Abstract
Solid-phase microextraction (SPME) based on carboxylated single-walled carbon nanotube fibers was used to extract several chlorophenols (CPs) and organochlorine pesticides (OCPs) from aqueous samples prior to their determination by GC with electron capture detection. The main parameters affecting microextraction (temperature, time, stirring rate and salting-out effect) and the conditions of the thermal desorption in the GC injector were optimized. Compared with commercial SPME fibers, the fiber presented better selectivity and sensitivity. Linear response was found for the concentration range between 2 and 1000 ng L−1 (20–1000 ng L−1 for CPs), and the limits of detection were in the range from 0.07 to 4.36 ng L−1. The repeatability expressed as relative standard deviation ranged from 4.1 % to 8.2 % and the fiber-to-fiber reproducibility for four prepared fibers was between 6.5 % and 10.8 %. The method was successfully applied to the analysis of CPs and OCPs in lake water and waste water samples. Recovery was tested with spiked lake water and waste water samples, with values ranging from 89.7 % to 101.2 % in case of waste water samples.




Raman spectra: (A) SWNTs, and (B) Oxidized SWNTs
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorophenols and organochlorine pesticides, respectively, are a large group of aromatic phenolic and chlorinated hydrocarbon residues exposed extensively to the environment as a consequence of the worldwide use of these chemicals as pesticides or insecticides, deodorants, and for other purposes. Toxicology studies have evidenced that long-term exposure to high levels of these chemicals may cause serious damage to human health [1, 2]. Some chlorinated insecticides and phenols show carcinogenic properties to humans, and higher levels of chlorophenols not only are nephrotoxic and interfere with blood formation, but also they can cause immunosuppressive effects [3]. Several substances have been included in the priority pollution list of the Environmental Protection Agency and the European Union [4, 5].
The conventional methods for sample clean-up and preconcentration of volatile or semi-volatile compounds in water, such as liquid-liquid extraction [6] or solid-phase extraction [7, 8], are rather time-consuming, low sensitivity, tedious, and require relatively large amounts of toxic solvents [9]. Thus it is desirable to enhance the sensitivity and reduce the sample handling procedure in analytical methods. SPME technology, developed by Pawliszyn in 1980s [10, 11], is an ideal analytical tool for the determination of these substances, due to its simplicity, rapidity, low expense, high extraction volume and low consumption of organic solvents.
Carbon nanotubes (CNTs), first discovered in 1991 by lijima [12], have drawn considerable attention for many attractive characteristics such as distinctive electric conductivity, strong physical adsorption ability, thermal stability, etc. Most of the reported applications of CNTs in analytical chemistry concern designs of novel gas sensors, enzymatic biosensors, voltammetry, and DNA probes [13]. Recently Cai [14–16] and Liu [17] have deeply investigated the potential of CNTs as adsorbents for solid-phase extraction of industrial pollutants in environment, the results have indicated that CNTs may be effective solid-phase extraction phases for a wide range of compounds. The application of CNTs as SPME coating has been also reported. Yan et al. [18] prepared a novel fiber with multi-walled carbon nanotubes (MWNTs) and used it to extract polybrominated diphenyl ethers (PBDEs) in water and milk samples. A SPME fiber coated with oxidized MWNTs was prepared and was found to be effective in carrying out extraction of phenols in aqueous samples [19], and the sol-gel-CNTs metal fiber coated with functionalized MWNTs was an attractive SPME tool because of its wide linear range and good extraction efficiency for broad classes of analytes. Theoretically, SWNTs are smaller than MWNTs in diameter and possess higher active surface area, thus they are expected to achieve higher extraction efficiency than MWNTs. Few articles have studied the performance of SWNTs fibers in aqueous samples and got satisfactory results [13, 20]. Our group has also investigated the performance of a prepared sol-gel SWNTs fiber for the determination of PBDEs [21].
Based on the previous work, the present work was to develop a novel carboxylated SWNTs (TSO/SWNTs) fiber with sol-gel method. The feasibility of the prepared fiber was investigated by determination of CPs and OCPs simultaneously in aqueous samples. Potential factors affecting the extraction efficiency were optimized, and comparison with commercial fibers was also carried out to evaluate the performance of the TSO/SWNTs fiber. Results indicated that the simplified procedure of the fiber preparation was convenient, and the prepared TSO/SWNTs fiber had higher extraction efficiency than commercial fibers. Finally, the developed method was applied to determine trace CPs and OCPs in lake water and waste water samples.
Experimental
Reagents and materials
The SWNTs (purity, SWNTs>50%) was purchased from Shenzhen Nanotech Port (Shenzhen, China, http://www.nanotubes.com.cn). The fused-silica fiber (140 μm, o.d.) with protective polyimide coating was obtained from the Academy of Post &Telecommunication (Wuhan, China). The commercial SPME fibers (100 μm Polydimethylsiloxane (PDMS) and 65 μm Polydimethylsiloxane/Divinylbenzene (PDMS/DVB)) were purchased from Supelco (Bellefonte, PA, USA, http://www.sigmaaldrich.com/). CPs (2, 3, 4, 6, - tetrachlorophenol (TeCP), pentachlorophenol (PCP)) and OCPs (o, p`-DDT, aldrine, dieldrin and endrine) were obtained from Dr.ehrenstorfer gmbh (Germany, http://www.ehrenstorfer.com/) and stored at 4 °C in the refrigerator. Trifluoroacetic acid (TFA, 99%), and hydroxyl-terminated silicone oil (TSO-OH) were purchased from Aldrich (Allentown, PA, USA, http://www.sigmaaldrich.com/). Tetraethoxysilane (TEOS, 99%), and poly (methylhydrosiloxane) (PMHS) were purchased from the Chemical Plant of Wuhan University (Wuhan, China, http://www.wdsilicone.cn/). All solvents used were of analytical grade from Sinopharm (Shanghai, China, http://www.sinopharm.com/).
Apparatus
The SPME devices for manual sampling were obtained from Supelco (Bellefonte, PA, USA, http://www.sigmaaldrich.com/). SPME-GC experiments were carried out on an Agilent HP6890 GC system (http://www.agilent.com/) equipped with 63Ni electron capture detection (ECD) system. A 30 m × 0.32 mm I.D., 0.25 μm SE-30 coating fused-silica capillary column (Dalian, China) was used for analysis. The carrier gas was nitrogen (99.999%). The injector was in splitless model and the temperature program conditions were as follows: Initial column temperature 80 °C (hold 1 min), increased to 220 °C at 10 °C min−1, and finally increased to 280 °C at 5 °C min−1 (hold 8 min). The injector and detector temperature were 250 °C and 300 °C respectively. Doubly deionized water was obtained from an Aquapro water purification system (Chongqing, China).
In order to mix the various ingredients in solution thoroughly, an Ultrasonator Model KQ2200-DB (Jiangsu, China, http://www.ks-csyq.com/) was used. A centrifuge model Eppendorf 5415R (Germany, http://www.eppendorf.cn/) was used to separate the sol solution from the precipitate during fiber preparation. A magnetic stirrer DF-101S (Wuhan, China) was employed for stirring the sample during headspace extraction. The thickness of TSO/SWNTs fiber was measured by Leica DME system (Shanghai, China, http://en.leica-camera.com/). The scanning electron micro-scopy images on the surface of the fiber were obtained by a JSM-6390LV scanning electron microscope (SEM) (Japan, http://www.jeolusa.com/).
Preparation of SPME fiber
Prior to sol–gel coating, 8 cm long fused-silica fiber was dipped into concentrated sulfuric acid (98%) for 1 h to remove the protective polyimide layer. The fiber was then dipped in 1 mol L−1 NaOH solution for 1 h to expose the maximum number of silanol groups on its surface, and then cleaned with distilled water. Finally it was dipped into 0.1 mol L−1 HCl solution for 30 min to neutralize the excess NaOH, cleaned with distilled water again, and air-dried at room temperature.
The coated fibers were prepared according to the method described by Matthew Giardina et al. [22]. First, 50 mg of carboxylated SWNTs powder was dispersed into 2 mL of dichloromethane under sonication. The sol–gel solution was prepared as follows: 120 mg of TSO-OH was dissolved in 200 μL of dichloromethane, and then 150 μL of TEOS, 15 mg of PMHS were added and mixed thoroughly for 5 min by ultrasonic agitation in a 2.5 mL plastic tube. 80 μL volume of TFA containing 5% water was sequentially added to the resulting solution with ultrasonic agitation for another 5 min. The mixture was centrifuged at 12000 rpm for 3 min. The precipitate at the bottom of the tube was removed and the supernatant was collected for fiber coating. Next, the fused-silica fiber was dipped vertically into the sol–gel solution for 1 min, and the sol–gel coating was formed on the outer surface of the fiber, then the fiber was transferred into the carboxylated SWNTs solution for another 1 min, the carboxylated SWNTs was also immobilized on the fiber, the above encapsulation procedure was repeated for 5–7 times until the desired thickness (80 μm) was obtained. Finally, the prepared fiber was placed in a desiccator for about 12 h at room temperature and conditioned in the GC injection port at 120–320 °C under gentle nitrogen protection for 3 h.
Sample preparation
Stock solutions for each standard were prepared in n-hexane at a concentration of 0.5 mg L−1 and stored at 4 °C. The working standard solution containing each compound was prepared by diluting the stock solutions in n-hexane to a concentration of 1.0 ng mL−1. Calibration standards with concentrations of 20, 50, 250, 500, 800 and 1000 ng L−1 were prepared by diluting the working standard solution directly in aqueous matrix, and for the optimization of headspace SPME process, 0.8 ng mL−1 of standard solution was chosen. Lake water sample was collected from the Nanhu Lake (Wuhan, China). Waste watera (treated) and Waste waterb (untreated) samples were both collected from a local waste water treatment plant (Wuhan, China). The three water samples were filtered and maintained at 4 °C before analysis.
Solid phase microextraction
A volume of 5 mL of sample solution was added into a 10 mL headspace vial containing a magnetic spin bar. To prevent sample evaporation, the vial was immediately sealed with PTFE septum and open centered aluminum cap and then placed in a heating bath for 15 min to reach the thermal equilibrium before extraction. The SPME fiber was exposed to the headspace of the vial, which was kept at a certain temperature and stirred. After extraction, the fiber was withdrawn into the needle, and was introduced into the heated GC injector port for desorption and analysis. The fiber was cleaned at 300 °C (250 °C for commercial fibers) for 5 min to eliminate the carry-over of analytes from previous extraction before sampling.
Results and discussion
Characteristics of the prepared SPME fiber
The morphological structure of the TSO/SWNT fiber was investigated by scanning electron micrograph technique. As can be seen from Fig. 1 that the surface of the prepared coating possessed a rough and porous structure, which could increase the available surface area on the fiber and improve the kinetics of extraction procedure, thus, enhanced extraction efficiency. Figure 1c was taken from the sectional part of the fiber, demonstrating the existence of SWNTs within TSO/SWNTs fiber.
Optimization of SPME procedure
Headspace sampling is commonly chosen to minimize the exposure of SPME fiber to the sample matrix and prolong the life of the fiber. Headspace SPME is an equilibrium process that involves the partitioning of analytes through three-phase system: aqueous matrix, headspace and the fiber coating [23]. Therefore optimization of parameters that influence the partition of analytes is extremely essential.
Extraction temperature and time
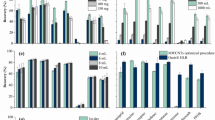
Extraction temperature can affect the partition coefficient of the analytes between fiber coating and sample matrix [24]. With the increase of temperature, the mass transfer of analytes will be accelerated. Increasing the temperature can significantly facilitate the analytes entering the gas phase and the coating from aqueous matrix. On the other hand, because of the fact that SPME is based on an exothermic process, high temperature could decrease the extraction recovery [25]. Figure 2 illustrated the peak areas of CPs and OCPs versus extraction temperatures ranged from 30 °C to 70 °C. The responses of most of the compounds exhibited the highest extraction yield when the temperature was 50 °C. To provide a higher extraction efficiency for all the compounds in this study, the optimum extraction temperature was set at 50 °C.
For this experiment, a wide range of extraction time from 15 to 75 min was evaluated. The results obtained showed that the changes of extraction efficiency were different among the six compounds (see Supplementary Material Fig. S3). The responses of all the analytes gradually increased when the extraction time increased from 15 to 30 min, while differences were observed between 30 and 75 min. Therefore, 30 min was adopted as extraction time based on the sensitivity and time efficiency.
Agitation and NaCl addition
Agitation, which can enhance mass transfer of the analytes from solution to fiber, is most widely used in SPME experiments to reduce the equilibration time. Study on the effect of stirring rate indicated that the chromatographic peak areas of the analytes increased as the stirring rate increased from 0 to 900 rpm. The results showed that the maximum extraction efficiency for the analytes was obtained when stirring rate was set at 900 rpm (see Supplementary Material Fig. S4). For further experiments, the stirring rate of 900 rpm was thus chosen.
In headspace SPME, salting-out effect is employed to increase the ionic strength of matrix and facilitate the extraction of analytes. In this experiment, the optimum concentration of NaCl was investigated by analyzing the solutions containing different concentrations of NaCl ranged from 0 to 3.6 g mL−1. The chromatographic peak areas over different concentrations of NaCl were profiled in Fig. 3. It was noted that the peak areas increased significantly as a function of NaCl addition except aldrine. Most of the compounds gave the best responses when the concentration of NaCl was 0.9 g mL−1. Thus, the best compromise for the group of compounds was 0.9 g mL−1 of NaCl.
Desorption temperature and time
For desorption procedure, two parameters including desorption temperature and time need to be optimized. Since SPME fibers are designed to be reusable, it is important to monitor the carry-over effects. Higher desorption temperature is apt to overcome carry-over effect. According to the boiling points of analytes and thermal stability of the commercial fibers, 250 °C was selected as the desorption temperature. Next, desorption time has a significant effect on the responses of analytes, especially for the compounds of high boiling point. In this wok, desorption time over the range of 1–5 min was investigated under the desorption temperature of 250 °C, the desorption was completed for each compound after 3 min at 250 °C (see Supplementary Material Fig. S5). Thereby, desorption time was set at 3 min.
Extraction quantity and selectivity
Comparison with commercial fibers
According to the previously optimized conditions, the optimum analytical conditions were 30 min of extraction at 50 °C, 3 min of desorption at 250 °C, 900 rpm of stirring rate and with the concentration of 0.9 g mL−1 of NaCl in aqueous samples. Figure 4 shows the comparison between commercial fibers and TSO/SWNTs fiber under the optimum conditions. It reveals that TSO/SWNTs fiber has higher extraction quantities than commercial PDMS/DVB and PDMS fibers. It is possibly due to the highly delocalized conjugate system of π-electron in SWNTs, the introduction of carboxylic groups on the surface of SWNTs may change the properties and polarity of the coating. Furthermore, the three-dimensional network and SWNTs in the sol–gel coating provide higher surface area and sample capacity, and the high thermal desorption temperature overcomes the sample carry-over effect. All of these indicate that TSO/SWNTs fiber possesses strong physical adsorption ability to CPs and OCPs.
Linearity, correlation coefficients and precision of the fiber
The analytical data for the determination of CPs and OCPs in aqueous samples are summarized in Table 1. The linearity of response was studied over a vast concentration ranged from 2 to 1000 ng L−1 (20–1000 ng L−1 for CPs), and the correlation coefficients were all greater than 0.996. For the tested compounds, the limits of detection (LODs) calculated were between 0.07 and 4.36 ng L−1. The repeatability study was performed by determining 0.8 ng mL−1 of standard solutions, and the relative standard deviation (RSD) calculated for one fiber was in the range from 4.17% to 8.12%. In addition, the fiber-to-fiber reproducibility of four prepared fibers, coated under the same conditions, was also investigated. Five replicate extractions were performed with each fiber in this study. The RSD for the four fibers was in the range 6.51%–10.79%. This indicated that the prepared TSO/SWNTs fiber provided good repeatability and reproducibility. The results of the developed method show higher sensitivity when compared with the literature data [26, 27].
Application to real samples
The optimized method was applied for the analysis of one lake water and two waste water samples using TSO/SWNTs fiber. Data are given in Table 2. Results showed that no analytes were detected in lake water samples, whereas in waste water samples, TeCP and PCP were found in waste waterb (see Supplementary Material Fig. S6 for peak identification), and PCP was detected in waste watera. Since the concentrations of most of the analytes in the real samples were lower than the detection limit of the method, the lake water and waste waterb were spiked with CPs and OCPs. The recoveries of analytes at 100 ng L−1 spiked for lake water and waste waterb were in the range from 90.4% to 100.3%, and from 89.7% to 101.2% respectively. Although waste water samples generally contain various matrices, the precisions of the tested compounds are acceptable. It can be concluded that TSO/SWNTs fiber is a promising tool for the determination of CP and OCPs from aqueous samples.
Conclusions
This work described a headspace SPME method using homemade TSO/SWNTs coated fiber that was applied for the analysis of CPs and OCPs in water matrices. Compared with commercial SPME fibers, the novel TSO/SWNTs fiber exhibited higher sensitivity and selectivity for the six analytes. The optimum conditions for extraction were investigated at an equilibrium time of 30 min at 50 °C and for desorption in a GC injector at 250 °C for 3 min. This method showed a precision below 8.2% (RSD), and the fiber-to fiber reproducibility was lower than 10.8%. Linearity was verified over a wide range from 2 to 1000 ng L−1 (20–1000 ng L−1 for CPs), and the LODs obtained were between 0.07 and 4.36 ng L−1. The developed method provided a very simple, rapid, sensitive and efficient tool for the analysis of trace CPs and OCPs pollutants in environmental water samples.
References
Michałowicz J, Majsterek I (2010) Chlorophenols, chlorocatechols and chloroguaiacols induce DNA base oxidation in human lymphocytes (in vitro). Toxicology 268:171
Cortada C, Vidal L, Tejada S, Romo A, Canals A (2009) Determination of organochlorine pesticides in complex matrices by single-drop microextraction coupled to gas chromatography-mass spectrometry. Anal Chim Acta 638:29
Veningerová M, Prachara V, Kovačičová J, Uhnáka J (1997) Analytical methods for the determination of organochlorine compounds. J Chromatogr A 774:333
Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008. Official journal of the European Union L348:84
National primary drinking water regulations, http://water.epa.gov/drink/contaminants/index. cfm. Accessed 6 September 2010
Fatoki OS, Awofolu RO (2003) Methods for selective determination of persistent organochlorine pesticide residues in water and sediments by capillary gas chromatography and electron capture detection. J Chromatogr A 983:225
Kim MS, Kang TW, Pyo H, Yoon J, Choic K, Hong J (2008) Determination of organochlorine pesticides in sediment using graphitized carbon black solid-phase extraction and gas chromatogramphy/mass spectrometry. J Chromatogr A 1208:25
Čonka K, Drobná B, Kočan A, Petrík J (2005) Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorine pesticides in human serum. J Chromatogr A 1084:33
Daneshfar A, Khezeli T (2009) Extraction of phenolic compounds from environmental water samples using oil-in-water emulsions. Microchim Acta 167:211
Belardi RP, Pawliszyn J (1989) The application of chemically modified fused silica fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Pollut Res J Can 24:179
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption fused silica optical fibers. Anal Chem 62:2145
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56
Lü JX, Liu JF, Wei Y, Jiang KL, Fan SS, Liu JY, Jiang GB (2007) Preparation of single-walled carbon nanotube fiber coating for solid-phasemicroextraction of organochlorine pesticides in lake water and waste water. J Sep Sci 30:2138
Cai YQ, Jiang GB, Liu JF, Zhou QX (2003) Multi-walled carbon nanotubes packed cartridge for the solid-phase extraction of several phthalate esters from water samples and their determination by high performance liquid chromatography. Anal Chim Acta 494:149
Cai YQ, Cai YE, Mou SF, Lu YQ (2005) Multi-walled carbon nanotubes as a solid-phase extraction adsorbent for the determination of chlorophenols in environmental water samples. J Chromatogr A 1081:245
Cai YQ, Jiang GB, Liu JF, Zhou QX (2003) Multiwalled carbon nanotubes as a solid-phase extraction adsorbent for the determination of bisphenol A, 4-n-nonylphenol, and 4-tert-octylphenol. Anal Chem 75:2517
Liu G, Wang J, Zhu Y, Zhang X (2004) Application of multiwalled carbon nanotubes as a solid-phase extraction sorbent for chlorobenzenes. Anal Lett 37:3085
Wang JX, Jiang DQ, Gu ZY, Yan XP (2006) Multiwalled carbon nanotubes coated fibers for solid-phase microextraction of polybrominated diphenyl ethers in water and milk samples before gas chromatography with electron-capture detection. J Chromatogr A 1137:8
Liu XY, Ji YS, Zhang YH, Zhang HX, Liu MC (2007) Oxidized multiwalled carbon nanotubes as a novel solid-phase microextraction fiber for determination of phenols in aqueous samples. J Chromatogr A 1165:10
Li QL, Wang XF, Yuan DX (2009) Preparation of solid-phase microextraction fiber coated with single-walled carbon nanotubes by electrophoretic deposition and its application in extracting phenols from aqueous samples. J Chromatogr A 1216:1305
Zhang W, Sun Y, Wu C, Xing J, Li J (2009) Polymer-functionalized single-walled carbon nanotubes as a novel sol gel solid-phase microextraction coated fiber for determination of polybrominated diphenyl ethers in water samples with gas chromatography electron capture detection. Anal Chem 81:2912
Matthew G, Susan VO (2001) Application of low-temperature glassy garbon films in solid phase microextraction. Anal Chem 73:5841
Liu M, Zeng Z, Fang H (2005) Preparation and application of the sol-gel-derived acrylate/silicone co-polymer coating for headspace solid-phase microextraction of 2-chloroethyl ethyl sulfide in soil. J Chromatogr A 1076:25
Martendal E, Budziak D, Debastini R, Carasek E (2007) Determination of haloanisoles in paper samples for food packaging by solid-phase microextraction and gas chromatography. Microchim Acta 159:229
Carasek E, Cudjoe E, Pawliszyn J (2007) Fast and sensitive method to determine chloroanisoles in cork using an internally cooled solid-phase microextraction fiber. J Chromatogr A 1138:10
Wei MC, Jen JF (2003) Determination of chlorophenols in soil samples by microwave-assisted extraction coupled to headspace solid-phase microextraction and gas chromatography-electron-capture detection. J Chromatogr A 1012:111
Beceiro-González E, Concha-Graña E, Guimaraes A, Gonçalves C, Muniategui-Lorenzo S, Alpendurada MF (2007) Optimisation and validation of a solid-phase microextraction method for simultaneous determination of different types of pesticides in water by gas chromatography-mass spectrometry. J Chromatogr A 1141:165
Acknowledgements
The authors wish to express their gratitude to Chang-Wen Ye, Yan-Chun Huang and Li-Hong Qin of Huazhong Agricultural University for the instrumental assistance in this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 381 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Zhang, WY., Xing, J. et al. Solid-phase microfibers based on modified single-walled carbon nanotubes for extraction of chlorophenols and organochlorine pesticides. Microchim Acta 173, 223–229 (2011). https://doi.org/10.1007/s00604-010-0526-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0526-7