Abstract
A novel method, based on the coupling of ionic liquid-based headspace single-drop microextraction (SDME) with gas chromatography (GC), is developed for the determination of chlorobenzene derivatives. For the SDME of five chlorobenzene derivatives, a 1.0 μL 1-octyl-3-methylimidazolium hexafluorophosphate microdrop is exposed for 20 min to the headspace of a 15 ml aqueous sample containing 20% (w/v) NaCl placed in 25 ml vial at 40 °C. Then, the extractant is directly injected into the injector block of the GC instrument. To avoid ionic liquid leaking into the chromatographic column, a small glass tube is placed in the injection block. Under optimized operation conditions, linear relation between peak areas and analyte concentrations up to 1.5 mg L−1 has been obtained The detection limits range from 0.1 to 0.5 μg L−1 for the various analytes. The relative standard deviations at 1.0 μg L−1 range from 7.7 to 12.4%, and the enrichment factors from 41 to 127. The method is simple and sensitive, and does not suffer from the influence of a solvent peak. Its applicability is demonstrated by the determination of chlorobenzenes in wastewater samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorobenzene derivatives are widely used as solvent, heat transfer agent, insect repellant, deodorant, degreaser and intermediate in dye and pesticide synthesis, and they are carelessly discharged in the environment at times. Chlorobenzene derivatives are toxic and are identified as primary pollutant [1, 2]. As it is difficult for chlorobenzene derivatives to decompose, they can accumulate in environment and their danger generally lasts for quite long time. Therefore, the detection of chlorobenzene derivatives in environment is quite important and gas chromatography is currently used for such purpose [3, 4]. However, their concentrations are generally quite low in environmental samples, thus some preconcentration procedures are required prior to their chromatographic determination.
Chlorobenzene derivatives are usually enriched by liquid–liquid extraction (LLE) [5] and solid-phase extraction (SPE) [6]. But LLE is laborious and solvent consuming; for SPE the column pretreatment is needed and organic solvent is required for elution. Recently, some new preconcentration methods, such as solid-phase microextraction (SPME) [7–9], and liquid-phase microextraction (LPME) [10–12], were developed. SPME shows advantages of solvent-free, fast, portable and simple [13], but it also has some shortcomings such as higher cost and sample carry-over. LPME is a simple, quick, inexpensive and solvent-minimized technique for sample preparation. It presents similar extraction efficiency and reproducibility to SPME. LPME can be performed with a drop of solvent [14], and a small length of hollow fiber-protected solvent [15, 16]. The former is also called single-drop microextraction (SDME), and was successfully applied in the determination of various substances [14, 17]. However, when SDME is coupled with gas chromatography the broad solvent peak can affect the separation and accurate determination of analytes sometimes. In addition, SDME suffers from the evaporation of organic solvent, which results in the change of solvent volume during the extraction process. Therefore, organic solvents suitable for SDME are limited.
Ionic liquids (ILs) are salts with melting point around room temperature. ILs possess many unique properties such as non-volatility, non-inflammability, high thermal stability and wide electrochemical window. Furthermore, their characteristics can be adjusted by varying the anion and cation. Hence they have great application potential in various fields. ILs are widely used as extractant and they give good performance [18]. Recently, ILs-based SDME was developed and used to pretreat samples for HPLC analysis [19–21]. However, ILs-based SDME has still not been coupled with GC, probably due to their nonvolatility.
The purpose of this work is to explore the feasibility to couple the ILs-based single-drop microextraction technique with GC. Thus chlorobenzene derivatives are chosen as model analytes, and dye wastewater is determined.
Experimental
Reagents and chemicals
1-Octyl-3-methylimidazolium hexafluorophosphate ([C8MIM][PF6], purity: 99%, boiling point: >300 °C, decomposition point: 376 °C; density: 1.235 g mL−1 (20 °C)) was purchased from Acros Organics (www.acros.com, New Jersey, USA) and used as received. Chlorobenzene (CB), o-chlorotoluene (o-CT), 1,4-dichlorobenzene (p-DCB), 1,2-dichlorobenzene (o-DCB), and 1,2,4-trichlorobenzene (1,2,4-TCB) were products of Beijing Chemical Industry Group Co. (www.bcigc.com, Beijing, China). Their stock solutions (1.0 mg mL−1) were prepared with methanol and stored in a refrigerator. Working solutions were prepared by diluting stock solutions with deionized water. Other chemicals used were of analytical grade and deionized water was used throughout. The wastewater sample was collected from a local dye company (Wuhan, China). It was filtered with micropore cellulose membrane (∅ = 0.45 μm) and stored at 4 °C.
Apparatus
The GC experiments were performed on a Model SP-6800A gas chromatography instrument with a flame ionization detector (FID) (www.lunan-gc.com, Rui Hong Chemistry Company, Sandong, China). A N2000 chromatographic workstation program for GC system (Zhejiang University, China) was used to process chromatographic data. A homemade SE-54 column (30 m × 0.25 mm i.d., film thickness: 0.25 μm) was used for separation. Its inlet was operated under the split mode with a split ratio of 10:1 and the on-column flow-rate of nitrogen gas (http://gas.wisco.com.cn, The Oxygen Co., Ltd. Of WISCO, Wuhan, China) was 12 cm s−1. The sample-injection part, FID detector and column temperature were kept at 250 °C, 250 °C and 80 °C, respectively.
Extraction procedure
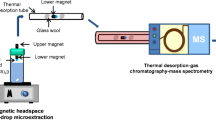
A headspace SDME procedure was adopted. For headspace SDME, 15 mL solution containing proper chlorobenzene derivatives and NaCl was transferred in a 25 mL vial with septum. After piercing the vial septum with a microsyringe, 1 μL [C8MIM][PF6] was pushed out. The microsyringe was fixed with a clamp so that the needle of the syringe was located at the same position. Then the magnetic stirrer was turned on. After extracting for certain time, the IL drop was retracted into the microsyringe and injected into the GC system. To avoid IL entering into the GC capillary column, a homemade small glass tube (o.d.: 2.0 mm, length: about 15 mm) was placed in the sample-injection part as shown in Fig. 1. The small tube could incept the IL microdrop when it was not successfully retracted. When the small tube was full, the IL was taken out with a syringe or the small tube was replaced, which was easy to conduct. Therefore, IL-based SDME could be coupled with GC.
Results and discussion
Some factors influencing the headspace SDME of chlorobenzene derivatives such as extraction time, extraction temperature, ionic strength, pH and solvent volume are optimized. The headspace SDME is evaluated on the basis of detection limit, linear range, reproducibility and enrichment factor for these compounds.
Influence of extraction time
Figure 2 shows the influence of extraction time on the extraction efficiency. As can be seen, the peak areas increase rapidly with extraction time increasing within 20 min. When it exceeds 20 min, the peak areas tend to unchanged, meaning that their extraction equilibriums are nearly achieved. For o-CT, however, the peak area decreases when extraction time is above 20 min. This can be ascribed to the influence of water vapor as well as other chlorobenzene derivatives. It is well known that headspace SDME is related to the partition equilibriums of analytes in gas phase and liquid phase, the adsorption of analytes at the microdrop surface and their boiling points. As the boiling point of o-CT is lower, it is extracted and adsorbed more rapidly. When the extraction time is long enough, the competition from other analytes and water vapor rises, which suppresses the extraction and adsorption of o-CT to some extent. Thus its extraction efficiency does not increase, but decreases. In the following experiment, the extraction time is fixed at 20 min.
Influence of extraction temperature and ionic intensity
The effect of temperature is tested from 0 °C to 60 °C. The extraction efficiency increases with raising temperature, it reaches maximum at 40 °C and then decreases. Therefore, an extraction temperature of 40 °C is adopted in this work. The concentration of NaCl is changed from 0% to 40% (w/v) to explore the effect of ionic intensity. It is found that the peak areas reach maximum around 20%. Hence, the NaCl concentration is fixed at 20%.
Influence of solution pH
To investigate the effect of acidity on extraction efficiency, the pH of working solution is adjusted with HCl and NaOH solution prior to extraction. The results are shown in Fig. 3. The influence of pH is marked and at about pH 7.0 the peak areas reach maximum. This is because these compounds are neutral around pH 7. When the solution becomes acidic or basic their solubility in aqueous solution may increase, so the extraction efficiency decreases.
Effect of pH on the extraction capacity. NaCl concentration: 20% (w/v); extraction time: 20 min; extraction temperature: 40 °C. Other conditions as in Fig. 2
Influence of solution volume
Figure 4 presents the variation of peak area with solution volume. For a 25 mL extraction bottle, when the solution volume is 15 mL the extraction efficiency is higher. This can be explained as follows: with the increase of solution volume, in the headspace part the equilibrium concentration is achieved more rapidly, which benefits the diffusion of the analytes to the IL drop. However, if the solution volume is too large, it will take more time for the analytes to transfer from the aqueous solution to the headspace part, thus the extraction efficiency decreases. Therefore, the ratio of the headspace volume and solution volume should be proper.
Effect of solution volume on the extraction capacity. Other conditions are the same as in Fig. 3
Evaluation
Figure 5 shows a typical chromatogram of the chlorobenzene derivatives under the optimized conditions. They are baseline separated, and no big “solvent peak” occurs, which is superior to that extracting with other organic solvents. To evaluate the ILs-based headspace SDME method, some parameters such as detection limit, linear range, reproducibility and enrichment factor (calculated according to the GC peak area of the analyte in IL microdrop and in corresponding aqueous solution with same volume) for the chlorobenzene derivatives are measured. The results are summarized in Table 1. The detection limits are 0.1–0.5 μg L−1 (S/N = 3), linear ranges are 1.0–1,500 μg L−1. At 1.0 μg L−1 the relative standard deviations (RSD, n = 8) of peak area range from 7.7% to 12.4%, and the enrichment factors from 41 to 127. The bigger RSD is related to the bigger deviation of microdrop-volume controlled with a common microsyringe.
Sample analysis
A 15 mL dye wastewater is transferred into a 25 mL vial. After adding 3 g NaCl and tuning on the magnetic stirrer, extraction is performed as described above. Then the extractant is determined with GC instrument. The results are shown in Table 2, and the recoveries are 88.9–110%.
Conclusions
Through placing a small glassy tube in the sample injection part, ionic liquid no long enters into the chromatographic column, thus ionic liquid based microextraction technique can be coupled with GC. To evaluate the proposed method, five chlorobenzene derivatives are used as model. Results show that the chlorobenzenes can be extracted effectively by ionic liquid [C8MIM][PF6] and the extractant exhibits good chromatographic behavior. The linear ranges are up to 1.5 mg L−1 and the detection limits range from 0.1 to 0.5 μg L−1. As ionic liquids have many unique properties, ionic liquid based microextraction technique coupled with GC has potential application in the detection of pollutants etc.
References
World Health Organisation (WHO) (2002) 10th Report on Carcinogens, National Toxicology Program, Public Health Service
EC (European Community) (2000) Directive 2000/60/EC of the European Parliament and of the council establishing a framework for community action in the field of water policy. Official Journal L 327, December 22, 2000
Oliver BG, Bothen KD (1980) Determination of chlorobenzenes in water by capillary gas chromatography. Anal Chem 52:2066
Wang Y, Lee HK (1998) Determination of chlorobenzenes in water by solid-phase extraction and gas chromatography-mass spectrometry. J Chromatogr A 803:219
Melcher RG, Morabito PL (1990) Membrane/gas chromatographic system for automated extraction and determination of trace organics in aqueous samples. Anal Chem 62:2183
Liu GH, Wang JL, Zhu YF, Zhang XR (2004) Application of multiwalled carbon nanotubes as a solid-phase extraction sorbent for chlorobenzenes. Anal Lett 37:3085
He Y, Wang Y, Lee HK (2000) Trace analysis of ten chlorinated benzenes in water by headspace solid-phase microextraction. J Chromatogr A 874:149
Paschke A, Popp P (2004) Diffusion-based calibration for solid-phase microextraction of benzene, toluene, ethylbenzene, p-xylene and chlorobenzenes from aqueous samples. J Chromatogr A 1025:11
Jose LV, Avismelsi P, Lilia A, Alberto N (2006) Application of isotope dilution to the determination of anthracene in environmental samples by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Microchim Acta 155:435
Shen G, Lee HK (2003) Headspace liquid-phase microextraction of chlorobenzenes in soil with gas chromatography-electron capture detection. Anal Chem 75:98
Khajeh M, Yamini Y, Hassan M (2006) Trace analysis of chlorobenzenes in water samples using headspace solvent microextraction and gas chromatography/electron capture detection. Talanta 69:1088
Chen X, Zhang T, Liang P, Li Y (2006) Application of continuous-flow liquid phase microextraction to the analysis of phenolic compounds in wastewater samples. Microchim Acta 155:415
Edmar M, Dilma B, Rafael D, Eduardo C (2007) Determination of haloanisoles in paper samples for food packaging by solid-phase microextraction and gas chromatography. Microchim Acta 159:229
Vidal L, Canals A, Kalogerakis N, Psillakis E (2005) Headspace single-drop microextraction for the analysis of chlorobenzenes in water samples. J Chromatogr A 1089:25
Zhao LM, Zhu LY, Lee HK (2002) Analysis of aromatic amines in water samples by liquid-liquid-liquid microextraction with hollow fibers and high-performance liquid chromatography. J Chromatogr A 963:239
Peng J, Lu J, Hu X, Liu J, Jiang G (2007) Determination of atrazine, desethyl atrazine and desisopropyl atrazine in environmental water samples using hollow fiber-protected liquid-phase microextraction and high performance liquid chromatography. Microchim Acta 158:181
Tor A (2006) Determination of chlorobenzenes in water by drop-based liquid-phase microextraction and gas chromatography-electron capture detection. J Chromatogr A 1125:129
Zhou Q, Ye C (2008) Ionic liquid for improved single-drop microextraction of aromatic amines in water samples. Microchim Acta 162:153
Vidal L, Psillakis E, Domini CE, Gran´e N, Marken F, Canals A (2007) An ionic liquid as a solvent for headspace single drop microextraction of chlorobenzenes from water samples. Anal Chim Acta 584:189
Ye CL, Zhou OX, Wang XM (2007) Determination of phenols in environmental water samples by ionic liquid-based headspace liquid-phase microextraction coupled with high-performance liquid chromatography. J Sep Sci 30:42
Liu JF, Chi YG, Jiang GB, Tai C, Peng JF, Hu JT (2004) Ionic liquid-based liquid-phase microextraction, a new sample enrichment procedure for liquid chromatography. J Chromatogr A 1026:143
Acknowledgement
The authors appreciate the support of the National Nature Science Foundation of China (No. 20173040).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 57 KB)
Rights and permissions
About this article
Cite this article
Zhao, F., Lu, S., Du, W. et al. Ionic liquid-based headspace single-drop microextraction coupled to gas chromatography for the determination of chlorobenzene derivatives. Microchim Acta 165, 29–33 (2009). https://doi.org/10.1007/s00604-008-0092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0092-4