Abstract
Purpose
Inflammation and the nutritional and immunologic status are known to be associated with the prognosis of malignant tumors. We aimed to examine inflammation–nutrition scores and predict the prognosis of colorectal cancer (CRC) patients by integrating nutritional and immunologic factors and tumor stage.
Methods
This study investigated 511 patients with CRC from 2007 to 2013: 380 in a training set (TS) and 131 in a validation set (VS). The Osaka Prognostic Score (OPS) used comprised 1 point each for C-reactive protein > 1.0 mg/dL, albumin (< 3.5 g/dL), and lymphocyte count < 1600. Patients were classified according to the total points. The modified Glasgow Prognostic Score and the Prognostic Nutritional Index were also examined. A nomogram for predicting the disease-free survival (DFS) and overall survival (OS) was constructed based on the OPS and TNM stage.
Results
In the TS, a high OPS and high TNM stage were significant predictors of the DFS and OS. The C-indexes of the OPS for the DFS and OS were higher than those of other reported scoring systems. The C-index of the nomogram for the DFS was 0.762 in the TS and 0.675 in the VS. The C-index of the nomogram for the OS was 0.805 in the TS and 0.743 in the VS.
Conclusion
Integrating the TNM stage and OPS accurately predicted the prognosis of patients with CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The inflammation, nutritional, and immune statuses are known to be associated with the prognosis of several malignant tumors [1,2,3]. Several prognostic scores have been reported, including the modified Glasgow Prognostic Score (mGPS) [4], the Prognostic Nutritional Index (PNI) [5,6,7], and the Systemic Inflammation Score (SIS) [3, 8]. C-reactive protein (CRP) and albumin are used in the mGPS, albumin and total lymphocyte count (TLC) in the PNI, and the lymphocyte-to-monocyte ratio and albumin in the SIS. Although the three factors of CRP, albumin, and TLC are known to be important indicators of the inflammation, nutritional, and immunologic status, no published scoring system incorporates all three of these variables.
Therefore, in the present study, we aimed to examine reported representative inflammation–nutrition scores, such as mGPS and PNI, and develop a simple prognostic score that incorporates these three key factors.
Although inflammation and nutritional status are very important factors in patients with malignant tumors, treatment for cancer is selected based on the tumor stage [9,10,11]. The combination of the TNM stage and mGPS was reported to stratify the outcomes of patients undergoing curative resection of colorectal cancer (CRC) [12]. However, there is no model to predict the outcomes based on a combination of the TNM stage and inflammation and nutritional status in each patient. Both the tumor status according to the TNM stage and patient status according to the inflammation and nutrition scores are thought to be important. Therefore, both should be taken into account when constructing a model for predicting the outcomes of patients with cancer.
The aim of this study was to examine the TNM stage and inflammation–nutrition scores as a prognostic prediction model. We assessed the correlation of the Osaka Prognostic Score (OPS), a new prognostic score that incorporates CRP and albumin and TLC, with clinicopathological factors and examined its association with the disease-free survival (DFS) and overall survival (OS) rates of patients with all stages of CRC. We also developed new prediction models for the DFS and OS by TNM stage and OPS and verified them in another data set. Combining the tumor status and patient status may be useful for future clinical research on the prognosis of patients with CRC.
Methods
Patients and datasets
For this retrospective study, 670 patients with CRC from 2007 to 2013 were identified from patient records, 159 of whom were excluded because of stage IV disease and/or inadequate data concerning histology and follow-up. All patients had histologically confirmed diagnoses of CRC and had undergone resection of primary CRC. The remaining 511 patients were divided into the following two groups: (i) a training set (TS) of 380 patients who had undergone resection of their primary tumors at the Osaka International Cancer Institute (OICI) from 2007 to 2013; and (ii) a validation set (VS) of 131 patients who had undergone resection at Osaka University from 2011 to 2012. This study was approved by the Institutional Review Boards of OICI and Osaka University.
The OPS
The OPS incorporates CRP, albumin, and TLC. The cut-off values were set as > 1.0 mg/dL for CRP, < 3.5 g/dl for albumin [4], and < 1600 for TLC. The cut-off value for TLC was chosen from the ROC curve for the OS in the TS (Supplementary Fig. 1). Each factor was used only once and allocated a single point. The points were summed and the patients were then divided into four groups according to the score (zero, one, two, and three).
The comparison of inflammation–nutrition scores
The mGPS and PNI were examined. According to a previous report [4], the mGPS score was determined using CRP and albumin as follows: patients with both an elevated CRP (> 1.0 mg/dL) and low albumin (< 3.5 g/dl) level were given an mGPS of 2, those with only an elevated CRP (> 1.0 mg/dL) level were given an mGPS of 1, and those without an elevated CRP (≤ 1.0 mg/dL) level were given an mGPS of 0. The PNI was calculated as 10 × albumin (g/dl) + 0.005 × TLC (/mm3). According to previous reports [5, 6], patients were divided into two groups: high PNI (> 45.5) and low PNI (< 45.5).
The clinicopathological evaluation and follow-up
Data on the age, sex, and pathological findings (e.g., histological grade, tumor invasion, lymph node metastases, lymphatic invasion, and venous invasion) were retrieved from patients’ medical records. The extent of tumor spread was assessed using computed tomography (CT), magnetic resonance imaging (MRI), and/or positron emission tomography (PET). Surgical specimens were fixed in formalin, processed through a graded series of ethanol washes, and embedded in paraffin. The sections were stained with hematoxylin and eosin (H&E) and Elastica van Gieson stain, and the histological grade and degrees of lymphatic and venous invasion were determined. Relevant clinicopathological factors were assessed according to the TNM classification of malignant tumors (Seventh edition, UICC). After surgery, follow-up included assessments of serum concentrations of the tumor markers CEA and carbohydrate antigen 19–9; further imaging with abdominal ultrasonography, CT, chest X-ray, and/or positron emission tomography every 3 to 6 months, and colonoscopy annually in accordance with Japanese guidelines [11]. The DFS was defined as the length of time after primary colorectal cancer surgery had ended that the patients survived without any signs or symptoms of that cancer.
Statistical analyses and the creation of the prediction model
A univariate analysis using a proportional hazards model was performed to identify the independent factors for the DFS and OS after primary curative resection. Two-sided P < 0.05 was considered to denote statistical significance. Kaplan–Meier survival curves were plotted and compared with the generalized log-rank test. All statistical analyses were performed using the JMP 13.0 statistical software program (SAS Institute, Cary, NC, USA). Nomograms were structured as prediction models for the OS by the TNM stage and OPS. Prediction models were structured using the R software program, ver. 3.1.3 (CRAN; the R Foundation for Statistical Computing, Vienna, Austria). All C-indices were calculated using R 3.1.3 (CRAN).
Results
Independent factors influencing the prognosis
The characteristics of all 511 study patients (TS and VS) are listed in Table 1. In the TS, no patients underwent preoperative treatment. In the VS, 11 patients underwent neoadjuvant chemotherapy. In the TS group, univariate and multivariate analyses of clinicopathological factors were performed. Univariate analyses revealed that a low histological grade (P = 0.017), lymphatic invasion (P < 0.001), vascular invasion (P < 0.001), tumor invasion (P < 0.001), lymph node metastasis (P < 0.001), a high CRP level (P < 0.001), and a low albumin level (P < 0.001) were significantly associated with the DFS (Table 2a). Multivariate analyses showed that lymph node metastasis (P < 0.001) and a low albumin level (P = 0.002) were independent prognostic factors for the DFS. Similarly, univariate analyses revealed that vascular invasion (P = 0.038), lymph node metastasis (P = 0.001), a high CRP level (P < 0.001), a low albumin level (P < 0.001), and low TLC (P = 0.002) were significantly associated with the OS (Table 2b). Multivariate analyses showed that lymph node metastasis (P = 0.003), a high CRP level (P = 0.035), a low albumin level (P < 0.001), and low TLC (P = 0.018) were independent prognostic factors for the OS. These results showed that patient’s inflammation, nutritional, and immune statuses were as important for the DFS and OS as the tumor status.
The comparison of the inflammation–nutrition scores
The C-indices of the inflammation–nutrition scores for the DFS and OS were compared (Table 3). The OPS used three factors (TLC, CRP, and albumin), the PNI used two factors (TLC and albumin), and the mGPS used two factors (CRP and albumin). The C-indices of the OPS for the DFS and OS were higher than those of the PNI and mGPS. Furthermore, the OPS nomogram was constructed using all three factors (TLC, CRP, and albumin) to evaluate the weights of continuous variables and their risks (Supplementary Fig. 2). The C-indices of the OPS nomogram for the DFS and OS were slightly higher than the OPS. However, the OPS was simple compared with the OPS nomogram.
A nomogram constructed using the TNM stage and OPS
Given that the TNM stage is defined by tumor invasion, lymph node metastasis and distant metastasis, and the OPS incorporates CRP, albumin, and TLC, it was unsurprising that the TNM stage and OPS were found to be independent prognostic factors for the DFS (Table 4a). Furthermore, the TNM stage and OPS were also independent prognostic factors for the OS (Table 4b). A total of 180 patients had an OPS of 0, 152 an OPS of 1, 36 an OPS of 2, and 9 an OPS of 3. Regarding the stage, 82 patients had Stage I disease, 135 Stage II, and 163 Stage III. Kaplan–Meier curves of the DFS and OS according to the tumor stage and OPS score are shown in Fig. 1. The C-index of the TNM stage and the combination of the OPS and TNM stage are summarized in Table 3. Nomograms for the DFS and OS constructed using the TNM stage and OPS are shown in Fig. 2. The C-index of the nomogram to predict the DFS was 0.762 in the TS group. It was validated in the VS group, with the C-index being 0.675. The C-index of the nomogram to predict the OS was 0.805 in the TS group. It was validated in the VS group, with the C-index being 0.743. The TNM stage was a strong predictor for the DFS, and the OPS was a strong predictor for the OS. We found that the OPS is an independent risk factor in all patients with CRC, and the combination of the TNM stage and OPS can predict the prognosis of CRC patients more accurately than only the TNM stage or OPS, especially the OS.
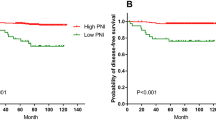
Survival curves for the disease-free survival (DFS) and overall survival (OS) by the TNM stage and Osaka Prognostic Score (OPS). a DFS curves by TNM stage (left) and OPS score (right). The 5-year DFS rate was 99% (n = 82) in patients with Stage I disease, 91% (n = 135) in those with Stage II, and 71% (n = 163) in those with Stage III. The 5-year DFS rate was 88% (n = 183) in those with an OPS of 0, 85% (n = 152) in those with an OPS of 1, 70% (n = 36) in those with an OPS of 2, and 50% (n = 9) in those with an OPS of 3. (b) OS curves by TNM stage (left) and OPS score (right). The 5-year OS rate was 94% (n = 82) in patients with Stage I disease, 94% (n = 135) in those with Stage II, and 84% (n = 163) in those with Stage III. The 5-year DFS rate was 98% (n = 183) in those with an OPS of 0, 90% (n = 152) in those with an OPS of 1, 63% (n = 36) in those with an OPS of 2, and 44% (n = 9) in those with an OPS of 3
Nomogram for predicting the disease-free survival (DFS) and overall survival (OS) after resection of colorectal cancer. (a, b) These nomograms were developed using the TNM stage and OPS to predict the DFS (a) and OS (b). The sum of these variable points was plotted on the total point axis, and the estimated OS rate was obtained by drawing a vertical line from the plotted total point axis down to the predicted probability axis
Discussion
In the present study, we detected a correlation between the OPS, which is a new, simple inflammation and nutrition scoring system, and the DFS and OS of patients with CRC. The C-indices of the OPS for the DFS and OS were higher than those of the PNI and mGPS in our data set, indicating that the OPS could serve as a useful prognostic indicator in patients with CRC. We used CRP, albumin, and TLC to reflect patient’s inflammation, nutritional, and immune statuses, respectively. According to our subgroup analyses, in patients with Stage III disease, a high OPS was associated with a poor prognosis (Supplementary Table 1) and was not correlated with adjuvant chemotherapy (Supplementary Table 2). While the OPS is very simple, the weights of continuous variables and their risks may not be reflected. Therefore, we compared the OPS with the nomogram constructed using CRP, albumin, and TLC. The C-indices were almost the same, suggesting that this simple scoring system fully reflected the importance of these three factors.
Inflammatory cytokines associated with malignant tumors have been reported to promote cancer proliferation and metastasis [13, 14]. The preoperative immune–nutritional status as indicated by the CRP, albumin, and TLC values may reflect the tumor status. The immune–nutritional status was also a predictor of the DFS. Immunotherapy has recently become widely performed, and the immune status of the host is thought to be related to the success rate of this treatment [15]. Further research will be required to explore the relationship between a patient’s immune–nutritional status and tumor immune–inflammation status.
Of note, the TNM stage, which is the most important prognostic factor for various cancers, was incorporated into our prediction model using a nomogram. Combining the TNM stage representing the tumor status and the OPS representing the patient’s overall condition enabled the more accurate prediction of the prognosis of patients with CRC than has been achieved using either indicator alone. Our findings indicate that both elements are important for predicting the prognosis of patients with CRC and that both should be considered when selecting treatment.
In the present study, we used a logistic regression model to develop a nomogram for predicting the prognosis [16]. Nomograms have been used to score and predict individual patients’ prognosis and risk [17, 18], and they should be more useful in identifying risks in each patient than the common classification such as TNM stage and NCCN classification. We clearly showed that the tumor status, such as that determined using the TNM stage, was a strong predictor for the DFS, and the patient’s immune–nutritional status, such as that determined using the OPS, was a strong predictor for the OS. The TNM stage is useful for predicting the DFS because it reflects the malignancy of cancer well, and the immune–nutritional status is considered to be useful for predicting the OS because it reflects the general condition of the patient.
However, this study has some limitations. First, it was a retrospective study. Second, the prediction models were validated using data from patients attending a single institute. More patients and multi-institutional studies are necessary to evaluate these models fully. However, for the time being, the prediction models we have developed will help identify high-risk patients with CRC. Careful follow-up, adjuvant chemotherapy, and/or interventions for improving the immune–nutritional status of high-risk patients with CRC may improve their prognosis.
Conclusions
We herein report a novel and reliable prognostic scoring system, the OPS, and a nomogram. Combining the TNM stage and OPS was useful for predicting an individual’s prognosis with high accuracy.
Abbreviations
- CRC:
-
Colorectal cancer
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- TNM stage:
-
Tumor node metastasis stage
- mGPS:
-
Modified Glasgow Prognostic Score
- PNI:
-
Prognostic Nutritional Index
- CRP:
-
C-reactive protein
- TLC:
-
Total lymphocyte counts
- OPS:
-
Osaka Prognostic Score
- TS:
-
Training set
- VS:
-
Validation set
References
Pan QX, Su ZJ, Zhang JH, Wang CR, Ke SY. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. Onco Targets Ther. 2015;8:1375–85. https://doi.org/10.2147/OTT.S82437.
Takeuchi H, Kawanaka H, Fukuyama S, Kubo N, Hiroshige S, Yano T. Comparison of the prognostic values of preoperative inflammation-based parameters in patients with breast cancer. PLoS ONE. 2017;12(5):e0177137. https://doi.org/10.1371/journal.pone.0177137.
Suzuki Y, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kondo T, et al. Comparison of preoperative inflammation-based prognostic scores in patients with colorectal cancer. Ann Surg. 2018;267(3):527–31. https://doi.org/10.1097/SLA.0000000000002115.
Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'Reilly DS, Foulis AK, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104(4):726–34. https://doi.org/10.1038/sj.bjc.6606087.
Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532–5. https://doi.org/10.1007/s00595-011-0061-0.
Park BK, Park JW, Han EC, Ryoo SB, Han SW, Kim TY, et al. Systemic inflammatory markers as prognostic factors in stage IIA colorectal cancer. J Surg Oncol. 2016;114(2):216–21. https://doi.org/10.1002/jso.24299.
Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20(8):2647–54. https://doi.org/10.1245/s10434-013-2926-5.
Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113(4):626–33. https://doi.org/10.1038/bjc.2015.241.
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, Group EGW. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–9. https://doi.org/10.1093/annonc/mdu260.
Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–72. https://doi.org/10.1093/annonc/mdt354.
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23(1):1–34. https://doi.org/10.1007/s10147-017-1101-6.
Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg. 2016;263(2):326–36. https://doi.org/10.1097/SLA.0000000000001122.
Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55(1):115–22. https://doi.org/10.1136/gut.2004.047100.
Yeung TM, Buskens C, Wang LM, Mortensen NJ, Bodmer WF. Myofibroblast activation in colorectal cancer lymph node metastases. Br J Cancer. 2013;108(10):2106–15. https://doi.org/10.1038/bjc.2013.209.
Blank CU, Haanen JB, Ribas A, Schumacher TN. CANCER IMMUNOLOGY. The "cancer immunogram". Science. 2016;352(6286):658–60.
Miyoshi N, Ohue M, Yasui M, Noura S, Shingai T, Sugimura K, et al. Novel prognostic prediction models for patients with stage IV colorectal cancer after concurrent curative resection. ESMO Open. 2016;1(3):e000052. https://doi.org/10.1136/esmoopen-2016-000052.
Yang P, Qiu J, Li J, Wu D, Wan X, Lau WY, et al. Nomograms for pre- and postoperative prediction of long-term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg. 2016;263(4):778–86. https://doi.org/10.1097/SLA.0000000000001339.
Fujino S, Miyoshi N, Ohue M, Yasui M, Sugimura K, Akita H, et al. A nomogram for predicting lymph node metastasis in submucosal colorectal cancer. Int Surg. 2017;102(102–8):16.
Acknowledgements
The authors would like to thank Ms. Aya Ito for special technical assistance and Dr Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This work was supported in part by a grant-in-aid for scientific research (C, 41040220, 17K16542) and a 38th Japan Medical Woman’s Association Academic Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujino, S., Myoshi, N., Saso, K. et al. The inflammation–nutrition score supports the prognostic prediction of the TNM stage for colorectal cancer patients after curative resection. Surg Today 50, 163–170 (2020). https://doi.org/10.1007/s00595-019-01861-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-01861-2