Abstract
Purpose
To identify the incidence of extraction site incisional hernia following gastrectomy for gastric cancer and its significant risk factors, including the subcutaneous fat area.
Methods
We reviewed data gathered prospectively on patients with gastric cancer, who underwent gastrectomy between 2008 and 2012 at Kyushu University Hospital, Fukuoka, Japan. The subcutaneous fat area (SFA) and visceral fat area (VFA) were measured using axial computed tomography at the level of the L4 and L3 transverse processes, and the L2–L3 intervertebral disc. The primary endpoint of the rate of extraction site incisional hernia was based on the computed tomography and clinical data including hospital follow-up reports.
Results
After applying the inclusion and exclusion criteria, 320 patients were included in this retrospective analysis: 3.1% (10/320) had extraction site incisional hernias after a mean follow-up of 11 months. Multivariate analysis revealed that age and the SFA were independent risk factors (age ≥ 70.5 years: P = .013, odds ratio: 9.116, 95% confidence interval 1.581–52.553; L4 SFA ≥ 124 cm2: P = .004, odds ratio: 13.752, 95% confidence interval 2.290–82.582).
Conclusion
Age and the SFA were independent risk factors for extraction site incisional hernia in patients undergoing gastrectomy for gastric cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Incisional hernia is a common cause of postoperative morbidity with a high recurrence rate (14–63%) despite developments in repair methods [1,2,3,4]. Approximately 23–60% of patients are asymptomatic [1, 5]; however, it can lead to life-threatening events such as incarceration (6–15%) and bowel strangulation (2%) [1, 2, 4, 6,7,8]. The incidence of incisional hernia is decreasing in line with the increasing popularity of laparoscopic surgery [9]. However, incisional hernia remains a major late postoperative complication that affects up to 2.8% of patients undergoing laparoscopic distal gastrectomy, 20% of those undergoing laparotomy, 8.5–29% of those undergoing laparoscopic colorectal surgery, and as many as 69.1% of those undergoing abdominal aortic occlusive and aneurysmal disease surgery [10,11,12,13,14,15].
Several studies have analyzed the risk factors for incisional hernia, including Jang et al., who found that female sex, higher body mass index (BMI), and the presence of comorbidities were significant risk factors in patients undergoing laparoscopic distal gastrectomy for gastric cancer [10]. Itatsu et al. reported that BMI and the thickness of the subcutaneous fat were independent risk factors for incisional hernia after abdominal surgery [1]. Yamada et al. identified age, open laparotomy, and subcutaneous fat area (SFA) as independent risk factors after colorectal surgery [16], while Yamamoto et.al found that an increased visceral fat area (VFA) and female sex were independent risk factors for incisional hernia [12].
The SFA as a risk factor for incisional hernia has been studied in other cancer surgeries, but rarely in gastric cancer surgery. It has also been studied in gastric cancer, but as a risk factor for early rather than late surgical complications [17]. Various studies used different abdominal levels to measure SFA and VFA. Yamada et al. used the umbilical level as slice-representative for SFA [16], Kim et al. used the L3 level for both SFA and VFA [18], Yoshikawa et al. used the umbilical or L4–L5 level for VFA [19], and So et al. recommended using the L2–L3 level for VFA and the L4–L5 level for SFA [20].
Data on the risk factors for incisional hernia after gastric cancer surgery are limited and the correlation between the fat area and incisional hernia as a risk factor is understood even less. The aim of this study was to identify the incidence and significant risk factors for extraction site incisional hernia in patients undergoing gastrectomy for gastric cancer, and to analyze the SFA as a risk factor.
Methods
Study population
This was a retrospective review of data gathered prospectively on consecutive patients with gastric cancer, who underwent gastrectomy between 2008 and 2012 at Kyushu University Hospital, Fukuoka, Japan. The patients included in this study had pathologically confirmed gastric cancer and underwent total, distal, proximal, or remnant gastrectomy with open or laparoscopic procedures, with or without other organ resection and after computed tomographic (CT) imaging was performed in our institution within 2 months preoperatively. We included both complicated and noncomplicated surgeries, with noncomplicated surgery defined as straightforward surgery and complicated surgery defined as a procedure with additional organ resection or repair, apart from the gallbladder and spleen, or a procedure with medical or surgical intraoperative complications, or laparoscopic procedures that were converted to open procedures. We excluded patients who underwent an abdominal reoperation unrelated to an incisional hernia within 1-year post-gastrectomy, and those with incomplete data.
Preoperative demographics and anthropometric measurements
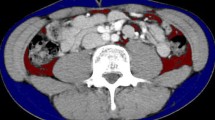
We identified incisional hernia risk factors by reviewing our electronic record database and imaging studies. An expert senior radiologist supervised the analysis of the CT images with CITA Clinical Finder Synapse Vincent volume analyzer version 5.0 (Fujifilm Medical, Tokyo, Japan). SFA and VFA were measured via axial-slice CT scans at three levels: L4 transverse processes, L3 transverse processes, and the L2–L3 intervertebral disc. We used a default setting of − 200 to − 50 HU, with manual adjustment of the boundary inclusion markers, as necessary (Fig. 1).
Specimen extraction and wound closure
During the study period, the extraction site varied as follows: umbilical port wound extension (range, 2.5–5 cm), separate upper median mini-laparotomy incision (range, 4–5 cm), curvilinear periumbilical incision, upper median laparotomy, and left vertical mini-laparotomy incision. Figure 2 shows illustrations of the various specimen extraction sites with incisions highlighted. All gastrectomies were performed or supervised by a single senior surgeon. We closed all incisions with #1 monofilament polydioxanone suture in a simple interrupted pattern through the muscle and fascia simultaneously.
Gastric cancer classification
The classifications of staging and lymph-node dissection are based on the Japanese gastric cancer treatment guidelines 2014 (ver. 4) [21]. Early gastric cancer comprises T1 tumors irrespective of lymph-node metastasis. T1 tumors are confined to the mucosa or submucosa. Any tumors extending beyond that were considered advanced gastric cancer in this study. In this study, lymph-node dissection was divided into D2 and < D2. Based on the same guidelines, D2 lymph-node dissection involved inclusion of all stations 9,11p, and 12a. Non-inclusion of those stations was classified < D2.
Follow-up
For stage I gastric cancer, patients underwent a follow-up medical examination and CT scan every 6 months for the first year postoperatively, and then yearly thereafter. For stage II and III gastric cancer, follow-up involved a medical examination every 3 months for 2 years, and then every 6 months, and CT scan every 6 months. For stage IV gastric cancer, patients underwent a medical examination and CT scan as frequently as every 3–4 months, depending on the attending physician. Post-gastrectomy patients from Kyushu University Hospital who transferred to another hospital for subsequent visits were also followed up, and any postoperative problems such as hernia were reported to our hospital.
Outcome assessment
The primary endpoint of the rate of extraction site incisional hernia was based on CT scans and clinical data, including follow-up reports from other hospitals. The CT scan definition of extraction site incisional hernia was a break in the abdominal fascia with accompanying bulging of the peritoneum containing an organ, bowel, or omentum in axial-slice evaluation at the extraction site, as noted in patients' surgical records.
Statistical analysis
Statistical significance was determined using SPSS software, version 23 (IBM Corp., Armonk, NY, USA). Results are expressed as means ± standard deviation or number (%). Continuous variables were evaluated using the independent-samples t test, and categorical data were compared using the Chi-square test. We used receiver-operating characteristic (ROC) curves to calculate the cut-off values for significant linear variables. Multivariate analysis of risk factors for incisional hernia was performed using binary logistic regression. P < 0.05 denoted significance and multivariate analysis was performed separately for each abdominal measurement level.
Results
A total of 344 patients underwent gastrectomy for gastric cancer in our hospital between 2008 and 2012. After the exclusion of 14 patients who underwent abdominal reoperation unrelated to an incisional hernia within 1 year post-gastrectomy and another 10 with incomplete data, we analyzed data for a final 320 patients. An extraction site incisional hernia developed in 10 (3.1%) of these 320 patients after a mean follow-up period of 11 months (Table 1). We found no significant difference in the duration of follow-up between the non-hernia and hernia groups. The ten extraction site incisional hernias were first diagnosed either by clinical examination followed by CT scan or ultrasound (7/10), or by an incidental CT scan finding as part of gastric cancer surveillance (3/10).
Three of the ten patients were considered to have undergone complicated surgery because of an additional procedure to gastrectomy. The first patient in the complicated group underwent simultaneous thoracoscopic lung resection for a metastatic tumor (skin appendage carcinoma); the second underwent simultaneous hiatal hernia repair, and the third underwent repair of a pre-existing umbilical incisional hernia resulting from previous colorectal surgery. Three of the ten patients with extraction site incisional hernia underwent elective hernia surgery. No incarceration or strangulation occurred. One of the three patients underwent hernia surgery at another hospital where they were being followed up.
Clinicopathological data
Table 2 summarizes the patients' clinicopathological characteristics. The incidence of extraction site incisional hernia was significantly higher with the following risk factors: advanced age (P = 0.046), presence or history of other hernias (inguinal, hiatal, and incisional) (P = 0.009), and higher SFA. All three SFA measurements were significant: both L4 SFA and L2–L3 SFA had P values of 0.007, and L3 SFA had a P value of 0.034. VFA was not a significant risk factor at any of the three measured levels, and the ratio of VFA/SFA for all three levels was also not significant. The total of VFA and SFA at L3 and L2–L3 were significant (P = 0.036 and P = 0.034, respectively).
Surgical outcome data
Table 3 shows the patients' surgical outcome variables. There was a significant difference between the non-hernia and hernia groups for the type of reconstruction (P = 0.045), which favored Roux-en-Y gastrojejunostomy and complicated surgery (P = 0.013). One of seven patients with an intra-abdominal infection had an extraction site incisional hernia, but it was not significant. There was only one recorded case of right upper quadrant cellulitis in a patient with intra-abdominal abscess, but there was no recorded case of extraction site surgical-site infection in the study population.
In this study, the majority of umbilical levels coincided with L4, rather than with L3 or L2–L3, and 90% (287/320) of the population underwent umbilical port extension as the extraction site. In our institution, we extract specimens through a 4-cm umbilical port extension for all types of gastrectomy. Therefore, further analysis and discussion will be focused on L4 SFA. To determine the cut-off point for the significant linear variables of age and L4 SFA, we used an ROC curve (Fig. 3a) identifying a cut-off of 124 cm2 for L4 SFA (sensitivity = 0.800 and 1-specificity = 0.274) and a cut-off of 70.5 years for age (sensitivity = 0.800 and 1-specificity = 0.342) (Fig. 3b). Five significant risk factors were used for the multivariate analysis: age, history of other hernia, reconstruction, complicated/noncomplicated surgery, and L4 SFA. Only age and L4 SFA remained independent risk factors (P = 0.013 and P = 0.004, respectively; Table 4).
Discussion
The risk factors for incisional hernia following cancer surgery have been studied extensively, not only for cosmetic concerns, but because of the complications and added morbidity to patients with existing critical cancer status, as well as their economic burden. Understanding the interrelation of modifiable and nonmodifiable factors can decrease the risk and, ultimately, the incidence of incisional hernia in less-often-studied oncological surgeries, such as gastrectomy for gastric cancer.
In the present study, extraction site incisional hernia developed in 3.1% (10/320) of patients after a mean interval of 11 months postoperatively, which is similar to the rate of 2.8% reported by Jang et al. Jang et al. also reported that the extraction site incisional hernia developed within 12 months postlaparoscopic distal gastrectomy in 93.7% of their patients. Similarly, a 2.7% incidence of incisional hernia was identified in a long-term Lebanese study of laparoscopic sleeve gastrectomy [22]. The mean follow-up of 4.6 years in our study is considered adequate and in accordance with the minimum 3-year recommended incisional hernia follow-up in a prospective multicenter study by Fink et al. [23].
Several risk factors for extraction site incisional hernia have been discussed in the literature. A Cochrane review found that monofilament sutures may reduce the risk of incisional hernia, with a moderate-quality body of evidence [11]. We used this type of suture in our study; however, the exact methods and other details of the incisional closure could not be analyzed because of its retrospective design. A colorectal cancer study compared trans-umbilical versus left lower abdominal incision as extraction sites, and a robotic-assisted laparoscopic radical prostatectomy study compared a vertical supraumbilical incision with an off-midline incision. Both studies concluded that the extraction site did not affect the risk of incisional hernia [24, 25]. Similarly, we found no significant difference between the different extraction sites in our study. Another large-scale study of 4305 abdominal operations showed the following independent risk factors for extraction site incisional hernia: age, sex, BMI, preoperative chemotherapy, increased subcutaneous fat thickness based on CT, elevated wound classification score, intraoperative blood transfusion, midline incision, and incisional surgical-site infection [1], while a Korean laparoscopic distal gastrectomy study listed female sex, higher BMI, and comorbidities as independent risk factors [10]. In our study, univariate analysis revealed five significant risk factors for extraction site incisional hernia: complicated surgery, presence or history of other hernia, reconstruction in favor of Roux-en-Y, age, and L4 SFA.
The cases of complicated surgery in the present study may be attributed to the fact that three patients underwent compound surgeries. Two were related to a concurrent other hernia (hiatal hernia repair in one and repair of a pre-existing umbilical incisional hernia in the other). Pathological studies report a link between abnormal collagen production and/or processing that is probably associated with different types of hernia development [26]. This may also contribute to recurrences such as that in one of our patients, although surgical factors on closure represent the most studied factor for recurrence [27, 28]. In our univariate analysis, reconstruction in favor of Roux-en-Y was a significant factor. A study from our institution by Noshiro et al. in 2003 found that Roux-en-Y anastomosis rather than Billroth I was adopted more often in patients with a high BMI than in the normal-BMI group because of difficulty in reconstruction during laparoscopy-assisted distal gastrectomy for early gastric cancer [29]. At that time, objective measurements of the fat area using CT had not yet been studied extensively. Although this study shows BMI as a nonsignificant variable, our results suggest that Roux-en-Y reconstruction may be related to the visceral or total visceral and subcutaneous fat area; however, larger sample sizes are needed. Perhaps secondary to our small-sample size, complicated surgery and a history of other hernias were not significant variables in our multivariate analysis. Nonetheless, the sample size in our study was large enough to verify age and L4 SFA as independent risk factors after binary logistic regression.
Age ≥ 70.5 years was a significant independent factor for incisional hernia (odds ratio: 9.116, 95% confidence interval 1.581–52.553) in our study. Similarly, age is also an independent risk factor for hernia following colorectal cancer surgery [16] as well as following abdominal surgery in general [1]. Yamamoto et al. identified an almost similar cut-off value of 72 years for incisional hernia among patients undergoing colorectal surgery [12]. The reason for the higher incidence of incisional hernias in older patients could be multifactorial [28]. Advanced age is associated with delayed wound healing and decreased collagen synthesis [1]. Medical comorbidities often associated with older age, such as diabetes, smoking, chronic obstructive pulmonary disease, renal disease, vascular disorders, malnutrition, immunosuppression, and obesity, can also be associated with incisional hernia [28, 30,31,32,33]. Although these comorbidities were not significant risk factors in our study, obesity is expected to increase SFA [34].
In our study, SFA was significant at all measured levels, and L4 SFA (≥ 124 cm2) was specifically an independent risk factor (odds ratio: 13.752, 95% confidence interval 2.290–82.582) for extraction site incisional hernia. This finding is consistent with the report by Itatsu et al. that subcutaneous fat thickness based on CT was a significant factor in incisional hernia development [1]. It also supports the results of Yamada et al. who analyzed both open and laparoscopic colorectal surgery for risk factors of incisional hernia. Both their findings and ours showed age and SFA as significant risk factors and that VFA was not significant. However, Yamada et al. reported a higher incidence of incisional hernia at 7.3% vs. 3.1% in our study. This may be due to a low proportion of cases of open surgery in our study, which Yamada et. al found to be an independently significant risk factor, as well as to our exclusivity on the extraction site [16]. During wound closure, increased SFA may cause mechanical hindrance during fascia closure; therefore, attention should be given to ensuring proper fascia exposure.
SFA alone may be a nonmodifiable factor; however, its relationships with VFA, intra-abdominal infection, and wound closure could be modifiable. In our study, total SFA-VFA was a significant risk factor for incisional hernia. Additionally, Tokunaga et al. described total SFA-VFA as a significant risk factor for postoperative intra-abdominal infection [17]. A retrospective study by Walming et al. of 1621 patients found that wound infection was a risk factor for both dehiscence and incisional hernia [35]. While our study found no record of extraction site surgical-site infection, there were seven patients with intra-abdominal infection, one of whom suffered an incisional hernia. However, intra-abdominal infection was not revealed as a significant variable perhaps because of the small-sample size. Moreover, the retrospective nature of this study precluded detailed wound closure technique and surgical-site infection classification. Studies of the relationships between SFA, total SFA-VFA, detailed wound closure technique, and surgical-site infection classification and prevention are recommended.
Some studies reported VFA as a significant factor for incisional hernia [12], and that an increased VFA is correlated with other risk factors for hernia such as surgical-site infection [17]. However, our results did not show VFA as a significant risk factor for incisional hernia at all three measurement levels. Future studies on the fat area, including the muscle area for sarcopenia assessment, should be conducted on a larger sample size. A systematic review of Kroese et al. on a total of 2986 patients revealed that 15–58% of incisional hernia were detected solely by imaging; hence, ultrasound or CT scanning will result in substantially more incisional hernia diagnoses [36]. The three cases identified on routine follow-up CT scans in the present study prompt us to recommend a low threshold for the request of imaging in patients with suspected asymptomatic incisional hernia.
This study has several limitations. First, it was a retrospective, single-center design, with a relatively small-sample size. The second limitation was the possible underreporting of patients with incisional hernia, especially those followed up at other hospitals. The third limitation was that we excluded preoperative data from CT scans performed at other hospitals because of incompatibilities with the Synapse Vincent software and because complete data were not available for ten patients, which decreased the number of patients included in the analysis.
Conclusion
Age and SFA were independent risk factors for extraction site incisional hernia following gastrectomy for gastric cancer. Strict perioperative care is important for patients with these risk factors.
References
Itatsu K, Yokoyama Y, Sugawara G, Kubota H, Tojima Y, Kurumiya Y, et al. Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg. 2014;101:1439–47.
Eker HH, Hansson BME, Buunen M, Janssen IMC, Pierik REGJM, Hop WC, et al. Laparoscopic vs open incisional hernia repair. JAMA Surg. 2013;148:259–63.
Burger JWA, Luijendijk RW, Hop WCJ, Halm JA, Verdaasdonk EGG, Jeekel J, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–85.
Sauerland S, Walgenbach M, Habermalz B, Seiler CM, Miserez M. Laparoscopic versus open surgical techniques for ventral hernia repair. Cochrane Database Syst Rev. 2009. https://doi.org/10.1002/14651858.CD007781.
Nieuwenhuizen J, Kleinrensink GJ, Hop WCJ, Jeekel J, Lange JF. Indications for incisional hernia repair: an international questionnaire among hernia surgeons. Hernia. 2008;12:223–5.
van ’t Riet M, Steyerberg EW, Nellensteyn J, Bonjer HJ, Jeekel J. Meta-analysis of techniques for closure of midline abdominal incisions. Br J Surg. 2003;89:1350–6.
Kingsnorth A, LeBlanc K. Inguinal and incisional hernias. Lancet. 2003;362:1561–71.
Diener MK, Voss S, Jensen K, Büchler MW, Seiler CM. Elective midline laparotomy closure. Ann Surg. 2010;251:843–56.
Shiroshita H, Inomata M, Bandoh T, Uchida H, Akira S, Hashizume M, et al. Endoscopic surgery in Japan: The 13th national survey (2014–2015) by the Japan Society for Endoscopic Surgery. Asian J Endosc Surg. 2019;12:7–18.
Jang EJ, Kim M-C, Nam S-H. Risk factors for the development of incisional hernia in mini-laparotomy wounds following laparoscopic distal gastrectomy in patients with gastric cancer. J Gastric Cancer. 2018;18:392.
Nelson RL, Patel SV, Steele SR, Paskar DD, Vedula SS. Closure methods for laparotomy incisions for preventing incisional hernias and other wound complications. Cochrane Database Syst Rev. 2017. https://doi.org/10.1002/14651858.CD005661.pub2.
Yamamoto M, Takakura Y, Ikeda S, Itamoto T, Urushihara T, Egi H. Visceral obesity is a significant risk factor for incisional hernia after laparoscopic colorectal surgery: a single-center review. Asian J Endosc Surg. 2018;11:373–7.
Lee L, Abou-Khalil M, Liberman S, Boutros M, Fried GM, Feldman LS. Incidence of incisional hernia in the specimen extraction site for laparoscopic colorectal surgery: systematic review and meta-analysis. Surg Endosc. 2017;31:5083–93.
Muysoms FE, Antoniou SA, Bury K, Campanelli G, Conze J, Cuccurullo D, et al. European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia. 2015;19:1–24.
Alnassar S, Bawahab M, Abdoh A, Guzman R, Al Tuwaijiri T, Louridas G. Incisional hernia postrepair of abdominal aortic occlusive and aneurysmal disease: five-year incidence. Vascular. 2012;20:273–7.
Yamada T, Okabayashi K, Hasegawa H, Tsuruta M, Abe Y, Ishida T, et al. Age, preoperative subcutaneous fat area, and open laparotomy are risk factors for incisional hernia following colorectal cancer surgery. Ann Surg Oncol. 2016;23:236–41.
Tokunaga M, Hiki N, Fukunaga T, Ogura T, Miyata S, Yamaguchi T. Effect of individual fat areas on early surgical outcomes after open gastrectomy for gastric cancer. Br J Surg. 2009;96:496–500.
Kim JH, Kim J, Lee WJ, Seong H, Choi H, Ahn JY, et al. A high visceral-to-subcutaneous fat ratio is an independent predictor of surgical site infection after gastrectomy. J Clin Med. 2019;8:494.
Yoshikawa K, Shimada M, Kurita N, Iwata T, Nishioka M, Morimoto S, et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg Endosc. 2011;25:3825–30.
So R, Matsuo T, Sasai H, Eto M, Tsujimoto T, Saotome K, et al. Best single-slice measurement site for estimating visceral adipose tissue volume after weight loss in obese. Japan men Nutr Metab. 2012;9:1.
Kodera Y, Sano T. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Dakour Aridi H, Alami R, Tamim H, Shamseddine G, Fouani T, Safadi B. Long-term outcomes of laparoscopic sleeve gastrectomy: a Lebanese center experience. Surg Obes Relat Dis. 2016;12:1689–96. https://doi.org/10.1016/j.soard.2015.11.025.
Fink C, Baumann P, Wente MN, Knebel P, Bruckner T, Ulrich A, et al. Incisional hernia rate 3 years after midline laparotomy. Br J Surg. 2014;101:51–4.
Morita Y, Yamaguchi S, Ishii T, Tashiro J, Kondo H, Suzuki A, et al. Does transumbilical incision increase incisional hernia at the extraction site of laparoscopic anterior resection? Am J Surg. 2015;209:1048–52. https://doi.org/10.1016/j.amjsurg.2014.06.023.
Seveso M, Melegari S, Bozzini G, De Francesco O, Mandressi A, Taverna G. Does site of specimen extraction affect incisional hernia rate after robot assisted laparoscopic radical prostatectomy? Int J Surg. 2017;47:96–100. https://doi.org/10.1016/j.ijsu.2017.09.065.
Harrison B, Sanniec K, Janis JE. Collagenopathies—implications for abdominal wall reconstruction. Plast Reconstr Surg Glob Open. 2016;4:e1036.
Radu P, Brãtucu M, Garofil D, Goleanu V, Popa F, Strâmbu V. The role of collagen metabolism in the formation and relapse of incisional hernia. Chirurgia (Bucur). 2015;110(3):224–30.
Caglià P, Tracia A, Borzì L, Amodeo L, Tracia L, Veroux M, et al. Incisional hernia in the elderly: Risk factors and clinical considerations. Int J Surg. 2014;12:S164–S169169.
Noshiro H, Shimizu S, Nagai E, Ohuchida K, Tanaka M. Laparoscopy-assisted distal gastrectomy for early gastric cancer: is it beneficial for patients of heavier weight? Ann Surg. 2003;238:680–5.
Amato B, Compagna R, Fappiano F, Rossi R, Bianco T, Danzi M, et al. Day-surgery inguinal hernia repair in the elderly: single centre experience. BMC Surg. 2013;13:S28.
Kim H, Bruen K, Vargo D. Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg. 2006;192:705–9.
Sauerland S, Korenkov M, Kleinen T, Arndt M, Paul A. Obesity is a risk factor for recurrence after incisional hernia repair. Hernia. 2004;8:42–6.
Fischer JP, Wink JD, Nelson JA, Kovach SJ. Among 1,706 cases of abdominal wall reconstruction, what factors influence the occurrence of major operative complications? Surg (United States). 2014;155:311–9. https://doi.org/10.1016/j.surg.2013.08.014.
Ha TK, Seo YK, Kang BK, Shin J, Ha E. Cardiovascular risk factors in gastric cancer patients decrease 1 year after gastrectomy. Obes Surg. 2016;26:2340–7. https://doi.org/10.1007/s11695-016-2085-4.
Walming S, Angenete E, Block M, Bock D, Gessler B, Haglind E. Retrospective review of risk factors for surgical wound dehiscence and incisional hernia. BMC Surg. 2017;17:1–6. https://doi.org/10.1186/s12893-017-0207-0.
Kroese LF, Sneiders D, Kleinrensink GJ, Muysoms F, Lange JF. Comparing different modalities for the diagnosis of incisional hernia: a systematic review. Hernia. 2018;22:229–42. https://doi.org/10.1007/s10029-017-1725-5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Valencia, S., Shindo, K., Moriyama, T. et al. Subcutaneous fat area as a risk factor for extraction site incisional hernia following gastrectomy for gastric cancer. Surg Today 50, 1418–1426 (2020). https://doi.org/10.1007/s00595-020-02039-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02039-x