Abstract
Aims
The aim of our study was to estimate the overall rate of first hospitalizations for diabetic foot (DF) regardless of the outcome in amputations, as well as the mortality rate with their determinants in the period 2012–2016 in Piedmont Region in Italy.
Methods
The study included all the subjects registered in the Regional Diabetes Registry and alive as at January 1, 2012. DF cases were identified by record linkage with the regional hospital discharge database. Incident cases of diabetic foot were followed up for mortality.
Results
The 5-year rates were 1762, 324, and 343 × 100,000 patients for first hospitalization without amputations, with major amputations, and with minor amputations, respectively. Patients not undergoing amputations were more than 70% of the cohort. Patients with the more severe stages of diabetes and those with low education were at higher risk of each type of hospitalization. The risk of death during a mean follow-up of 2.5 years was about 16, 18, and 30% among patients without amputations, with major amputations, and with minor amputations, respectively. Males, insulin-treated patients, those affected with severe diabetes complications, particularly on dialysis, and those with lower levels of education were at higher risk.
Conclusions
The heavier burden of DF on hospitalizations is due to cases without amputation, a condition that is seldom considered in the diabetes literature. The severity of diabetes, preexisting complications, and low educational levels are associated with both first hospitalization and subsequent survival at any level of severity of DF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcers as well as both major and minor amputations represent the main outcomes in the diabetic foot (DF) syndrome [1,2,3,4,5,6].
DF ulcerations are characterized by high recurrence: About 40% of the patients with a healed ulcer have a recurrence within 1 year, whereas 65% at 5 years [1].
The presence of DF ulcerations is characterized by poor prognosis: 85% of amputations stem from an ulcer [7].
Notably, the presence of an ulcer in diabetic patients is considered an independent risk factor for cardiovascular mortality at 10 years [8, 9], and in patients with a new ulcer, the 5-year survival has been calculated to be 50–60% that represents a lower life expectancy than that in some common neoplasms.
The amputation rate cannot be considered as good marker of the quality of clinical care as, theoretically, the most appropriate end point would be the complete healing without amputation, even if this result is in many cases difficult to achieve [10].
Although if it is relatively simple to get from the hospital discharge sheets (HDSs) the number of hospital admissions related to major and minor amputations, the amount of admissions due to DF that do not result in amputation is difficult to calculate, and it is even more difficult to describe the polymorphic vascular/neurological/infective components typical of the ulcerative diseases and of the diabetic neuroarthropathy.
The principal objective of this study was to estimate, in a large Italian Region, the overall rate of first hospitalizations for DF regardless of the outcome in amputations, the mortality rate following the hospitalization, and their determinants in the period 2012–2016.
Research design and methods
Study population
The study included all the subjects registered as affected by diabetes mellitus in the Regional Diabetes Registry (RDR) of the Piedmont Region (4,400,000 inhabitants in Northwestern Italy) and alive as at January 1, 2012. RDR records all subjects who obtained exemption from payment of drugs, diabetes devices, and laboratory testing due to a confirmed diagnosis of diabetes mellitus [11].
The type of diabetes was retrieved from the RDR. The severity of the disease was estimated from the prescribed therapy as well as from the presence of cardiovascular disease (CVD) or chronic kidney failure (CKF) [12, 13]. Data on therapy were retrieved via record linkage with the regional drug prescriptions database. Treatment was categorized as: no drug therapy, oral antidiabetics (separately secretagogue or not secretagogue drugs), and insulin (separately short acting or long acting). Patients prescribed both insulin and oral antidiabetics were included in the “insulin treatment” category. A patient was defined as being on therapy if he/she had filled at least two drug prescriptions in the previous 12 months [12]. Data on existing cardiovascular disease (CVD) were obtained by record linkage with the regional hospital discharge database, which contains data on all hospitalizations of Piedmont residents wherever they may have been hospitalized in Italy. All patients discharged from hospital in the previous 5 years with a primary or secondary diagnosis of coronary heart disease (ICD 9-CM: 410–414) or cerebrovascular disease (ICD 9-CM: 430–438) were defined as having CVD. Similarly, we considered as affected from CKF all the patients who had a dialytic treatment in the previous 5 years [12].
Educational level for each individual was obtained by record linkage with the 2011 National Census and was classified into three levels: high (university or high school), medium (middle school), and low (primary school or no formal education).
Diabetic foot definition and classification
DF cases were identified through a deterministic record linkage between the cohort of persons with diabetes and the regional hospital discharge database. Hospital discharges with DRGs (24th version) or ICD-9-CM codes reported in Table 1 were considered as having a DF, classified according to three different levels of severity: hospitalization without amputations, with minor (below ankle) amputations, and with major (above ankle) amputations [13].
Individuals without a previous hospitalization for the causes reported in Table 1 during the period 2007–2011 were considered as incident cases and were included in the study from January 1, 2012, to December 31, 2016. Patients who were classified in more than one level of severity were included separately in every level (i.e., a patient with an evolving level of severity can be included in different levels of severity, therefore contributing to different levels of incidence).
Outcomes
Patients considered as incident cases of DF were followed up for mortality. Information on death (or transfer out of Piedmont) was obtained by record linkage with the Unique Regional Archive of residents covered by the regional health system.
Statistical analysis
The crude (cumulative) incidence of DF was calculated by dividing the number of patients with diabetes classified in the three levels of severity by the total number of residents in Piedmont in the period 2012–2016. Incidence was standardized by age using Poisson regression, and 95% confidence intervals were calculated.
The start of follow-up was defined as the date of incidence (i.e., the first hospital admission) and ended at the date of death or transfer out of Piedmont or December 31, 2016, whichever came first. We defined as lost to follow-up patients who moved out of the region during the study period. Days of follow-up were calculated as the difference between the incidence date and the date of the event under study, loss to follow-up, death, or December 31, 2016. Cox regression was used to estimate adjusted hazard ratios (HR), after testing for proportionality of risks, and 95% confidence intervals were calculated. Statistical analyses were performed using SAS version 9.4 and STATA version 13.
Results
As of January 1, 2012, we identified 246,877 residents in Piedmont with diagnosed diabetes. Within this cohort, from 2012 to 2016, we identified 5999 incident cases (i.e., first hospitalization) of DF: 848 cases of major lower limb amputations (65.8% men), 801 of minor amputations (69.8% men), and 4350 without amputation (61.3% men), thus resulting in a 5-year crude incidence of, respectively, 343.5 × 100,000 (IC 95% 320.4–366.6), 324.4 × 100,000 (IC 95% 302–347), and 1762.1 × 100,000 (IC 95% 1709.7–1814.4). Table 2 reports the 5-year, age-adjusted, cumulative incidence of DF by selected clinical characteristics and educational level in the two genders. Among men, considering the three levels of severity together, the incidence of DF complication was 3102 × 100,000, i.e., 3% 5-year chance of developing this complication. As expected, the incidence was much higher for DF without amputation compared to the two other more severe conditions. Incidence was higher for type 1 diabetes, but the difference with type 2 was significantly different only for major amputations. Incidence increased with increasing severity of the disease as detected either by therapy, or previous cardiovascular disease or dialytic treatment: DF without amputations was nearly five times higher among insulin-treated patients compared to those with no drug therapies, and this difference rose to ten times when considering major amputations. The highest incidence of DF, for each of the three levels of severity, was recorded among patients undergoing dialytic treatment. DF complications were higher among low-educated patients, particularly for amputations (both minor and major).
Incidence among women was lower than among men, being 1775 × 100,000 (three levels together), i.e., 1.7% 5-year chance of developing a DF. No statistically significant difference was evident between types of diabetes, while, similarly to men, the incidence of the complications rose with the severity of the disease, being the highest in insulin-treated patients and in those on dialysis, for whom the risk of major amputations was more than twenty times higher than that in patients not on dialysis. Differently from men, the incidence of DF was higher among low-educated women only in the “no amputation” group, while no differences were evident in case of amputations.
During the 2.5-year mean period of follow-up from the onset of a DF complication, 1121 deaths occurred. Not surprisingly, mortality increased with increasing severity of DF, being 165.5 × 1000 in patients without amputations, 183.5 × 1000 in patiets with minor amputations, and 299.0 × 1000 in those with major amputations. The results of Cox models for the three different levels of severity of DF are shown in Table 3. Due to the small number of cases, diabetes types other than 1 and 2 have been excluded from the analysis, while different levels of therapy have been grouped in “none”, “oral therapy”, and “insulin”. HRs were also adjusted for local health unit of residence of patients to take into account the role of geographical variability due to different access to specialist care [14]. After the onset of a DF complication, mortality was higher in men compared to women, being double in case of amputations (both minor and major), and increased with increasing age, in particular for major amputations: Patients aged 80 or more were more than four and a half times likely to die if affected by a major amputation than patients below 60 years old. The risk of death did not differ by type of diabetes, while was strongly associated with the severity of the disease: The risk was higher in insulin-treated patients, in those with cardiovascular disease, and in patients undergoing dialytic treatment. Educational level was inversely related to mortality, and this association was stronger in the more severe levels of the complication: Among patients suffering from amputations, the risk of death in low-educated patients was nearly double than that in high-educated ones.
Discussion
In this study, based on 250,000 individuals with diabetes, we report that the 5-year incidence of hospitalization for DF without amputations is 1762 × 100,000 patients, is 324 × 100,000 patients for hospitalization with major amputations, and is 343 × 100,000 for hospitalization with minor amputations. Patients affected with the more severe stages of diabetes as well as those with low education were at higher risk within each of the three types of hospitalization.
We also showed that the prognosis after the onset of DF complications worsens with the worsening of the severity of DF. The risk of death during a mean follow-up of two and a half years was about 16% among patients without amputations, 18% among patients with minor amputations, and 30% among those with major amputations. Other than age, the risk of death was higher in men, in insulin-treated patients, in those affected with severe diabetes complications, particularly on dialysis, and in those with lower levels of education.
Even if the comparisons of our data with the other studies available in the literature are difficult because of different populations, length of observation time, and, for the cases of DF without amputations, the paucity of published data, our results show lower incidence of DF and higher survival than that previously reported by other authors [7, 15,16,17].
In England, the incidence of amputations in diabetic patients from 2007 to 2010 was 2.51 per 1000 persons/year with a relative risk of 23.3 compared to non-diabetic subjects [18]. The rate of minor amputations in a sample of 1232 diabetic subjects with DFU from the Eurodiale prospective cohort study reaches 18% in a single year of observation, with a wide variation from 2.4 to 34% depending on the DF center [19]. In Italy, from 2001 to 2010, the standardized discharge rate for amputation in the hospitalized diabetic population increased from 12 to 13.3 (per 100,000). The trend was substantially stable over time, with a slight reduction in the major amputations and a growing trend in minor amputations [20].
In the OECD countries (that included Italy), the amputation rates in diabetes patients during the period 2000–2011 underwent a progressive decrease of − 0.27 per 1000.000 per year [21]. However, surprisingly, in the USA, after two decades of decline in non-traumatic lower-extremity amputations in adults with diabetes, a reversal in trend was observed from 2009. The increase in hospitalizations for non-traumatic lower-extremity amputations is driven by a 62% increment of the rate of minor amputations and a 29% of major amputations, particularly in young- and middle-aged adults [22].
Minor amputations may indicate a better quality of care as being an intervention potentially able to prevent from major ones and hence to salvage lower extremities.
As stated by Jeffcoate and Harding, amputation is a marker not just of disease, but also of disease management [15]. In a long follow-up of 82.6 ± 26.5 months, Giurato et al. demonstrated that using a limb salvage protocol—shared by many centers in Italy—is not just a temporary solution, but can change the patient’s life, with long-term benefits [23].
In our study, in the period 2012–2016, more than 70% out of almost 6000 registered admissions for DF were not accompanied by a surgical amputation procedure.
It is common in clinical practice to detect repeated hospitalizations for subsequent treatment phases (for example, percutaneous revascularization, treatment of infection, surgical debridement, amputation, aggravation with more proximal amputation).
Therefore, the hospitalizations without amputation can be the result of either effective treatments (medical and surgical), conservative choices justified by the general conditions of the patient or decisions taken by the patient himself. It could also be the result of hospitalizations that have not completed the therapeutic procedure by discharging patients simply after stabilization of the clinical picture, and postponing amputation to another hospitalization, a conservative approach that is not always appropriate.
The high number of hospitalizations without amputation could probably be reduced, with a decrease in costs for the National Health Service, through the full enhancement and/or strengthening of multi-specialist/professional human resources dedicated to the problem of DF, but, above all, through the application of diagnostic and therapeutic flowcharts.
Consistently with other studies [16, 24], our data show gender-related differences with an higher incidence of hospitalizations for DF in males.
Type 1 diabetes affected 7.9% of our cohort. Despite the differences in the etiopathogenesis, treatment, and duration of the disease, the type of diabetes did not seem to influence the incidence of hospitalizations.
As far as therapy is concerned, the increase in its complexity from diet to multi-injective basal-bolus therapy, theoretically reflecting the complexity and severity of the disease, was associated with an higher incidence of hospitalizations in both sexes.
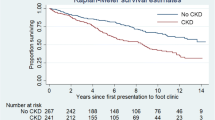
Dialysis is known to be an independent risk factor for foot ulcer, its healing, and amputation [24, 25]; the increased risk of DF in the presence of cardiovascular disease or dialysis treatment, therefore, is not surprising, given that these are indicators of systemic vascular damage [9, 10]. In Piedmont, in line to other studies, the incidence of minor amputations in dialyzed patients is tenfold higher in both sexes, while for major amputations is tenfold in men and even twenty times in women, further confirming the strongly negative effect of dialysis on the prognosis of patients with DF [26].
The higher incidence of DF in patients with lower levels of education calls into question of the complexity of diabetic disease management. In many countries, including Italy, unhealthy behavior, non-adherence to treatment, and difficulty in accessing treatment are more frequent in socially disadvantaged groups, and therefore, social differences in the onset of complications are not a surprise [27, 28].
As for mortality, the risk at 2.5 years from hospitalization for DF ranges from 16% in the absence of amputations to 30% in the presence of greater amputations. Mortality was higher for men in all three classes of disease severity, with a risk doubled in groups undergoing amputation. Unlike other studies, these values are lower than those observed in other series of patients with DF [9, 10, 16, 17].
Mortality progressively increases with age in all the groups, but the increase is more evident in those with major amputations: There, the risk is almost five times higher in subjects aged > 80 years compared to those < 60. Data can reflect both a greater severity of the pathology and the choice of more definitive surgical procedures in conditions where effective walking rehabilitation is not possible (bedridden patients, severe obesity, etc.). Minor amputation could select patients with a more favorable prognosis in which a more conservative choice was made, regardless of age.
As for the incidence, the type of diabetes does not change the risk of death, while insulin therapy or previous cardiovascular diseases [29] are accompanied by an increased risk of mortality in all the three classes.
The risk of death in patients on dialysis who have undergone minor and major amputation is ten times higher than for non-dialyzed patients, a data higher than that already registered in other studies [29, 30].
As with the incidence of DF, high schooling, even if only of medium level, has a protective effect against mortality in all three classes of severity.
The main strength of our work is that, to our knowledge, it is the first population-based study (i.e., not from selected clinical cohorts) to report data on incidence of DF by different levels of severity (including patients without amputations) and their mortality after the onset of the complication.
However, our study has also some limitations: We do not have any information about some relevant clinical data as HbA1c levels or duration of diabetes, which can be only partially approximated by the type of therapy or comorbidities. Furthermore, data on behavioral risk factors, such as body mass index or physical inactivity, are not available from administrative database. However, all our analyses were either stratified or adjusted for educational level that is a reasonable proxy of unhealthy life styles (27). Finally, an underestimation of minor amputations is certain, as these are often performed in an outpatient setting and therefore not recorded as interventions during hospitalization.
Conclusions
Our work confirms that the presence of a DF weighs on mortality and highlights the worsening and prognostically unfavorable role of cardiovascular disease, dialysis, as well as of low social position.
Notably, our study also focuses on hospitalizations without amputation, a quite common condition that has been so far not adequately analyzed in the available literature. Future studies should better define the meaning of this heterogeneous group.
References
Armstrong DG, Boulton AJ, Bus SA (2017) Diabetic foot ulcers and their recurrence. N Engl J Med 376:2367–2375
Abbott CA, Carrington AL, Ashe H et al (2002) The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 19:377–384
Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y (2017) Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 49:106–116
Boyko EJ, Ahroni JH, Cohen V, Nelson KM, Heagerty PJ (2006) Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care 29:1202–1207
Stoekenbroek RM, Lokin JLC, Nielen MM, Stroes ESG, Koelemay MJW (2017) How common are foot problems among individuals with diabetes? Diabetic foot ulcers in the Dutch population. Diabetologia 60:1271–1275
Margolis DJ, Jeffcoate W (2013) Epidemiology of foot ulceration and amputation: can global variation be explained? Med Clin North Am 97:791–805
Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J (2005) The global burden of diabetic foot disease. Lancet 366:1719–1724
Morbach S, Furchert H, Gröblinghoff U et al (2012) Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care 35:2021–2027
Iversen MM, Tell GS, Riise T et al (2009) History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trøndelag Health Study, Norway. Diabetes Care 32:2193–2199
Moxey PW, Gogalniceanu P, Hinchliffe RJ et al (2011) Lower extremity amputations—a review of global variability in incidence. Diabet Med 28:1144–1153
Gnavi R, Karaghiosoff L, Dalmasso M, Bruno G (2006) Validazione dell’archivio di esenzione per diabete della Regione Piemonte: vantaggi e limiti per un uso epidemiologico. Epidemiol Prev 30:59–64
Giorda C, Carnà P, Salomone M et al (2018) Ten-year comparative analysis of incidence, prognosis and associated factors for dialysis and renal transplantation in type 1 and type 2 diabetes versus non-diabetes. Acta Diabetol 55:733–740
Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ, Schaper NC (2016) The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev 32:2–6
Giorda C, Petrelli A, Gnavi R, the Regional Board for Diabetes Care of Piemonte (2006) The impact of second-level specialised care on hospitalisation among persons with diabetes: a multilevel population based study. Diabet Med 23:377–383
Jeffcoate WJ, Harding KG (2003) Diabetic foot ulcers. Lancet 361:1545–1551
Martins-Mendes D, Monteiro-Soares M, Boyko EJ et al (2014) The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complicat 28:632–638
Brennan MB, Hessa TM, Bartle B et al (2017) Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complicat 31:556–561
Holman N, Young RJ, Jeffcoate WJ (2012) Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia 55:1919–1925
van Battum P, Schaper N, Prompers L et al (2011) Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diabet Med 28:199–205
Lombardo FL, Maggini M, De Bellis A, Seghieri G, Anichini R (2014) Lower extremity amputations in persons with and without diabetes in Italy: 2001–2010. PLoS ONE 9:e86405
Carinci F, Massi Benedetti M, Klazinga NS, Uccioli L (2016) Lower extremity amputation rates in people with diabetes as an indicator of health systems performance. A critical appraisal of the data collection 2000-2011 by the Organization for Economic Cooperation and Development (OECD). Acta Diabetol 53:825–832
Geiss LS, Li Y, Hora I, Albright A, Rolka D, Gregg EW (2019) Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care 42:50–54
Giurato L, Vainieri E, Meloni M et al (2015) Limb salvage in patients with diabetes is not a temporary solution but a life changing procedure. Diabetes Care 38:e156–e157
Pscherer S, Dippel F-W, Lauterbach S, Kostev K (2012) Amputation rate and risk factors in type 2 patients with diabetic foot syndrome under real-life conditions in Germany. Primary Care Diabetes 6:241–246
Ndip A, Lavery LA, Boulton AJ (2010) Diabetic foot disease in people with advanced nephropathy and those on renal dialysis. Curr Diab Rep 10:283–290
Meloni M, Giurato L, Izzo V et al (2016) Long term outcomes of diabetic haemodialysis patients with critical limb ischemia and foot ulcer. Diabetes Res Clin Pract 116:117–122
Piccinelli C, Carnà P, Stringhini S et al (2018) The contribution of behavioural and metabolic risk factors to socioeconomic inequalities in mortality: the Italian Longitudinal Study. Int J Public Health 63:325–335
Petrelli A, De Luca G, Landriscina T, Costa G, Gnavi R (2018) Effect of socioeconomic status on surgery waiting times and mortality after hip fractures in Italy. J Health Qual 40:209–216
Faglia E, Clerici G, Clerissi J et al (2006) Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: data of a cohort study of 564 patients. Eur J Vasc Endovasc Surg 32:484–490
Meloni M, Izzo V, Giurato L, Cervelli V, Gandini R, Uccioli L (2018) Impact of heart failure and dialysis in the prognosis of diabetic patients with ischemic foot ulcers. J Clin Transl Endocrinol 11:31–35
Author information
Authors and Affiliations
Contributions
ML and GR conceived of and guided the analysis and wrote the manuscript. CP and GR conducted the analysis. BF, GC, CP and BG contributed to the data collection and reviewed and edited the manuscript. ML and GR are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Ethical standard
This is an observational study and data were collected retrospectively. All data were anonymously linked and no personal identifiers were available to researchers. No ethical approval was required according to Italian law 211/2003, which explains that no ethics committee permission is required for this kind of study.
Informed consent
Data were drawn from anonymous regional database and informed consent was impossible to obtain. According to Italian privacy law, no patient’s or relative’s consent is required for large retrospective population-based studies and if data are published only in aggregated form.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monge, L., Gnavi, R., Carnà, P. et al. Incidence of hospitalization and mortality in patients with diabetic foot regardless of amputation: a population study. Acta Diabetol 57, 221–228 (2020). https://doi.org/10.1007/s00592-019-01412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01412-8