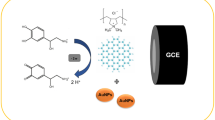
Abstract
A simple, cost-effective and efficient method was developed for the determination of glycine, gamma-aminobutyrate and taurine in rat brain using graphene as a sorbent for solid-phase extraction. The analytes were eluted from a graphene-packed solid-phase extraction cartridge with methanol, derivatized at their amino groups with the fluorescent label 4-carboxy-2,6-dimethylquinoline N-hydroxysuccinimide ester, and then separated and fluorescently detected by HPLC. The type and volume of eluent, sample pH, extraction time and sample volume were optimized with respect to sensitivity and precision. Under optimal conditions, linear response is obtained in the concentration range from 0.1 to 50 μg g−1, with correlation coefficients of >0.990. The limits of detection are 23.4 ng g−1 (gamma-aminobutyrate), 45.3 ng g−1 (glycine) and 67.5 ng g−1 (taurine) (S/N = 3). The results reveal the potential of graphene as a sorbent in the analysis of biological samples.

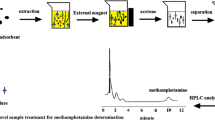
Representative chromatogram of NAAs derivatives obtained in rat brain samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurotransmitters are widely distributed in central neural system, brain tissues and body fluids of mammals. The role of neurotransmitter amino acids (NAAs) in the brain has been the focus of increasingly intense research over the past several years [1]. The varied level of NAAs has been reported associating with diseases such as Alzheimer’s disease [2–4]. Therefore, the quantitative detection of NAAs is important for the research of physiological and pathological mechanism of life. Some methods such as liquid chromatography–mass spectrometry (LC-MS) [5, 6] and capillary electrophoresis (CE) [7] have been developed for NAAs determination.
Sample pretreatment is a key step in the process of biological and chemical analysis. Especially in biological analysis, sample pretreatment is usually the most important and laborious step due to the complex matrices of biosamples and the low concentration of analytes. Solid-phase extraction (SPE) is a common technique that is widely used for pretreatment of analytes because of its advantages of high enrichment factor, rapid phase separation, low cost, low consumption of organic solvent and the ability of combination with different detection techniques in the form of on-line or off-line mode [8, 9]. In SPE procedure, the choice of appropriate adsorbent is a critical factor to obtain good recovery and high enrichment factor [10, 11].
Carbon nanomaterials [12–15] recently are widely used as adsorbents in SPE techniques, because they usually display large specific surface areas, high adsorption capacity and good chemical and thermal stability. Graphene is a novel and fascinating carbon nanomaterial, which posses a single layer of carbon atoms in a closely packed honeycomb two-dimensional lattice. Recently, it has attracted increasing attention from researchers because of its unique mechanical and electronic properties. Much research effort has been made to explore its fascinating applications in fabricating various electrical devices, such as batteries [16], field-effect transistors [17], ultrasensitive sensors [18], electromechanical resonators [19] and electrochemical biosensors [24, 25]. Actually, graphene is suitable to be used as SPE adsorbent. Some reports have proved that graphene can be used as adsorbent in SPE for different compounds enrichment [22–26].
The main purpose of this work is to develop a method for the sensitive determination of NAAs in biological samples. 2,6-Dimethyl-4-quinolinecarboxylic acid N-hydroxysuccinimide ester (DMQC-OSu) not only reacts readily with primary and secondary amines with good selectivity in aqueous solution, but also provides the advantages of few by-products and mild reaction conditions [27]. In this work, DMQC-OSu was used to label NAAs including γ-aminobutyric acid (GABA), taurine (Tau) and glycine (Gly). Graphene was selected as SPE adsorbent for the enrichment of NAAs derivatives. Several key parameters were investigated for good SPE efficiency. The method was demonstrated to be applicable for the analysis of NAAs in rat brain samples.
Experimental
Chemicals
DMQC-OSu was synthesized according to the reference [27]. Gly, GABA and Tau were purchased from Sigma (Saint Louis, MO, USA, http://www.sigmaaldrich.com). The stock standard solutions were prepared in double-distilled water. Working solutions were prepared daily by proper dilution of the stock solution with double-distilled water. Graphite powder (99.95%, 325 mesh), hydrazine solution (50 wt.%) and ammonia solution (28 wt.%) were obtained from from Aaladdin Reagent Co., Ltd. (Shanghai, China, http://www.aladdin-reagent.com). Single-walled carbon nanotubes (SWCNTs, >90%, outer diameter 1–2 nm, length ~20 μm) and multi-walled carbon nanotubes (MWCNTs) (>95%, outer diameter <8 nm, length ~50 μm) were obtained from Nanotech Part Co. (Shenzhen, China, http://www.nanotubes.com). Na2B4O7-H3BO3 buffer was obtained by mixing 0.05 M Na2B4O7 and 0.20 M H3BO3 solutions. Unless otherwise specified, all reagents were of analytical reagent grade and used without further purification. All solutions were prepared with double-distilled water and were stored in the refrigerator at 4 °C before used.
Instrumentation
An Agilent 1200 Series of LC system, which consisted of a quaternary pump, a vacuum degasser and a fluorescent detector were used (Agilent, Palo Alto, CA, USA¸ http://www.home.agilent.com). A reversed-phase Eclipse XDB-C18 column (150 mm × 4.6 mm i.d., 5 μm, Agilent, USA, http://www.home.agilent.com) was used. A manual sample injector with a 20 μL loop was used. Agilent ChemStation for LC system was employed to acquire and process chromatographic data. The mobile phase was a mixture of methanol-water (25/75, v/v) containing 10 mmol L−1 H3Cit-Na2HPO4 and delivered at a flow rate of 1.0 mL min−1. The detection wave length was set at λ ex/λ em = 326/412 nm.
Preparation of graphene
Graphene oxide (GO) was synthesized from graphite according to Hummers and Offeman method [28]. Graphene was prepared as described previously [29] by reduction of a GO dispersion with hydrazine. In short, GO dispersion (100 mL) was mixed with 70 μL of hydrazine solution (50 wt.% in water) and 0.7 mL of ammonia solution (28 wt.% in water). The mixture was stirred for 1 h at 95 °C. Finally, black graphene was obtained by filtration and dried in vacuum.
Preparation of stock solutions and calibration standards
Individual stock solution of each NAAs standard was prepared by accurate weighing from each compound (1 mg mL−1 in double-distilled water as the stock solution). The standards were spiked in the brain homogenates to prepare the calibrating solutions of Gly, Tau and GABA (0.1, 0.3, 0.6, 1, 3, 6, 10, 25, 50 μg g−1).
Derivatization procedure
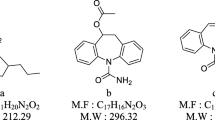
Three NAAs standards were spiked in the brain homogenates to prepare a mixed NAAs solution (the concentration of each NAAs for 1 mg g−1). 5-mL aliquot of the mixed NAAs solution was mixed with 2 mL of DMQC-OSu (2.5 mg mL−1) and 3 mL of H3BO3-Na2B4O7 buffer (pH 8.3) in test tubes. The mixture was then incubated at 40 °C for 50 min. The chemical structure of DMQC-OSu and its reaction with NAAs are shown in Fig. 1.
Solid-phase extraction procedure
Graphene packed mini-column was prepared by modifying a SPE column (polypropylene). The C18 packing was removed and 30 mg of graphene was packed in the column. The polypropylene upper frit was reset at the upper end of the column to prevent the loss of graphene during the operation process. In order to reduce the possible contamination, the SPE column was washed with 10 mL of 0.3 mol L−1 ascorbic acid, 10 mL of 1 mol L−1 nitric acid and 10 mL of water, respectively. Before loading samples, the SPE cartridge was first conditioned with 10 mL n-hexane and methanol (MeOH). Then it was washed with 10 mL water to equilibrate the phase. 5 mL sample solution was passed through the cartridge. Then the analytes retained on the cartridge were eluted with 0.3 mL of MeOH. The eluate was collected and 20-μL solution was injected in LC system for analysis.
Sample preparation
Three male rats were decapitated and the whole brain was immediately removed. The brain samples were immediately weighed, homogenized with 10 volumes of 0.4 mol L−1 perchloric acid containing 0.05% EDTA-2Na, and centrifuged at 2300 × g for 20 min at 4 °C. The supernatant was centrifuged at 10000 × g at 4 °C for 20 min. The solution was adjusted to pH 8.3 with 0.2 M NaOH solution. Then the derivatization and extraction procedure was performed as described above.
Method validation
The calibration curves were constructed by spiking tissue homogenates in adequate amounts with appropriate volumes of stock solutions of NAAs in order to give a final concentration in the range of 0.1–50 μg g−1. These calibration samples were then taken through the sample preparation procedure described above. The Calibration curves were prepared by plotting peak area as a function of analyte concentrations, and were used to determine the analyte concentrations in the samples and the detection and quantitation limits.
The recovery was determined at low, medium and high levels (0.1, 1, 50 μg g−1). The recovery was expressed as the mean area of analyte with NAAs standard solution added before the sample preparation divided by the mean area of the same NAAs standard solution added after the sample preparation. The precision in the conditions of intra-day repeatability (one analyst prepared six replicates of spiked samples at three different levels on a single day) and inter-day repeatability (different analysts prepared six replicates of spiked samples at three levels on three different days), expressed as relative standard deviations (RSD), was determined over 3 weeks.
The sensitivity was evaluated by the limit of detection (LOD) and the limit of quantitation (LOQ). LOD and LOQ were defined as concentrations where the signal/noise ratios were equal to 3 and 10, respectively.
Results and discussion
Morphology of graphene
The morphology of the obtained graphene was observed by SEM (Fig. 2). It exhibits the SEM image of graphene agglomerate, consisting of almost transparent carbon nanosheets with thin wrinkled structure that graphene owns intrinsically.
Optimization of derivatization procedure
In order to achieve the maximum derivatization yield, the derivatization conditions including the concentration of DMQC-OSu, pH value of buffer, the reaction temperature and time were optimized.
The effect of DMQC-OSu concentration on the derivatization yield was evaluated from 1 to 3 mg mL−1. The concentration of each NAAs was kept for 1 mg g−1. As shown in Fig. 3a, the peak areas of three derivatives reached the maximum when the concentration of DMQC-OSu was 2.5 mg mL−1. When the concentration of DMQC-OSu further increased, the peak areas of the analytes almost kept unchanged. Therefore, 2.5 mg mL−1 of DMQC-OSu was chosen for NAAs derivatization.
The pH values of H3BO3-Na2B4O7 buffers in the range of 7.0–10.0 were tested. The maximum peak area of NAAs derivatives was obtained in the range of pH 8.0–8.6. When pH value exceeded 8.6, peak areas of the derivatives decreased. Therefore, pH 8.3 was selected for the further experiments. The effect of the buffer volume was also investigated. It is found that the peak areas of the derivatives reach the maximum when 2.5–3.5 mL of H3BO3-Na2B4O7 buffer is used (Fig. 3b). The peak areas then decrease when the amount of buffer further increase. Thus, 3 mL of H3BO3-Na2B4O7 buffer (pH 8.3) was selected.
The effect of temperature was studied in range of 20–60 °C (Fig. 3c). When the temperature was below 40 °C, NAAs could not be labeled completely. When the temperature was at 40 °C, the yields of three derivatives reached the maximum, and then they decreased with the increase of temperature. Therefore, 40 °C was selected.
The effect of the reaction time was evaluated in the range of 10–60 min. Fig. 3d showed that the analytical signals increased quickly with the reaction time until 40 min for all the derivatives. No significant increase was obtained with additional reaction time. Therefore, 40 min were chosen in the following studies.
Under the optimized conditions stated above, the yield of NAAs derivatization was 82%.
Optimization of separation procedure
The separation of the NAAs derivatives was studied. The buffer in mobile phase significantly affected peak resolution. When the mobile phase was methanol–water (25:75, v/v), three derivatives of NAAS could not be baseline separated in 30-min LC analysis. When H3Cit-Na2HPO4 buffer was added in the mobile phase, the peaks of all the derivatives were obtained with well separation. The concentration of H3Cit-Na2HPO4 buffer was evaluated in the range of 1–20 mmol L−1. The results showed 10 mmol L−1 was the optimum concentration.
By fixing the concentration of H3Cit-Na2HPO4 buffer as 10 mmol L−1, the effect of the content of methanol on the separation of NAAs derivatives was examined by changing its content between 20 and 40% (v%). The results showed 25% was suitable. Reducing methanol content resulted in a longer retention time of all the analytes. When the content of methanol was more than 25%, peaks of Gly and Tau derivatives overlapped in chromatogram. So 25% of methanol was selected.
Optimization of SPE procedure
In order to obtain the maximal extraction efficiency, several factors such as eluent and elution volume, sample volume and pH value, and extraction time were optimized.
The effect of eluent solvents was studied. Four different organic solvents including MeOH, ACN, THF, and n-hexane were used to elute the analytes from graphene-packed cartridge. As shown in Fig. 4a, MeOH yields the higher recoveries (93.1–106.2%) than those of ACN (83.2–88.6%). THF also could not satisfactorily elute the analytes with the recoveries of 83.1–90.3%. n-Hexane had a poor eluting ability toward the derivatives with recoveries of 45.4–56.1%. Therefore, MeOH was selected for eluting analytes from the SPE cartridge. The effect of elution volume of MeOH was also evaluated. A series of experiments were designed to get the proper volume without loss of extraction efficiency. It was found that the highest recovery was obtained when 0.3 mL of MeOH was used (Fig. 4b). So 0.3-mL MeOH was selected.
The effect of sample pH was investigated. Sample pH plays an important role in the SPE procedure, because the pH value of the solution determined the present state of analytes in solution as ionic or molecular form, and thus determined the extraction efficiency of the target analytes. Herein, a range of sample pH values from 3 to 9 were evaluated. No significant effect was observed with sample pH change. It suggested that non-electrostatic interaction such as pi-pi interaction played an important role in the adsorption of NAAs derivatives to graphene. To facilitate the extraction process, no adjustment of sample pH was performed in the further experiments.
To obtain reliable analytical results and high enrichment efficiency, it was necessary to obtain the breakthrough volume in the SPE process. Seven volumes (0.3, 10, 30, 60, 80 and 100 mL) of samples were investigated. The results were presented in Fig. 4c. It was believed that the breakthrough volumes of NAAs derivatives were more than 80 mL at least. In practice, the sample volume was chosen according to the required sensitivity and the time acceptable for a whole analysis. Generally, further increasing of the sample volume was not desirable for routine analysis since the total time needed for one analysis would be longer. So a sample volume of 5 mL was selected for subsequent analysis with satisfactory sensitivity.
The alkyl chain length on the chemical structure of NAAs has influence on its physical and chemical properties, such as density and viscosity. GABA has the longer alkyl chain length than that of Gly and Tau. This may be the reason that the recoveries of GABA in Fig. 4 were more than 100%.
Extraction time is an important parameter because SPE is an equilibrium-based technique. Generally, extraction time is regarded as the interval time between injecting sample and before elution. In this work, different extraction time was tested. When the extraction time ranged from 1 to 7 min, the recoveries of the derivatives kept almost unchanged. So, 2 min was selected as the extraction time based on the sensitivity and analysis time.
Comparison study
For comparative studies, NAAs derivatives were extracted using graphene, C18 silica, graphitic carbon, SWCNTs and MWCNTs as SPE adsorbents, respectively. Following the same experimental procedure and optimized conditions as the graphene sorbents packed cartridges (the methods were not optimized for the other materials), the enrichment factor, recovery and breakthrough volume of each analyte were investigated. The results were listed in Table 1. It showed that the enrichment factors, recoveries and breakthrough volumes of NAAs derivatives extracted by graphene were obviously higher than other adsorbents. Although the recoveries and breakthrough volumes obtained by SWCNTs and MWCNTs were close to that by graphene, the elution of the analytes from the MWCNTs and SWCNTs-packed SPE cartridge consumed more time than from graphene-packed SPE cartridge. The reason may be that CNTs are composed of rolled-up graphite sheets and hexagonal arrays of carbon atoms. They adsorbed NAAs derivatives mainly by the coaction of delocalized pi bond interaction and physical adsorption. The delocalized pi bond interaction between the derivatives and CNTs was strong and their tube-like structure may hinder the objective molecules elution. The good performances of graphene-packed SPE cartridge may be due to the characteristic of graphene. Graphene has a large specific surface area (theoretical value 2630 m2 g−1) which suggests a high sorption capacity [30]. The special structure makes both sides of the planar sheets of graphene are available for molecules adsorption. Furthermore, the hexagonal arrays of carbon atoms in the graphene sheets may have a strong pi–pi interaction with the target molecules. Although being chemically reduced, the graphene sheets still have some hydrophilic groups such as hydroxyl and carboxyl groups, and these hydrophilic groups can enhance the retention and elution of polar compounds. These are not only favorable for the adsorption of target molecules, but also helpful for the elution of analytes from SPE cartridge.
Figures of merit
The method was further tested by applying the developed method to analysis of spiked animal tissues. Mixed standard solution of Gly, Tau and GABA was spiked to animal tissues at nine different concentrations ranging from 0.1 to 50 μg g−1. The results were summarized in Table 2. The matrix spike curves show good linearity (r > 0.990) within the tested range. LODs and LOQs were in the range of 23.4–67.5 and 78–225 ng g−1, respectively. Consequently, all these results gave evidence to demonstrate that the method was unbiased and could be applied to the testing of the samples for monitoring.
The recoveries in tissue homogenates were in the range of 94.2–112.1% for NAAs at three concentration levels (Table 3). Table 3 also summarized the intra- and inter-day precision for NAAs in tissue homogenates. The validation of the developed method in the three rat brain homogenates demonstrates that the method was accurate and precise. Intra-day (R.S.D.) of and inter-day precisions (R.S.D.) of the analytes determinations were below 6% for rat tissue homogenates at all concentration levels.
Under the optimized conditions, the recommended method was applied to detect NAAs in rat brain samples. Every sample was analyzed for three replicates. The results were showed in Table 4. The chromatograms obtained by graphene-based SPE-LC method were shown in Fig. 5. As listed in Table 4, the NAAs values reported in this work were in good agreement with the range of previously reported literature values.
Conclusions
The experimental work reported in the present article has been aimed at the sensitive determination of NAAs in rat brain samples based on SPE-LC method using graphene as adsorbent. The results obtained demonstrated its applicability for the determination of NAAs in rat brain matrices. Concerning the SPE procedure, it was demonstrated for the first time that graphene could be used as an effective SPE material for the extraction of NAAs derivatives. The interaction established between analytes and graphene simplified the isolation step due to the low retention of interferents, leading to a high selectivity and ruggedness of the extraction with clean extracts without chromatographic interferences. Moreover, the low amount of stationary phase of the cartridge was a great advantage as it notably reduced the cost of the analysis. This made the developed method a valid alternative for the determination of NAAs in biological samples.
References
Sandlin ZD, Shou MS, Shackman JG, Kennedy RT (2005) Microfluidic electrophoresis chip coupled to microdialysis for in vivo monitoring of amino acid neurotransmitters. Anal Chem 77:7702
Soto J, Ulibarri I, Jauregui JV, Ballesteros J, Meana JJ (1999) Dissociation between I2-imidazoline receptors and MAO-B activity in platelets of patients with Alzheimers type dementia. J Psychiatr Res 33:251
Burke WJ, Li SW, Schmitt CA, Xia P, Chung HD, Gillespie KN (1999) Accumulation of 3,4-dihydroxyphenylglycolaldehyde, the neurotoxic monoamine oxidase A metabolite of norepinephrine, in locus ceruleus cell bodies in Alzheimer’s disease: mechanism of neuron death. Brain Res 816:633
Bruhlmann C, Ooms F, Carrupt PA, Testa B, Catto M, Leonetti F, Altomare C, Carotti A (2001) Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase. J Med Chem 44:3195
Cai HL, Zhu RH, Li HD (2010) Determination of dansylated monoamine and amino acid neurotransmitters and their metabolites in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Biochem 396:103
Zhao XE, Suo YR (2008) Simultaneous determination of monoamine and amino acid neurotransmitters in rat endbrain tissues by pre-column derivatization with high-performance liquid chromatographic fluorescence detection and mass spectrometric identification. Talanta 76:690
Denoroy L, Parrot S, Renaud L, Renaud B, Zimmer L (2008) In-capillary derivatization and capillary electrophoresis separation of amino acid neurotransmitters from brain microdialysis samples. J Chromatogr A 1205:144
Pyrzynska K, Trojanowicz M (1999) Functionalized cellulose sorbents for preconcentration of trace metals in environmental analysis. Crit Rev Anal Chem 29: 313.
Cai YQ, Jiang GB, Liu JF, Zhou QX (2003) Multiwalled carbon nanotubes as a solid-phase extraction adsorbent for the determination of bisphenol A, 4-n-nonylphenol, and 4-tert-octylphenol. Anal Chem 75: 2517.
Poole CF (2003) New trends in solid-phase extraction. Trends in Anal Chem 22:362
Zang ZP, Hu Z, Li ZH, He Q, Chang XJ (2009) Synthesis, characterization and application of ethylenediamine-modified multiwalled carbon nanotubes for selective solid-phase extraction and preconcentration of metal ions. J Hazard Mater 172:958
Vallant RM, Szabo Z, Bachmann S, Bakry R, Najam-ul-Haq M, Rainer M, Heigl N, Petter C, Huck CW, Bonn GK (2007) Development and application of C60-fullerene bound silica for solid-phase extraction of biomolecules. Anal Chem 79:8144
Ravelo-Perez LM, Herrera-Herrera AV, Hernandez-Borges J, Rodriguez-Delgado MA (2010) Carbon nanotubes: solid-phase extraction. J Chromatogr A 1217:2618
Zhu S, Niu W, Li H, Han S, Xu G (2009) Single-walled carbon nanohorn as new solid-phase extraction adsorbent for determination of 4-nitrophenol in water sample. Talanta 79:1441
Jimenez-Soto JM, Cardenas S, Valcarcel M (2009) Evaluation of carbon nanocones/disks as sorbent material for solid-phase extraction. J Chromatogr A 1216:5626
Xu Y, Bai H, Lu GW, Li C, Shi GQ (2008) Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J Am Chem Soc 130:5856
Cassagneau T, Fendler JH (1998) High density rechargeable lithium-ion batteries self-assembled from graphite oxide nanoplatelets and polyelectrolytes. Adv Mater 10:877
Gilje S, Han S, Wang M, Wang KL, Kaner RB (2007) A chemical route to graphene for device applications. Nano Lett 7:3394
Schedin F, Geim AK, Morozov SV, Hill EW, Blake P, Katsnelson MI, Novosenlov KS (2007) Detection of individual gas molecules adsorbed on graphene. Nature Mater 6:652
Bunch JS, Van der Zande AM, Verbridge SS, Frank IW, Tanenbaum DM, Parpia JM, Craighead HG, McEuen PL (2007) Electromechanical resonators from graphene sheets. Science 315:490
Shan CS, Yang HF, Han DX, Zhang QX, Ivaska A, Niu L (2010) Electrochemical determination of NADH and ethanol based on ionic liquid-functionalized graphene. Biosen Bioelectron 25:1504
Liu Q, Shi JB, Zeng LX, Wang T, Cai YQ, Jiang GB (2011) Evaluation of graphene as an advantageous adsorbent for solid-phase extraction with chlorophenols as model analytes. J Chromatogr A 1218:197
Basri G, Yasun E, Shukoor MI, Zhu Z, You MX, Tan XH, Sanchez H, Powell DH, Dai HJ, Tan WH (2010) A dual platform for selective analyte enrichment and ionization in mass spectrometry using aptamer-conjugated graphene oxide. J Am Chem Soc 132:17408
Tang LAL, Wang JZ, Loh KP (2010) Graphene-based SELDI probe with ultrahigh extraction and sensitivity for DNA oligomer. J Am Chem Soc 132:10976
Dong XL, Cheng JS, Li JH, Wang YS (2010) Graphene as a novel matrix for the analysis of small molecules by MALDI-TOF MS. Anal Chem 82:6208
Luo YB, Shi ZG, Gao Q, Feng YQ (2011) Magnetic retrieval of graphene: extraction of sulfonamide antibiotics from environmental water samples. J Chromatogr A 1218:1353
Cao LW, Wang H, Liu X, Zhang HS (2003) Spectrofluorimetric determination of aliphatic amines using a new fluorigenic reagent: 2,6-dimethylquinoline-4-(N-succinimidyl)-formate. Talanta 59:973
Hummers W, Offeman R (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Huang KJ, Niu DJ, Sun JY, Han CH, Wu Z, Li YL, Xiong XQ (2011) Novel electrochemical sensor based on functionalized graphene for simultaneous determination of adenine and guanine in DNA. Colloids Surf B: Biointerfaces 82:543
Stoller MD, Park SJ, Zhu YW, An JH, Ruoff RS (2008) Graphene-based ultracapacitors. Nano Lett 8:3498
Acosta GB (1998) Administration of cholecystokinin sulphated octapeptide (CCK-8S) induces changes on rat amino acid tissue levels and on a behavioral test for anxiety. Gen Pharmacol 31:637
Aburawi SM, Elhwuegi AS, Ahmed SS, Saad SF, Attia AS (2000) Behavioral effects of acute and chronic triazolam treatments in albino rats. Life Sci 73:3095
Harvey BH, Jonker LP, Brand L, Heenop M, Stein DJ (2002) NMDA receptor involvement in imipramine withdrawal-associated effects on swim stress, GABA levels and NMDA receptor binding in rat hippocampus. Life Sci 71:43
Clarke G, O’Mahony S, Malone G, Dinan TG (2007) An isocratic high performance liquid chromatography method for the determination of GABA and glutamate in discrete regions of the rodent brain. J Neurosci Methods 160:223
Li H, Wang H, Chen JH, Wang LH, Zhang HS, Fan Y (2003) Determination of amino acid neurotransmitters in cerebral cortex of rats administered with baicalin prior to cerebral ischemia by capillary electrophoresis–laser-induced fluorescence detection. J Chromatogr B 788:93
O’Donnell T, Rotzinger S, Ulrich M, Hanstock CC, Nakashima TT (2003) Effects of chronic lithium and sodium valproate on concentrations of brain amino acids. Eur Neuropsychopharmacol 13:220
Li Q, Jin CL, Xu LS, Zu-Ge ZB, Yang LX, Liu LY, Chen Z (2005) Histidine enhances carbamazepine action against seizures and improves spatial memory deficits induced by chronic transauricular kindling in rats. Acta Pharmacol Sin 11:1297
Acknowledgments
This work was supported by the National Natural Science Foundation of China (20805040), Program for Science & Technology Innovation Talents in Universities of Henan Province (2010HASTIT025), and Excellent Youth Foundation of He’nan Scientific Committee (104100510020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, KJ., Yu, S., Li, J. et al. Extraction of neurotransmitters from rat brain using graphene as a solid-phase sorbent, and their fluorescent detection by HPLC. Microchim Acta 176, 327–335 (2012). https://doi.org/10.1007/s00604-011-0719-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0719-8