Abstract
Introduction
After adopting preoperative assessment of the perigastric vessels using 3D-CT and standardization of the procedures, obesity still influences smooth laparoscopy assisted gastrectomy (LAG). We evaluated the impact of body mass index (BMI) and area of visceral fat tissue on the risks of LAG.
Methods
Sixty-six patients who underwent LAG for gastric cancer were included. The patients were divided into two groups by BMI (<25 BMI-L group: n = 53; ≥25 BMI-H group: n = 13) and area of intraperitoneal fat tissue (<100 cm2 AF-L group: n = 35; ≥100 cm2 AF-H group: n = 31), respectively. Fat scan®, which was computer software operating on abdominal CT, was used to measure the visceral fat areas (VFA). The incidence of postoperative complications, operation time, intraoperative blood loss, and number of dissected lymph nodes were compared between each two groups.
Results
The incidence of postoperative complications of BMI-L and BMI-H groups was 11.3% and 30%, respectively (p = 0.18). The mean blood loss was 85 and 134 g, respectively (p = 0.21). There were no significant differences in operation time and the number of retrieved LNs. The incidence postoperative complications (29%) and mean blood loss (148 g) of then VFA-H group were significantly higher than those of the VFA-L group (5.7%, 48 g). The number of retrieved LNs of the VFA-H group (n = 25) was significantly lower than that of the VFA-L group (n = 34). There was no significant difference in operation time.
Conclusions
In the VFA-H group, the incidence of postoperative complications and intraoperative blood loss increased, and the dissected number of LNs decreased. The area of visceral fat tissue was useful to predict risks of LAG and postoperative complications with higher precision compared with BMI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
A laparoscopically assisted gastrectomy (LAG) is less invasive and has better cosmetic outcome. Moreover, several prospective, randomized, controlled trials have concluded that the long-term and disease-free survival of patients who undergo laparoscopically assisted gastrectomy is similar to that for patients who undergo conventional open surgery. Although the demand for laparoscopically assisted gastrectomy seems to be increasing, laparoscopy for gastric surgery has not been as readily embraced into surgical practice as other procedures, partly because of the requirement for advanced laparoscopic surgical skills and partly because of initial concerns about potential risks of tumor dissemination in malignant neoplasms. These concerns have been addressed in randomized, controlled trials, which have shown that the laparoscopic approach is associated with the same significant short-term benefits without a compromise of oncological long-term outcomes. However, operative time is longer, and the procedures are considered to be technically complicated compared with the open method.
Gastrointestinal surgeons know that the degree of intra-abdominal adhesion or the amount of fat can greatly influence the technical difficulty during abdominal surgery. Body mass index (BMI) has been widely used as an indicator to express the degree of a patient’s obesity. However, BMI does not always properly reflect the degree of a patient’s visceral fat, because the distribution of fat tissue differs greatly between individuals. Complex surgical procedures of gastrectomy involving lymph node dissection are difficult for inexperienced surgeons to perform. Moreover, reports have suggested high incidence of intraoperative and postoperative surgical complications for obese patients, irrespective of the operative methods used. For this reason, some surgeons do not recommend laparoscopy-assisted distal gastrectomy (LADG) for patients with high BMI. Few reports have evaluated the predictive factors for surgical complications of LADG. It is important to evaluate these factors to perform LADG safely.
Several studies have shown that visceral fat areas (VFA) from a single scan obtained at the level of the umbilicus (the approximate level of L4 and L5) closely correlate with the total volume of visceral fat. On the basis of all these findings, we have developed a practical, standardized technique for determining the VFA using a single CT scan, while taking into consideration that the average CT number for fat tissue varies considerably depending on the individual and on the kind of CT scanner used. There is a high morbidity rate during and after surgery in obese patients. Although it is thought that the quick recovery after laparoscopic surgery would benefit obese patients, few data exist that support this conclusion. In this retrospective study, using an originally designed software package to quantify the VFA, we evaluated the impact that the degree of VFA had on technical difficulty during a laparoscopic resection of gastric cancer.
Patients and methods
This investigation was a single-center study. Sixty-six patients who underwent laparoscopic gastrectomy in the Department of Surgery, University of Tokushima, between 2007 and 2009 were investigated.
Prophylactic intravenous administration of cefamezin was performed upon induction of general anesthesia and repeated during surgery if the operative time exceeded 3 h. Water could be taken orally on the next day of surgery. Feeding began 5 days after surgery and started with a low-residue diet, progressing to a regular diet on the following day.
Gastrectomy procedures
The surgical team consisted of an operating surgeon, one assistant surgeon, and one scopist (surgeon). Laparoscopic gastrectomy was treated by LADG and laparoscopic total gastrectomy (LATG).
Quantification of visceral fat area
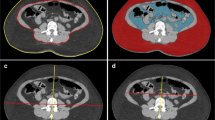
During the past two decades, quantification of VFA has been developed to evaluate visceral adipose tissue accumulation by imaging studies, primarily with computed tomography (CT). In this study, all patients underwent an abdominal CT scan for the preoperative assessment of the extent of disease. The CT scanner is linked to a networked medical imaging system through which images are electronically transferred to a centralized data system and then retrieved at a workstation. Software Fatscan® ver 3.0, N2 system, Japan) enables multiple image rendering and geometric measurements of a specific region with a specified CT number (in Hounsfield units). A single cross-sectional scan at the level of the umbilicus was selected for quantification (Fig. 1A). Adipose tissue was determined by setting the attenuation level within the range of −190 to −30 Hounsfield units, and the acquired image corresponded to the total fat region (Fig. 1B). The region of visceral fat was defined by manual tracing of its contour, and then the total fat region was divided into visceral and subcutaneous fat regions (Fig. 1C). Finally, the VFA was calculated by the software (Fig. 1D).
Quantification of visceral fat area (VFA). A Method for determining abdominal fat distribution on a computed tomography (CT) scan obtained at the umbilicus. B Outlines the intraperitoneal tissue. The line, made with the cursor automatically or manually, outlines the subcutaneous fat layer, in which attenuation is measured. C Histogram of CT numbers (in Hounsfield units) in the lesion outlined in B (mean ± 2 SD). D Within the region outlined in A, tissue with mean attenuation plus or minus 2 standard deviations was regarded as visceral fat tissue. The total fat area was calculated in the region outlining the circumference of the abdominal wall. The visceral fat area was subtracted, and the remainder was regarded as the subcutaneous fat area
Definition of obesity
Patients were classified as obese or nonobese by using both VFA and BMI criteria. We used the definition of visceral obesity as VFA ≥ 100 cm2, because this threshold value has been previously proven to be associated with elevated cardiovascular risk and with a substantial deterioration of metabolic variables predictive of the metabolic syndrome. Accordingly, patients with VFA ≥ 100 cm2 were classified as obese (VFA-H) and the remainder as nonobese (VFA-L). BMI was calculated, and obese patients were defined as those with BMI ≥ 25 kg/m2 (BMI-H), and the remainder as nonobese (BMI-L), in accordance with the criteria of the Japan Society for the Study of Obesity. For both of these definitions, patient and tumor characteristics, intraoperative variables, and postoperative course were compared between obese and nonobese patients.
Statistical analysis
The study endpoints were duration of operation, blood loss, intraoperative and postoperative complications, and the number of the retrieved lymph node. Statistical analysis included one-way ANOVA, Kruskal–Wallis test and, for multiple post hoc comparisons, Tamhane’s T2 test. Dichotomous or categorical endpoints were assessed by using χ2 or Fisher’s exact test as indicated.
Results
Distribution of VFA and BMI
There were 35 (53%) VFA-H patients and 31 (46.9%) VFA-L patients, and there were 13 (19.6%) BMI-H patients and 53 (80.4%) BMI-L patients. Twenty-two (33.3%) BMI-low patients were VFA-high, and none of BMI-H patients were VFA-L. This variation in the distribution of VFA and BMI implies that BMI-defined obesity does not necessarily correspond to visceral obesity. Figure 2 demonstrates images of a “viscerally obese” patient and a “subcutaneously obese” patient with a nearly similar BMI value.
Patient characteristics and tumor stage
Tables 1 and 2 summarize the patient characteristics and tumor stage. There was no significant difference in age and stage between low and high patients defined by VFA or BMI. VFA-H patients included a significantly higher proportion of men, whereas the gender ratio was identical in BMI-L and BMI-H patients.
Intraoperative difficulties
Intraoperative variables used to assess technical difficulty are shown in Figs. 3A–C. Operation time did not differ statistically between low and high patients defined by VFA and BMI (367 vs. 336, 348 vs. 363 min). In contrast, VFA-H patients had a significantly higher estimated blood loss (48 vs. 148 ml, p < 0.05) and lower number of retrieved lymph nodes (34 vs. 25, p < 0.05), but these differences were not recognized in BMI.
Operation time. A There was no significant difference for BMI and VFA. B Estimated blood loss. Although there was no significant difference in BMI, VFA-H group had a much estimated blood loss significantly. C Number of retrieved LNs. Although there was no significant difference in BMI, VFA-H group had a much number of retrieved LNs. The average number of retrieved LNs of VFA-H is over 25
Postoperative complications and mortality
There were 11 postoperative complications in 66 patients, including stasis of remnant stomach in 5 patients, pancreatic juice leakage in 1 patient, wound infection in 2 patients, and pneumonia in 3 patients. The incidence of postoperative complications of BMI-L and BMI-H groups were 11.3 and 30%, respectively (p = 0.18). The incidence of postoperative complications of VFA-L and VFA-H groups was 5.7 and 29%, respectively (p = 0.01).There was no postoperative mortality. No patient required reoperation or readmission within 30 days after discharge.
Discussion
In Japan, dietary changes favoring more western eating habits have resulted in an increased rate of obesity in the population [1]. Previous reports have shown that obese patients have unfavorable surgical outcomes, including longer operative time, increased postoperative complication rate, increased conversion rate, and prolonged hospital stay. In contrast, more recent studies have shown no significant differences between obese and nonobese patients in terms of operative time, conversion rate, and incidence of postoperative complications, and similar or shorter hospital stay in obese patients [2, 3]. The question of whether obesity is a significant risk factor for postoperative morbidity in patients who undergo gastrectomy remains controversial.
BMI has been used as one of the most reliable anthropometric indices of obesity. BMI is simply calculated as weight in kilograms divided by height in meters squared (kg/m2); therefore, it is an easily available, objective value. BMI does not always reflect the degree of visceral fat, because the distribution of tat tissue differs greatly between individuals [2]. BMI is an indicator of obesity, and higher BMI seems to be related to increased morbidity and mortality rates. However, it is calculated simply from the patient’s height and weight and may not accurately reflect the intra-abdominal fat area [4].
Intra-abdominal visceral fat is associated with various obesity-related complications [2]. Although VFA is measured on one cross-sectional scan obtained at the level of the umbilicus, the VFA may reflect the fat volume in the upper abdomen as well as the lower abdomen [5]. Body fat is more likely to accumulate in the abdomen in men, whereas it accumulates more in the subcutaneous area in women [6]. In men, fat is predominantly distributed in the upper body, whereas in women, it is predominant in the hip and thigh areas [2].
In general, surgeons consider laparoscopic surgery difficult for obese patients because of limited visualization of surgical field with cumbersome fat tissue. Thus, surgeons are hesitant about selecting laparoscopic surgery for the corpulent patient [7]. An excess of fatty tissue necessitates more complex lymph node dissection and a large cutting area, which can sometimes be associated with hemorrhaging [8]; as a result, a longer operation time could negatively influence the patient’s surgical outcome [7]. In fact, the JCOG 9501 study, which compared D2 and D2 plus para-aortic LN dissections among gastric cancer patients, identified only obesity and age as significant patient-related factors associated with major surgical complications. Many large-scale studies have demonstrated repeatedly that obesity influences surgical outcomes after gastric cancer surgery [6].
Various reports have indicated that pancreatic fistula frequently occur in obese patients. The reason that patients with high VFA are more likely to develop pancreatic fistula could be due to the surgical difficulty associated with deeper and poorer view of the surgical field as well as the fragile, easily bleeding tissue in the high VFA group. Previous studies have discussed the effect of visceral obesity on surgical difficulty. Excess counter of the pancreas to distinguish the pancreatic tissue from the surrounding fat tissue may increase the chance of injuring the pancreatic tissue during a lymph node dissection [2].
During the LADG, the fat tissue of the intra-abdominal cavity may hinder surgeons from performing extensive lymphadenectomy [9]. The massive and fragile adipose tissue can be easily torn, and this further results in a poor view of the surgical field and more difficulty in achieving precision of the complete procedure [9]. Gastrectomy with D2 lymph node dissection is considered more difficult in obese patients, because intra-abdominal fat, in which the second-level lymph nodes are usually embedded, interferes with dissection [4]. With the exception of subcutaneous fat, an increase in individual fat areas correlated significantly with a decrease in the number of retrieved lymph nodes, indicating that excessive intra-abdominal visceral fat precludes the complete dissection of LN. For complete LN dissection, the surrounding fat tissue needs to be removed and this is more difficult in patients with excessive visceral fat [4]. Because the LNs and major vessels are covered with abundant adipose tissue in obese patients, accurate retrieval of LNs could be more difficult than in nonobese patients [10].
LADG with systemic lymphadenectomy is considered technically more complicated than other laparoscopic procedures, because numerous great vessels must be identified and extensive lymph node dissection is necessary for radical gastrectomy [7]. The anatomy is unclear because of abundant fat tissue, and manipulation of this tissue is difficult [11]. The careful division of the right gastroepiploic vessels was especially important in obese patients, because of the unclear border of the pancreas [11]. In most obese patients, physiological adhesions attributable to excessive adipose tissue make anatomy unclear, particularly around the right gastroepiploic vein. Dissection of physiological adhesion is important for precise lymph node dissection and preventing intraoperative complications in LADG [7].
According to the German Gastric Cancer study, 25 lymph nodes are necessary to obtain valid information about lymph node status [7]. Actually, the number of resected lymph nodes exceeded 25 in the high VFA group. In this study, even for the obese patient, we keep the surgical complete resection. In this study, the 5-year survival rate after curative gastrectomy was better for overweight than non-overweight Japanese patients, especially for early-stage gastric cancer [12]. Surgeons should not hesitate to perform gastrectomy because of obesity.
Conclusions
Although VFA is one constituent of BMI, it better reflects the likelihood of technical difficulties in LAG for gastric cancer than BMI itself. Differences in VFA between patients with and without surgical complications were more significant than differences in BMI. VFA is superior to BMI as a predictive factor for surgical complications.
References
Noshiro H, Shimizu S, Nagai E, Ohuchida K, Tanaka M (2003) Laparoscopy-assisted distal gastrectomy for early gastric cancer. Ann Aurg 238:680–685
Tanaka K, Miyashiro I, Yano M, Kishi K, Motoori M, Seki Y, Noura S, Ohue M, Yamada T, Ohigashi H, Ishikawa O (2009) Accumulation of excess visceral fat is a risk factor for pancreatic fistula formation after total gastrectomy. Ann Surg Oncol 16:1520–1525
Lee JH, Paik YH, Lee JS, Kim CG, Park SR, Kim YW, Kook MC, Nam B, Bae JM (2007) Abdominal shape of gastric cancer patients influences short-term surgical outcomes. Ann Surg Oncol 14(4):1288–1294
Tokunaga M, Hiki N, Fukunaga T, Ohyama S, Yamaguchi T, Nakajima T (2009) Effect of individual fat areas on early surgical outcomes after open gastrectomy for gastric cancer. Br J Surg 96:496–500
Kunisaki C, Makino H, Takagawa R, Sato K, Kawamata M, Kanazawa A, Yamamoto N, Nagano Y, Fujii S, Shimada H (2009) Predictive factors for surgical complications of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc 23:2085–2093
Lee HJ, Kim HH, Kim MC, Ryu SY, Kim W, Hyung WJ, Ryu SW (2009) The impact of a high body mass index on laparoscopy assisted gastrectomy for gastric cancer. Surg Endosc 23:2473–2479
Yamada H, Kojima K, Inokuchi M, Kawano T, Sugihara K (2008) Effect of obesity on technical feasibility and postoperative outcomes of laparoscopy-assisted distal gastrectomy. Comparison with open distal gastrectomy. J Gastrointest Surg 12:997–1004
Makino H, Kunisaki C, Akiyama H, Ono H, Kosaka T, Takagawa R, Nagano Y, Shimada H (2008) Effect of obesity on intraoperative bleeding volume in open gastrectomy with D2 lymph-node dissection for gastric cancer. Patient Saf Surg 2:7
Kim K, Kim M, Jung G, Kim H (2006) The impact of obesity on LADG for early gastric cancer. Gastric Cancer 9:303–307
Hiki N, Fukunaga T, Yamaguchi T, Ogura T, Miyata S, Tokunaga M, Ohyama S, Sano T (2009) Increased fat content and body shape have little effect on the accuracy of lymph node retrieval and blood loss in laparoscopic distal gastrectomy for gastric cancer. J Gastrointest Surg 13:626–633
Yasuda K, Inomata M, Shiraishi N, Kitano S (2004) Laparoscopy-assisted distal gastrectomy for early gastric cancer in obese and nonobese patients. Surg Endosc 18:1253–1256
Tokunaga M, Hiki N, Fukunaga T, Yamaguchi T, Ogura T, Miyata S, Tokunaga M, Ohyama S, Sano T (2009) Better 5-year survival rate following curative gastrectomy in overweight patients. Ann Surg Oncol 16:3245–3251
Disclosures
Dr. Yoshikawa, Dr. Shimada, Dr. Kurita, Dr. Iwata, Dr. Nishioka, Dr. Morimoto, Dr. Miyatani, Dr. Komatsu, Dr. Mikami, Dr. Kashihara have no conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshikawa, K., Shimada, M., Kurita, N. et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg Endosc 25, 3825–3830 (2011). https://doi.org/10.1007/s00464-011-1798-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-1798-7