amino group which led to low extraction efficiency. By increasing the sample pH, the analytes were still in the protonated form (pKa, MAMP = 9.8, pKa, AMP = 9.8 and pKa,MDMA = 9.9), while MAA lost H+ ion (pKa = 4.7) and was converted to its anionic form. Therefore, a relatively strong interaction based on ionic interactions could be formed between cationic analytes and anionic carboxylate groups of MIP. At the higher pH values the analytes and MAA were in their molecular and anionic forms, respectively. Therefore, only hydrogen bonding could be established between them which caused lower extraction efficiency. Therefore, in the following studies, pH 8 was selected as the optimum pH.
The effect of pH of the aqueous solution on the extraction efficiency of MAMP, AMP and MDMA by fabricated needle. The concentration and volume of model solution were 500 ng mL−1and 5 mL, respectively. Polymerization time, 12 h; porogen, acetonitrile (30 mL). The bars indicate the maximum and minimum of three extractions and determinations
Salting effect
In most cases, addition of a salt plays an important role in the conventional extraction procedures. By increasing ionic strength of aqueous sample, solubility of organic compounds in the aqueous phase is decreased and more analyte molecules are transferred into the organic phase. So extraction recovery is often increased in the presence of a salt. To investigate the effect of ionic strength, the extraction procedure was performed on the model solutions containing different amounts of NaCl (0–10 %, w/v). The extraction efficiencies relating to the extracted amount of the analytes against NaCl concentration are presented in Fig. 2. Regarding the extraction efficiency, increasing with the increasing of the NaCl concentration, the salting-out effect is thought to be responsible, which is also commonly found in the extraction process involving hydrophobic interaction. By more increasing the percentage of salt, the extraction efficiencies were decreased slightly and reached plateaus approximately at 4 % (w/v). It was proved that the analytes may interact with the salts, thus reducing their capacities to be retained in the coated layer. Therefore, in this work ionic competition between Na+ and HMAMP+ to establish ionic interaction with carboxylate ion of MIP matrix could be increased and the extraction efficiency thereby decreased. According to the results obtained, salt concentration had unfavorable effect on the analytical signal. But, because of presence of salt in the real samples, calibration graph and other analytical characterization of the method should be established in the presence of 4 % NaCl.
Effect of salt concentration on the efficiency of extraction of MAMP, AMP and MDMA. Sample pH 8 (adjusted by phosphate buffer, 50 mM); and other experimental conditions were as given for Fig. 1. The bars indicate the maximum and minimum of three extractions and determinations
Effect of sample flow rate
The flow rate of the sample solution through the fabricated extraction needle is an important factor because it controls the total analysis time and must be enough to prevent waste of time. The flow rate, on the one hand, must be low enough to make an effective retention of the analytes possible. Therefore, to evaluate the influence of the time of contact between the MIP-coated layer and the sample solution on the recovery, the effect of the sample loading flow rate has been studied in the range of 0.1–2 mL min−1, with other conditions kept constant. Considering the extraction time and relatively higher extraction efficiency, 0.4 mL min−1 was selected as the optimum sample flow rate for further studies.
Effect of sample volume (break through)
The effect of sample volume was studied by taking different volumes of 1 mg L−1 solution of the analytes (5–150 mL). As it can be seen from Fig. 3, peak areas of MAMP increased up to 75 mL (for other analytes, 50 mL) and after that remained constant. These results show that the coated layer gets saturated for the volumes more than 75 mL of MAMP. Therefore, the volumes less than 75 mL for MAMP should be selected. On the other hand the time needed to reach equilibrium in the extraction stage also increased. To reach a compromise between the sample volume and the extraction time it was decided to fix the sample volume at 12 mL (sampling time, 30 min), because of the obtained response for the analytes exhibited good reproducibility and was considered suitable for the determination of trace levels. So, it was selected as the optimum sample size.
The effect of sample volume (and breakthrough) on the extraction ability of the fabricated needles for different volumes of the solution containing MAMP, AMP and MDMA (1 μg mL−1 of each); sample pH, 8; NaCl, 4 % (w/v); and other experimental conditions were as given for Fig. 1. The bars indicate the maximum and minimum of three extractions and determinations
Thickness and extraction capacity of the coated MAMP-MIP layer
To investigate the thickness, the weight of the coated MIP-layer was firstly calculated. For this purpose, weights of the six treated extraction needles were measured before and after coating with MAMP-MIP layer. The differences in the weights, 10.1 ± 0.3 mg, correspond to the amount of the coating MIP film. To calculate the density of the polymer, 1 mL of the pre-polymer solution was poured into a test tube. It was immediately sealed with a rubber cap and the solution was then cured in a water bath in the optimized conditions. The resultant bulk polymer was crushed, ground and sieved to collect the particles sized 74–105 μm. Then, density of the polymer was calculated by dividing mass of the dry synthesized particles by volume of the particles and found to be 1.78 g mL−1. Considering dimensions of the extraction needle (60 mm × 1.15 mm i.d.) and MIP density, the average thickness of the MIP layer was calculated to be approximately 26 μm. On the other hand, the analytical signal of MAMP increased by the volume of the solutions up to 75 mL and then reached a plateau. Therefore, maximum adsorbed amount of MAMP on the layer was 75 μg. By taking into the weight of the layer (10.1 mg), molecular weight of MAMP (149 g mol−1), and the maximum adsorbed amount (75 μg); the extraction capacity of the coated MIP layer was obtained 49.5 μmol g−1.
Investigation of chemical and thermal stability
Different experiments showed that the fabricated needles were chemically stable and the extraction ability of the fabricated needles did not change until 300 °C which can be regarded as the limiting temperature of application for these extraction needles (discussed in detail in ESM).
Selectivity of the fabricated MAMP-MIP coated needles
To investigate the selectivity, SPDE followed by GC procedures was carried out using series of the selected abused drugs and other drugs as reference samples (C = 500 μg mL−1 of each drug) in the optimized conditions. The ratios of peak areas of each studied compound to MAMP peak area by considering detector response factors (calculated from direct injection) are presented in Table 1. These results can prove that the fabricated MAMP-MIP coated exhibits high selectivity for MAMP and other analogues while other structurally unrelated compounds show almost no extraction. They revealed that the fabricated MAMP-MIP extraction needle had different imprinting behaviors towards MAMP and the related compounds, and its ability toward the template molecule was higher than the analogues compounds. It was clearly induced during the imprinting process.
Lifetime of the fabricated MIP-coated extraction needle
Lifetime of the fabricated extraction needle was evaluated by comparing the results of the model solution during continues usages. It was observed that the extraction efficiencies of the MAMP, AMP and MDMA were similar for more than 75 times with total RSDs of 5.8, 6.9 and 8.1 %, respectively. This means that the fabricated needle was stable and could be used for a number of tests and was durable for longer time spans and there is no need to prepare new sorbent for each experiment. This could decrease analysis time and expanse.
Repeatability of the method
To evaluate repeatability of the developed method, two experiments sets were performed: (i) extractions carried out using one MAMP-MIP coated extraction needle on the six separate solutions, and (ii) extractions carried out using four coated needles (same sizes). The results are shown in Table 1. Relative standard deviations (RSDs) for peak areas obtained by one coated needle are excellent and are between 4.9 and 6.9 %, which indicates that in spite of more steps, repeatability of the method is satisfactory. RSDs for different coated needles are between 5.8 and 8.7 %.
Quantitative features of the method
Analytical performance of the presented method was validated by calculation of linear dynamic range, square of correlation coefficient, limit of detection (LOD), and limit of quantification (LOQ) for the target analytes and was summarized in Table 1. The results showed that wide linear ranges with good linearity (R2 > 0.991) were achievable for the selected drugs. The detection limits (LODs) were calculated by progressively reducing the amount of extracted and desorbed analytes until the response had a peak height three times as large as the average noise around the peak. Low detection and quantification limits for MAMP were obtained by GC–FID. Repeatability of the method was another advantage of the method which was discussed in the previous section.
Samples analysis
The method was applied for determining the selected abused drugs in urine samples. Drug-free urine samples were collected from healthy volunteers. Typical GC–FID chromatograms of non-spiked and spiked urine, after performing the extraction method on them, are shown in Fig. 4. Figure 4 (I) shows the chromatogram using the method (MAMP-MIP-SPDE) from non-spiked and non historical urine sample. Figures 4 (II) and 4 (III) illustrate the chromatograms resulted after the extraction from the sample spiked with 500 ng mL−1 of the analytes using NIP-SPDE and MIP-SPDE procedures, respectively.
Typical chromatograms corresponding to the extraction of MAMP, AMP and MDMA in the optimum condition from human urine sample. Lower chromatogram (I) belong to non-spiked samples after extraction using MAMP-MIP coated needle and the other chromatograms, (II) and (III), are related to spiked samples with MAMP, AMP and MDMA (each 0.5 μg mL−1) and extraction using the NIP-coated needle and MAMP-MIP coated needle, respectively
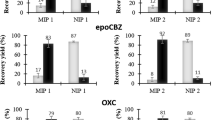
Like many other sample preparation approaches for trace analysis, the efficiency of the method may be affected by the complexity of the matrix involved. In order to evaluate the matrix effect, the three samples were spiked with the analytes at three levels (0.5, 1 and 1.5 μg mL−1 of each drug) and the method was applied to them (three times for each concentration). The recoveries, in comparison with those obtained for standard solutions at the same concentrations, are summarized in Table 2. As it is clear from the results, there is a relatively low matrix effect.
Comparison of the method with other methods
To assay the performance of the method, MAMP analytical parameters were compared with those of other methods employed for its analysis. For this purpose, linear range, limit of detection, limit of quantification and relative standard deviation of the reported methods along with those of the presented method were listed in Table 3. It was noted that in the reported methods in Table 3, highly sensitive methods such as GC or LC coupled with mass spectrometry are inherently more sensitive than GC-FID. Repeatability of the method reported as RSDs was very good compared to the other methods. This method has been validated in accordance with LOD, linearity and precision. Generally the presented method can be considered as an inexpensive, simple, and reliable analytical technique in determination of analytes of interest in aqueous samples.
Conclusions
In this paper MAMP-MIP was coated on the inner surface of the stainless steel extraction needle through chemical bonding. By investigation and optimization of pretreatment and polymerization conditions, an MIP coating of high strength was obtained with very good thermal and chemical stabilities. The fragility of the monolithic MIP fibres was improved by using this technique. It can be used for selective extraction and pre-concentration of MAMP, AMP and MDMA, and can be directly inserted into the GC injection port. In addition, unlike the conventional method, this method had no need for performing pretreatment step and prevents the loss of the analytes. It allows field sampling with portable field sampler. The efficiency of the fabricated extraction needle to extract MAMP and other analogs in urine samples was investigated by implementation of extraction in the non-spiked and spiked urine samples. High extraction efficiency was obtained for the studied drugs, resulting in low detection limits and high quantities of recoveries. The results indicated that the method has some advantages in respect of pretreatment time, organic solvent consumption, simplicity, and extraction efficiency. Hence, it seems possible to extend this method to the extraction of interest analytes in other similar samples (such as saliva and heir) by varying the extraction conditions.
References
Khajeamiri AR, Faizi M, Sohani F, Baheri T, Kobarfard F (2012) Determination of impurities in illicit methamphetamine samples seized in Iran. Forensic Sci Int 217:204–206
International Narcotics Control Board, Report 2009, United Nations, New York, 2010
Cook JD, Schanberg SM (1970) The effects of methamphetamine on behavior and on the uptake, release and metabolism of norepinephrine. Biochem Pharmacol 19:1162–1179
Sato M (1986) Acute exacerbation of methamphetamine psychosis and lasting dopaminergic. Psychopharmcol Bull 22:751–756
Kalant H (2001) The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. Can Med Assoc J 165:917–928
Huestis MA, Cho RE (2002) Drug abuse’s smallest victims: in utero drug exposure. Forensic Sci Int 128:20–30
Kumazawa T, Sato K, Seno H, Suzuki O (1993) Rapid extraction of methamphetamine and amphetamine in body fluids with bond elut SCX cartridges before capillary gas chromatography. Jpn J Legal Med 47:129–133
Myung SW, Min HK, Kim S, Kim M, Cho JB, Kim TJ (1998) Determination of amphetamine, methamphetamine and dimethamphetamine in human urine by solid-phase microextraction (SPME)-gas chromatography/mass spectrometry. J Chromatogr B 716:359–365
Raikos N, Christopoulou K, Theodoridis G, Tsoukali H, Psaroulis D (2003) Determination of amphetamines in human urine by headspace solid-phase microextraction and gas chromatography. J Chromatogr B 789:59–63
Hendrickson HP, Milesi-Halle A, Laurenzana EM, Owens SM (2004) Development of a liquid chromatography–tandem mass spectrometric method for the determination of methamphetamine and amphetamine using small volumes of rat serum. J Chromatogr B 806:81–87
Ming-Ren F, Ti-Yu W, Tzuen-Yeuan L (2006) Determination of amphetamine and methamphetamine in urine by solid phase extraction and ion-pair liquid chromatography–electrospray–tandem mass spectrometry. Talanta 68:987–991
Sato M, Mitsui T (1997) Rapid and simple determination of methamphetamine and amphetamine in blood by simultaneous extraction-derivatization. J Pharm Biomed Anal 16:139–145
Yamada H, Yamahara A, Yasuda S, Abe M, Oguri K, Fukushima S, Ikeda-Wada S (2002) Dansyl chloride derivatization of methamphetamine: a method with advantages for screening and analysis of methamphetamine in urine. J Anal Toxicol 266:17–22
Ramseier A, Caslavska J, Thormann W (1998) Screening for urinary amphetamine and analogs by capillary electrophoretic immunoassays and confirmation by capillary electrophoresis with on-column multiwave-length absorbance detection. Electrophoresis 19:2956–2966
Ramseier A, Siethoff C, Caslavska J, Thormann W (2000) Confirmation testing of amphetamines and designer drugs in human urine by capillary electrophoresis-ion trap mass spectrometry. Electrophoresis 21:380–387
Bagheri H, Mir A, Babanezhad E (2005) An electropolymerized aniline-based fiber coating for solid phase microextraction of phenols from water. Anal Chim Acta 532:89–95
Djozan D, Assadi Y (1999) Monitoring of polycyclic aromatic hydrocarbons in water using headspace solid-phase microextraction and capillary gas chromatography. Microchem J 63:276–284
Han D, Row KH (2012) Trends in liquid-phase microextraction, and its application to environmental and biological samples. Microchim Acta 176:1–22
Saraji M, Boroujeni MK (2011) Analysis of narcotic drugs in biological samples using hollow fiber liquid–phase microextraction and gas chromatography with nitrogen phosphorus detection. Microchim Acta 174:159–166
Djozan D, Baheri T, Pournaghi-Azar MH (2007) Development of electro solid-phase microextraction and application to methamphetamine analysis. Chromatographia 65:45–50
Djozan D, Farajzadeh MA, Sorouraddin SM, Baheri T (2011) Synthesis and application of high selective monolithic fibers based on molecularly imprinted polymer for SPME of trace methamphetamine. Chromatographia 73:975–983
Namera A, Yashiki M, Liu J, Okajima K, Hara K, Imamura T, Kojim T (2000) Simple and simultaneous analysis of fenfluramine, amphetamine and methamphetamine in whole blood by gas chromatography–mass spectrometry after headspace–solid phase microextraction and derivatization. Forensic Sci Int 109:215–223
Jochman MA, Kmiecik MP, Schmidt TC (2006) Solid-phase dynamic extraction for the enrichment of polar volatile organic compounds from water. J Chromatogr A 1115:208–216
Musshoff F, Madea B (2007) New trends in hair analysis and scientific demands on validation and technical notes. Forensic Sci Int 165:204–215
Eom IY, Niri VH, Pawliszyn J (2008) Development of a syringe pump assisted dynamic headspace sampling technique for needle trap device. J Chromatogr A 1196–1197:10–14
Eom IY, Tugulea AM, Pawliszyn J (2008) Development and application of needle trap devices. J Chromatogr A 1196–1197:3–9
Tamayo FG, Titirici MM, Esteban AM, Sellergren B (2005) Synthesis and evaluation of new propazine-imprinted polymer formats for use as stationary phases in liquid chromatography. Anal Chim Acta 542:38–46
Jiang X, Zhao C, Jiang N, Zhang H, Liu M (2008) Selective solid-phase extraction using molecular imprinted polymer for the analysis of diethylstilbestrol. Food Chem 108:1061–1067
Djozan D, Baheri T, Pournaghi Azar MH, Mahkam M (2007) Preparation of new fibers on the basis of codeine imprinted polymer. Mater Manuf Processes 22:758–763
Djozan D, Ebrahimi B (2008) Preparation of new solid phase micro extraction fiber on the basis of atrazine-molecular imprinted polymer: application for GC and GC/MS screening of triazine herbicides in water, rice and onion. Anal Chim Acta 616:152–159
Djozan D, Farajzadeh MA, Sorouraddin SM, Baheri T, Norouzi J (2012) Development of an inside needle extraction method based on molecularly imprinted polymer for solid-phase dynamic extraction and preconcentration of triazine herbicides followed by GC–FID determination. Chromatographia 75:139–148
Acknowledgments
The authors thank the Research Council of University of Tabriz for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 67 kb)
Rights and permissions
About this article
Cite this article
Djozan, D., Farajzadeh, M.A., Sorouraddin, S.M. et al. Determination of methamphetamine, amphetamine and ecstasy by inside-needle adsorption trap based on molecularly imprinted polymer followed by GC-FID determination. Microchim Acta 179, 209–217 (2012). https://doi.org/10.1007/s00604-012-0879-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0879-1