Abstract
In this study, a novel method was reported for the extraction of methamphetamine from urine samples using magnetic solid phase extraction (MSPE) technique. Magnetic nano graphene oxide (MNGO) was synthesized and applied as a new adsorbent for the extraction of methamphetamine from urine samples. The successful synthesis of MNGO was confirmed by Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), powder X-ray diffraction (XRD) and vibrating sample magnetometer (VSM). The main factors (the amounts of sample volume, amount of adsorbent, type and amount of extraction organic solvent, time of extraction and desorption, pH, the ionic strength of extraction medium, and agitation rate) influencing the extraction efficiency were investigated and optimized. Under optimized extraction conditions, a good linearity was observed in the range of 100–1500 ng mL−1 with the correlation coefficient of 0.9973 (r 2). Limit of detection (LOD) and Limit of quantification (LOQ) were 30 and 100 ng mL−1, respectively. The inter-day and intra-day precisions were within 8.18 and 9.21 %, respectively. The inter-day and intra-day biases were −0.39 and −0.44 %, respectively. The recovery of spiked samples was 91.76 %. The method was applied for determination of methamphetamine in abused drug urine samples with the recovery of 93.47 %. It was concluded that the proposed method can be applied in forensic clinics for the determination of methamphetamine in addicted subjects.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methamphetamine is a central nervous system stimulant used as a recreational drug for its euphoric properties. It produces severe adverse effects that include withdrawal, irritability, physiological disorders, anorexia, insomnia, and a high risk of addiction [1]. Methamphetamine abuse has continued to increase worldwide and is the cause of significant social problems. This drug is abused as capsules or tablets; moreover, it might be injected or sniffed by abusers. Determination of methamphetamine is a major concern in clinical and forensic laboratories. Urine is a proper medium for the determination of methamphetamine because 43 % of administered methamphetamine is excreted in urine without any change [2]. Urine is commonly used in clinical and toxicological laboratories because it is easily available and is useful for diagnosis of pharmaceuticals used for pain management and in addiction [3]. Sample extraction is necessary because of the complexity of the urine matrix which causes low sensitivity and selectivity of determination. The low dosages of analyte in a urine sample require a preconcentration step, making a sample treatment stage indispensible before analysis. Liquid–liquid extraction is a common drug extraction method, but its application has been limited because it produces hazardous waste, is time-consuming, and laborious. Solid phase extraction (SPE) has a high recovery rate, greater selectivity and sensitivity, and produces less toxic waste. Magnetic solid phase extraction (MSPE) is a novel sample preparation method [4, 5] based on SPE in which magnetic nanoparticles (MNPs) are applied. MNPs are composite materials with magnetic and nano properties that affect extraction. Nanoparticles have a high surface-area-to-volume ratio that increases extraction over other adsorbents. The MNPs can be separated using an external magnetic field, making it easy to separate the analytes adhering to superparamagnetic particles in aqueous solution or in biological matrices without filtration or centrifuging. Graphene (G) is a carbon material with unique mechanical, thermal, and electrical properties with a large surface area which increases graphene application in modified electrochemical sensors [6–9]. The presence of a large delocalized π-electron system causes a strong π stacking interaction with the benzene ring [10]. The combination of magnetism and the strong π stacking interaction of G results in preconcentration of the target analyte and convenient separation. Magnetic nano graphene composites (MNG) have been used as a MSPE adsorbent [11–13]. Moreover, MNG is applied as an electrochemical sensor for determination of various drugs [14]. Graphene oxide (GO) is easily dispersed in aqueous media because of the presence of hydroxyl, carboxyl, and epoxy polar groups on the surface of the lamellar structure [15, 16]. The large surface area of nano-GO increases its adsorption capacity and recommends GO as a novel adsorbent in SPE. The introduction of these magnetic properties combined with the adsorption capacity of GO allows comfortable separation of MNPs. Magnetic nano graphene oxide (MNGO) was applied for MSPE for preconcentration of a water sample [17]. Moreover, electrochemical application of graphene oxide in determination of drugs as a modified sensor is interesting [18]. The chemical structure of methamphetamine in Fig. 1a makes it a good candidate for MSPE with MNG because of the strong π-π interaction with the benzene rings of methamphetamine and G. MNGO also adsorbs methamphetamine using potential of the negative surface charge for interaction with amine groups in addition to benzene ring π-π interaction. No study thus far has reported on the determination of methamphetamine in biological matrices using MSPE in which MNG and MNGO are used as adsorbents. Comparison of the performance of MSPE with MNPs, MNG, and MNGO is an interesting topic for analytical chemistry. Identification and quantification of methamphetamine can be done using techniques such as immunoassay, gas chromatography, and electrophoresis [19–25]. The present study established a new system for analysis of methamphetamine in urine using the HPLC–UV method that is suitable for analysis of non-volatile or semi-volatile compounds [26, 27]. For the sake of brevity, an effective sample treatment was carried out to enrich and isolate the methamphetamine from complex urine matrix instead of derivatization of the analyte.
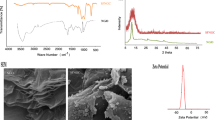
Chemical structure of methamphetamine (a), Fourier transform infrared spectroscopy spectrums (b), X-ray diffraction patterns (c), zeta potential values (d), magnetization hysteresis loops (e) and scanning electron microscope images of graphene oxide (GO), magnetic nano graphene oxide (MNGO), graphene (G) and magnetic nano graphene (MNG) (f)
In this study, nano-GO was synthesized and functionalized using MNPs. After full characterization and confirmation of MNGO synthesis, it was applied as an adsorbent for the extraction of methamphetamine in urine for determination and quantification by HPLC–UV.
Experimental
Reagents and materials
Expanded graphite powder, KH2PO4, H2SO4 (98 %), H2O2 (30 %), KMNO4, FeCl2.4H2O, FeCl3.6H2O, KOH, NaOH, NH3 (25 %), and HCl were obtained from Merck (Germany). Methamphetamine hydrochloride and amphetamine hydrochloride stock solutions (1000 µg mL−1) in methanol were obtained from Sigma-Aldrich (USA). Pseudoephedrine hydrochloride and methadone were kindly donated from Zahravi Pharmaceutical Co. (Tabriz, Iran). Graphene powder was purchased from Iranian Nano-Fanavaran (Iran). HPLC grade acetonitrile, acetone, methanol, and dichloromethane were supplied by Duksan (South Korea). Double distilled water was obtained from Shahid Ghazi Pharmaceutical Co. (Tabriz, Iran).
Characterization and analytical conditions
HPLC–UV analysis was performed on a Knauer system equipped with a UV–visible detector (K-2600, Knauer, Germany) and pump (K-1001, Knauer, Germany) and a Knauer injector consisting of a 20 µL loop. Separation was conducted on an analytical C18 column (10 µm particle diameter, 4.6 mm id × 25 cm) (Knauer, Germany) at room temperature. The mobile phase of acetonitrile/phosphate buffer solution (10 mM) at a ratio of 15:85 (v/v) and final pH adjustment of 3.5 was used in the isocratic mode at a flow rate of 1.5 mL min−1.
Fourier transform infrared (FT-IR) spectrometry (Tensor 27; Bruker; Germany) was applied at 400–4000 cm−1 to characterize the synthesized MNGO. Probe sonication (U 200H; Heielscher; Germany) was used for dispersion of the GO. Scanning electron microscopy (SEM; Mira 3 FEG-SEM; Tescan; Czech Republic) was used for the morphologic survey. Magnetization curves were recorded using (VSM—4 inch, Daghigh Meghnatis Kashan Co., Iran) at room temperature. Zeta potential was measured using a Zetasizer (Nanotrac Wave; Microtrac; Germany). Powder X-ray diffraction was carried out using a D5000 (Siemens; Germany) with a Cu tube anode.
GO was prepared according to the improved Hummers’ method for eco-friendly synthesis (green synthesis) [28]. NaNO3 is eliminated; thus, NO2 and N2O4 toxic gases are not produced. Briefly, 0.5 g expanded graphite powder was weighed and 12 mL concentrated H2SO4 was added and stirred in an ice bath. Under vigorous agitation 1.5 g KMnO4 was added gradually to the solution, the system was transferred to an oil bath maintained at 35 °C. After 30 min of stirring, the color of the solution changed to light brown, after which 15 mL·H2O was added. The temperature was increased to 98 °C and the mixture was stirred for 30 min.
The reaction was terminated by the addition of 1 mL·H2O2 (30 %) as determined by a change in color of the solution to yellowish brown. The mixture was filtered and washed repeatedly with HCl (5 %) and double distilled water to remove metal ions. Water was added to the final product, which was then well vortexed to produce a homogenous suspension. The suspension was probe sonicated for 30 min with on-off cycles of 5 min at 100 % power (200 W), which resulted in the nano-GO.
Synthesis of G and GO MNPs
The synthesis of MNG was carried out by in situ chemical co-precipitation of Fe+2 and Fe+3 in an alkaline solution in the presence of G suspension [29]. Briefly, 30 mL of G suspension (3 mg mL−1) was sonicated for 15 min, then 10 mL solution of Fe+3 (100 mg) and Fe+2 (45 mg) was purged with nitrogen for 30 min. This solution was added to the G dispersion dropwise under a nitrogen atmosphere at room temperature and stirring. Ammonia solution was added to the system dropwise until the pH of the solution reached 10–11 and then the temperature was increased to 65 °C and stirred for 2 h. The solution was cooled at room temperature and the resulted black solids were separated by magnet and washed with double distilled water repeatedly then dried in 70 °C for 6 h. This process was repeated for 30 mL of GO suspension (3 mg mL−1) as well.
Urine sample
Drug free urine samples were collected from a healthy volunteer. Positive urine samples were obtained from Mahan drug abuse therapy center (Tabriz, Iran). The pH of urine samples were adjusted to 11 and centrifuged (Universal 320, England) for 15 min until white lipidic solid was sedimented in the bottom of the tube [30], and then supernatants were transferred into a clean tube and spiked with methamphetamine. All samples were stored in 4 °C and directly used for MSPE.
MSPE procedure
MSPE procedure was carried out as follows: first, 40 mg of MNGO was weighted and dispersed in 10 mL of urine and vortexed for 25 min to make a chance for drug–MNGO interaction. Analyte-loaded adsorbent were isolated from solution by a strong magnet. Subsequently the isolated MNGO were sonicated in 600 µL of proper organic solvent for 15 min to desorb the analyte, then magnet was positioned outside of the glass to isolate MNGO from solution. Finally, 20 µL of sample was injected into HPLC system for analysis. This procedure was repeated for MNG and MNP identically.
Magnetic nano graphene oxide is selected as MSPE adsorbent for methamphetamine extraction in urine media probably due to the presence of a large delocalized π-electron system that causes a strong π stacking interaction with the benzene ring of the methamphetamine chemical structure, moreover, negative surface charge for interaction with amine groups of the methamphetamine in addition to benzene ring π-π interaction might help to drug adsorption on the MSPE surface. Methamphetamine pKa value is 10.1 and mainly exists in undissociated molecular form at higher pH values, which contributes to the extraction in optimized pH value due to π-π interaction of benzene ring of the methamphetamine with those of MNGO and leads to maximum extraction efficiency. The preconcentration factor (PF) is calculated as follows: The AUC of the 0.1 µg mL−1 methamphetamine spiked urine and extracted during our developed MSPE procedure is calculated and this amount is divided to the AUC of the 0.1 µg mL−1 methamphetamine standard solution without any extraction process. PF is calculated as 168 for the proposed method.
Results and discussion
Characterization of nano-GO, MNGO, and MNG
Figure 1b shows the FT-IR spectra of GO, MNGO, G, and MNG, respectively. Peaks at 3447 and 1231 cm−1 that denote stretching and bending bands of the O–H group in the GO spectra. The peak at 1704 cm−1 denotes stretching of the C=O band of the carboxyl group. The peak at 1634 cm−1 denotes the C=C aromatic band or possibly the skeletal vibration of the unoxidized graphitic domains. The peak at 1073 cm−1 denotes deformation of the C–O band. The MNGO spectrum differed from that for GO. The peak at 1704 cm−1 weakened and a peak appeared at about 619 cm−1 that denotes formation of Fe–O and the presence of Fe3O4 on the GO. An absorption peak at about 1560 cm−1 related to the skeletal vibrations of the G sheets. The weak peaks at about 1255 and 3411 cm−1 denote the C–O and O–H bands, respectively. The FT-IR spectrum of MNG shows the presence of the characteristic Fe–O stretching peak at about 623 cm−1 proves that Fe3O4 is anchored to the G sheet. Skeletal vibration peaks for MNG appear at about 1515 and 1615 cm−1. These results are in agreement with those of previous works confirming synthesis of GO, MNGO, and MNG [31–33].
The XRD patterns Fig. 1c were assessed for the crystalline structure of GO, MNGO, G, MNG, and MNPs. The XRD pattern of GO shows a main peak at 2θ = 11 indicating and increase in (002) inter-planar spacing caused by the oxide treatment and the presence of a peak at 2θ = 26 from residual unoxidized graphite [7, 9, 14, 18, 34]. The broad peak at 2θ = 25.8 corresponds to the (002) reflection of G. Diffraction lines at 2θ = 30.25, 35.58, 43.21, 54.39, 57.9, and 62.92 are related to (220), (311), (400), (422), (511), and (440) reflections and confirm the pure cubic spinel crystalline structure of the MNPs. The successful synthesis of MNGO and MNG was proven by the emergence of a main peak for MNP at 2θ = 35.58.
The zeta potential of the 1 mg mL−1 GO and MNGO dispersions were −36.6 and −33.7 mV, respectively (Fig. 1d). The presence of a large delocalized π-electron system on the graphene oxide layers leads to negative potentials of the GO and MNGO suspensions which causes repulsion between GO layers and increases its well dispersibility in the medium. This property increases its drug adsorption ability in the medium. The results are sufficiently negative to confirm the colloidal stability of dispersion. It has been reported that a zeta potential of ±30 mV greatly improves the long-term stability of dispersed NPs [35].
VSM characterization of MNGO, MNG, and MNPs of Fe3O4 was carried out at room temperature Fig. 1e. S-like magnetization hysteresis loops appeared as proof of superparamagnetization of the nanocomposites. The values for saturation magnetization were M s = 51 and 24.32 emu g−1 for MNGO and MNG, respectively. These confirm that both nanosorbents easily dispersed in solution and that analyte adsorption can be readily isolated from the solution using an external magnetic field. The nanocomposites easily redispersed in solution when the magnet field was removed.
Figure 1f shows SEM images of GO. Plate-like forms that are quite smooth and without amorphous or other crystallized phase particles can be seen. The average size of the GO was estimated from the SEM images to be about 25 nm. SEM of the MNGO was also carried out. The Fe3O4 nanoparticles added to the GO solution appear as bright dots on the surface of the MNGO. The estimated size of the MNGO was about 35 nm.
Optimization of MSPE extraction conditions
The extraction and desorption parameters of the MSPE adsorbent, pH of sample, sample volume, volume of organic solvent used for drug extraction, organic solvent type, times of extraction and desorption, ionic strength, and agitation rate were optimized using a systematic, one-factor-at-a-time experimental design.
Comparison of extraction efficiency of MNG, MNGO, MNPs adsorbents, and adsorbent content
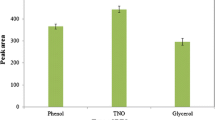
Figure 2a compares the extraction efficiency and absorbance of the MNG, MNGO, and MNPs. It is clear that MNGO has high extraction efficiency in comparison with MNG. This can be attributed to the higher surface area of MNGO and the presence of polar groups on the surface of the MNGO sheets that separate them from each other. The unilamellar structure of MNGO allows high adsorption of methamphetamine onto its surface. This interaction is more intense in MNGO than in the π-π interaction which dominates the MNG surface. Comparison of drug extraction efficiencies from administration of MNG and MNPs indicates that the adsorption by MNG was about 2.5-fold that of the MNPs because of the π-π interaction. The drug adsorption value for MNPs can be attributed to its large surface area. These preliminary studies were performed in aqueous solution and resulted in the selection of MNGO as the best candidate for MSPE in vivo. The MNGO content was optimized at 10–60 mg to optimize extraction efficiency of the analyte in the urine sample. Figure 2b shows that the maximum peak was achieved for 40 mg of MNGO added to the spiked urine (0.1 µg mL−1). Increasing the solid phase to 40 mg provided an adequate surface for drug adsorption. At higher solid phases, the low extraction efficiency obtained was probably the result of MNGO aggregation, which decreased the effective adsorption surface area. The remaining experiments were carried out at 40 mg of MNGO.
Comparison of extraction efficiency with magnetic nano graphene oxide (MNGO), magnetic nano graphene (MNG) and magnetic nanoparticles (MNPs) adsorbents, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; volume of aqueous solution, 10 mL; extraction time, 25 min; desorption time, 15 min; no salt addition, pH, not adjusted; desorption solvent, acetone; amount of desorption solvent, 600 µL; amount of adsorbent, 40 mg; stirring speed, 1300 rpm (a). Effect of various amount of magnetic nano graphene oxide (mg) as an adsorbents, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; volume of urine sample, 10 mL; extraction time, 25 min; desorption time, 15 min; no salt addition, pH, not adjusted; desorption solvent, acetone; amount of desorption solvent, 600 µL; stirring speed, 1300 rpm (b)
Effect of organic solvent type and volume on drug extraction
After extraction, the proper organic solvent is required for desorption of the analyte. Four organic solvents (acetone (AC), methanol (MET), acetonitrile (ACN), and dichloromethane (DCM)) were tested Fig. 3a. The presence of polar groups on the surface of the MNGO provided suitable dispersion of the MNGO in the water miscible solvents of acetonitrile, methanol, and acetone. As expected, dispersion in the non-polar solvent of dichloromethane was poor, provided the lowest surface area of MNGO available for drug adsorption, and resulted in minimum extraction efficiency. Acetone had the best drug extraction efficiency of the polar solvents. High dispersion of MNGO in acetone provided a well-matched medium for polarity. Polar mediums such as methanol and acetonitrile and non-polar mediums such as dichloromethane were not suitable for the dispersion of MNGO because of the presence of a non-polar backbone of GO and polar groups on the GO surface. Acetone provided the most well-balanced medium for dispersion of MNGO and was selected as the optimal solvent for the remaining experiments. Acetone contents of 300–800 µL were examined for drug extraction efficiency. It was found that increasing the acetone content increased extraction efficiency because of the high dispersion of MNGO in acetone. Higher volumes of acetone diluted the drug and decreased extraction efficiency Fig. 3b. Indicates that a volume of 600 µL resulted in the highest peak.
The effect of various extraction solvent, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; volume of urine sample, 10 mL; extraction time, 25 min; desorption time, 15 min; amount of desorption solvent, 600 µL; no salt addition, pH, not adjusted; amount of adsorbent, 40 mg; stirring speed, 1300 rpm (a). Effect of desorption solvent volumes, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; volume of urine sample, 10 mL; extraction time, 25 min; desorption time, 15 min; desorption solvent, acetone; no salt addition, pH, not adjusted; amount of adsorbent, 40 mg; stirring speed, 1300 rpm (b)
Effect of sample volume, sample pH and ionic strength
The influence of sample volume on extraction efficiency was investigated using different volumes (1–12 mL) of urine. The 10 mL volume exhibited maximum extraction efficiency Fig. 4a. Increasing the sample volume facilitated dispersion of the MNGO and increased possible drug–MNGO interaction. The unilamellar structure of MNGO Fig. 1f promoted aggregation of particles at low sample volumes. Higher sample volumes allowed the nanoparticles to better disperse and created a larger surface area available for drug interaction. At 12 mL, the volume of the urine sample did not further nanoparticles separation and decreased the tendency of the drug to interact with nanoparticles. The pH of the sample should be adjusted to ensure that the analyte is electrically neutral, can efficiently be adsorbed, and that desorption is unaffected by charges on the surface of the adsorbent. The pH of the spiked urine samples were adjusted to between 4 and 13 using 0.1 M·HCl and NaOH. Low extraction efficiency was observed at the lower pH values (pH = 4) and can be attributed to the non-ionized characteristics of the carboxylic acid groups of MNGO which increase aggregation and lower the surface available for drug extraction. The carboxylic acid groups were ionized at higher pH values (pH = 6), which helped unilamellar dispersion of the MNGO. The surface of the adsorbent should increase dramatically for proper drug extraction. In addition to good dispersibility of the adsorbent at higher pH values, ionic interaction of the negatively charged adsorbent and positively charged drug was also responsible for increasing drug extraction efficiency at pH values of up to 10. The drug lost its positive charge above pH = 12. Interestingly, the highest drug extraction efficiency was achieved at this pH. Methamphetamine is an alkaline compound with a pKa value of 10.1 and mainly exists in undissociated molecular form at higher pH values, which contributes to extraction. The strong drug–MNGO π-π interaction could be responsible for the results. In addition to polar groups, MNGO contains a large sheet of non-polar benzene rings. When methamphetamine is undissociated, its benzene ring approaches those of the MNGO and contributes to the strong π-π interaction. Maximum extraction efficiency was achieved when the pH was adjusted to 12 Fig. 4b. At higher alkaline pH values (pH = 13), the negative charge of the drug surface was repulsed by the high negative charge of the MNGO and extraction efficiency decreased. Salting out caused by ionic strength can affect extraction efficiency. In urine, the optimum pH value for maximum extraction was found to be 12. At this pH value, the effect of ionic strength 0–10 % (w/w) NaCl was investigated. No obvious change in the amount of drug extraction was found because the drug is in undissociated form at pH = 12; thus, the increase in ionic strength did not affect the extraction efficiency.
Effect of various volumes of urine, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; extraction time, 25 min; desorption time, 15 min; desorption solvent, acetone; amount of desorption solvent, 600 µL, no salt addition, pH, not adjusted; amount of adsorbent, 40 mg; stirring speed, 1300 rpm (a). Effect of different pH of urine, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; extraction time, 25 min; desorption time, 15 min; volume of urine sample, 10 mL; desorption solvent, acetone; amount of desorption solvent, 600 µL, no salt addition, amount of adsorbent, 40 mg; stirring speed, 1300 rpm (b)
Effect of extraction and desorption time
MSPE is an equilibrium-based technique. Increasing the extraction time increased the extraction efficiency up to extraction equilibrium. Extraction times of 10–30 min were applied to the spiked samples. The results showed that 25 min resulted in maximum extraction efficiency; thus, the remaining tests were carried out at a 25 min extraction time Fig. 5a. Desorption time was investigated at 1–15 min of sonication Fig. 5b. The increase in sonication time for desorption increased the amount of drug desorbed up to a maximum of 10 min. Continuing sonication up to 15 min had a negative effect on drug extraction, probably because of the aggregation of MNGO; thus, 10 min was selected for the remaining testing.
Effect of different time of drug extraction, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; desorption time, 15 min; volume of urine sample, 10 mL; desorption solvent, acetone; amount of desorption solvent, 600 µL, no salt addition, pH, 10; amount of adsorbent, 40 mg; stirring speed, 1300 rpm (a). Effect of various time of desorption, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; extraction time, 25 min; volume of urine sample, 10 mL; desorption solvent, acetone; amount of desorption solvent, 600 µL, no salt addition, pH, 10; amount of adsorbent, 40 mg; stirring speed, 1300 rpm (b)
Effect of stirring speed
The speed at which the sample is stirred can affect the equilibrium achieved between the analyte in the sample and MSPE adsorbent. The extraction efficiency was investigated at stirring speeds ranging from 900 to 1500 rpm. The results showed that the extraction efficiency reached a maximum at 1300 rpm and decreased at higher stirring speeds, probably because of the aggregation of MNGO Fig. 6. The samples of subsequent experiments were stirred at 1300 rpm.
Effect of different stirring speed of urine sample, extraction conditions: concentration of spiked methamphetamine, 0.1 µg mL−1; extraction time, 25 min; desorption time, 15 min, volume of urine sample, 10 mL; desorption solvent, acetone; amount of desorption solvent, 600 µL, no salt addition, pH, 10; amount of adsorbent, 40 mg
Survey of adsorbent reusability
MNGO adsorbent can disperse in urine medium; in the presence of a magnetic field the adsorbent was easily separated. After the using of MNGO under optimized extraction conditions for urine samples spiked with 0.1 µg mL−1 methamphetamine, the adsorbent was washed with acetone extraction solvent three times, sonicated for 10 min, and wash again for three times using double-distilled water. The adsorbent was applied for extraction 10 times to blank urine samples. The results of chromatograms showed no peak for the presence of methamphetamine.
Method validation
The calibration curve was constructed by plotting the mean peak of five concentrations using three measurements each. Blank urine spiked with methamphetamine at concentrations from 0.1 to 1.5 µg mL−1 was analyzed using the MSPE. The figures of merit are shown in Table 1.
Table 2 compares the proposed method and existing methods for methamphetamine determination. The good capabilities of the MNGO in the complex matrix of urine showed a low detection limit in comparison with existing methods. The reported LOD in the proposed method is slightly higher than reported LOD in [2], this might be due to the application of electric potential beside the presence of agitation which increases the analyte migration and leads to higher extraction efficiency. The commercial availability of nano-GO and MNPs of Fe3O4 and the simple chemical reaction method for MNGO production encourages the application of MNGO for extraction of methamphetamine. Analytical recovery, accuracy, and precision testing were performed at three concentrations covering the calibration range (Table 3).
The results were satisfactory considering the complexity of the biological matrix.
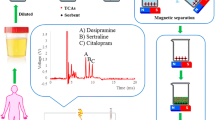
Application of MSPE to real samples and method selectivity
The applicability of the proposed method was tested using a urine sample from individuals addicted to methamphetamine. Figure 7a, b show the chromatograms of blank urine and of the urine samples of addicted persons. The mean concentration of methamphetamine in the real samples was 1.43 µg mL−1. The lowest methamphetamine concentration in urine was about 0.5 µg mL−1 [36]. The level in the evaluated individuals was found to be more than 1 µg mL−1. The LOQ of the proposed method was 100 ng mL−1, which indicates that it is sufficiently accurate for determination of methamphetamine in urine.
As we know methamphetamine has two primary metabolites, namely amphetamine and 4–hydroxymethamphetamine. Unfortunately, official governmental institutes had not 4–hydroxymethamphetamine meantime to be used in our project and its preparation from regular import–export chemical companies is very difficult and needs many paper works. Therefore, we could not carry out the selectivity test in the presence of 4-hydroxymethamphetamine. Therefore, the effect of interference on the proposed method for identification and quantification of methamphetamine was investigated by adding 0.1 µg mL−1 of different drugs that are commonly abused to the urine Fig. 7c. The ability of MNGO adsorbent for extraction of each abused drug separately and without overlapping confirms the selectivity of the MNGO adsorbent in methamphetamine extraction. Table 4 lists the rates of recovery.
Conclusion
MSPE is a new alternative to routinely used LLE and SPE sample preparation methods for the detection of methamphetamine in biological samples. MNGO was applied as a novel and sensitive adsorbent for the extraction of methamphetamine from urine. This method was successfully applied for the determination of the presence of methamphetamine in urine samples of drug abusers. The high extraction efficiency achieved resulting in detection at minimum levels of the drug.
References
L.-W. Chung, G.-J. Liu, Z.-G. Li, Y.-Z. Chang, M.-R. Lee, J. Chromatogr. B 874, 115 (2008)
H. Bagheri, A.F. Zavareh, M.H. Koruni, J. Sep. Sci. 39, 1182 (2016)
H.A. Heit, D.L. Gourlay, J. Pain Symptom Manag. 27, 260 (2004)
K. Aguilar-Arteaga, J. Rodriguez, E. Barrado, Anal. Chim. Acta 674, 157 (2010)
L. Chen, T. Wang, J. Tong, TrAC-TREND. Anal. Chem. 30, 1095 (2011)
A. Afkhami, H. Khoshsafar, H. Bagheri, T. Madrakian, Sens. Actuator B-Chem. 203, 909 (2014)
A. Afkhami, H. Khoshsafar, H. Bagheri, T. Madrakian, Anal. Chim. Acta 831, 50 (2014)
A. Afkhami, A. Shirzadmehr, T. Madrakian, H. Bagheri, Talanta 131, 548 (2015)
H. Bagheri, A. Afkhami, H. Khoshsafar, M. Rezaei, S.J. Sabounchei, M. Sarlakifar, Anal. Chim. Acta 870, 56 (2015)
J. Chen, J. Zou, J. Zeng, X. Song, J. Ji, Y. Wang, J. Ha, X. Chen, Anal. Chim. Acta 678, 44 (2010)
W. Wang, Y. Li, Q. Wu, C. Wang, X. Zang, Z. Wang, Anal. Methods 4, 766 (2012)
W. Lu, Z. Xiao-Huan, W. Chun, W. Zhi, Chinese. J. Anal. Chem. 42, 136 (2014)
J. Yang, J.Q. Qiao, S.H. Cui, J.Y. Li, J.J. Zhu, H.X. Yin, C.Y. Zhan, H.Z. Lian, J. Sep. Sci. 38, 1969 (2015)
H. Bagheri, A. Afkhami, P. Hashemi, M. Ghanei, RSC Adv. 5, 21659 (2015)
H. He, J. Klinowski, M. Forster, A. Lerf, Chem. Phys. Lett. 287, 53 (1998)
A. Lerf, A. Buchsteiner, J. Pieper, S. Schöttl, I. Dekany, T. Szabo, H. Boehm, J. Phy. Chem. Solids 67, 1106 (2006)
Q. Han, Z. Wang, J. Xia, S. Chen, X. Zhang, M. Ding, Talanta 101, 388 (2012)
H. Bagheri, S.M. Arab, H. Khoshsafar, A. Afkhami, New J. Chem. 39, 3875 (2015)
Z. Huang, S. Zhang, J. Chromatogr. B 792, 241 (2003)
N.A. Santagati, G. Ferrara, A. Marrazzo, G. Ronsisvalle, J. Pharm. Biomed. Anal. 30, 247 (2002)
N. Alizadeh, A. Mohammadi, M. Tabrizchi, J. Chromatogr. A 1183, 21 (2008)
M. Pujadas, S. Pichini, S. Poudevida, E. Menoyo, P. Zuccaro, M. Farré, R. de la Torre, J. Chromatogr. B 798, 249 (2003)
M. Nishida, A. Namera, M. Yashiki, T. Kojima, J. Chromatogr. B 789, 65 (2003)
G. Boatto, M.V. Faedda, A. Pau, B. Asproni, S. Menconi, R. Cerri, J. Pharm. Biomed. Anal. 29, 1073 (2002)
V. Piette, F. Parmentier, J. Chromatogr. A 979, 345 (2002)
Y. He, Y.-J. Kang, J. Chromatogr. A 1133, 35 (2006)
R. Herraez-Hernandez, P. Campíns-Falcó, Chromatographia 52, 169 (2000)
J. Chen, B. Yao, C. Li, G. Shi, Carbon 64, 225 (2013)
S. Zeng, N. Gan, R. Weideman-Mera, Y. Cao, T. Li, W. Sang, Chem. Eng. J. 218, 108 (2013)
M. Cruz-Vera, R. Lucena, S. Cárdenas, M. Valcárcel, TrAC-Trends. Anal. Chem. 28, 1164 (2009)
Z. Ma, Y. Guan, H. Liu, J. Polym. Sci. A: Polym. Chem. 43, 3433 (2005)
N. Rahmanian, H. Hamishehkar, J.E.N. Dolatabadi, N. Arsalani, Colloids Surf. B 123, 331 (2014)
P. Teo, H. Lim, N. Huang, C. Chia, I. Harrison, Ceram. Int. 38, 6411 (2012)
M. Kassaee, E. Motamedi, M. Majdi, Chem. Eng. J. 172, 540 (2011)
V. Mohanraj, Y. Chen, Trop. J. Pharm. Res. 5, 561 (2007)
J. Xiong, J. Chen, M. He, B. Hu, Talanta 82, 969 (2010)
M. Nishida, A. Namera, M. Yashiki, K. Kimura, Forensic Sci. Int. 143, 163 (2004)
A. Namera, A. Nakamoto, M. Nishida, T. Saito, I. Kishiyama, S. Miyazaki, M. Yahata, M. Yashiki, M. Nagao, J. Chromatogr. A 1208, 71 (2008)
C. Chafer-Pericas, P. Campıns-Falcó, R. Herraez-Hernandez, Anal. Biochem. 333, 328 (2004)
N. Raikos, K. Christopoulou, G. Theodoridis, H. Tsoukali, D. Psaroulis, J. Chromatogr. B 789, 59 (2003)
Acknowledgments
Drug Applied Research Center of Tabriz University of Medical Science is greatly appreciated for supporting the study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Taghvimi, A., Hamishehkar, H. & Ebrahimi, M. The application of magnetic nano graphene oxide in determination of methamphetamine by high performance liquid chromatography of urine samples. J IRAN CHEM SOC 13, 1471–1480 (2016). https://doi.org/10.1007/s13738-016-0862-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0862-6