Abstract
Aim
To assess the performance of HbA1c and the 1-h plasma glucose (PG ≥ 155 mg/dl; 8.6 mmol/l) in identifying dysglycemia based on the oral glucose tolerance test (OGTT) from a real-world clinical care setting.
Methods
This was a diagnostic test accuracy study. For this analysis, we tested the HbA1c diagnostic criteria advocated by the American Diabetes Association (ADA 5.7–6.4 %) and International Expert Committee (IEC 6.0–6.4 %) against conventional OGTT criteria. We also tested the utility of 1-h PG ≥ mg/dl; 8.6 mmol/l. Prediabetes was defined according to ADA-OGTT guidelines. Spearman correlation tests were used to determine the relationships between HbA1c, 1-h PG with fasting, 2-h PG and indices of insulin sensitivity and β-cell function. The levels of agreement between diagnostic methods were ascertained using Cohen’s kappa coefficient (Κ). Receiver operating characteristic (ROC) curve was used to analyze the performance of the HbA1c and 1-h PG test in identifying prediabetes considering OGTT as reference diagnostic criteria. The diagnostic properties of different HbA1c thresholds were contrasted by determining sensitivity, specificity and likelihood ratios (LR).
Results
Of the 212 high-risk individuals, 70 (33 %) were identified with prediabetes, and 1-h PG showed a stronger association with 2-h PG, insulin sensitivity index, and β-cell function than HbA1c (P < 0.05). Furthermore, the level of agreement between 1-h PG ≥ 155 mg/dl (8.6 mmol/l) and the OGTT (Κ[95 % CI]: 0.40[0.28–0.53]) diagnostic test was stronger than that of ADA-HbA1c criteria 0.1[0.03–0.16] and IEC criteria (0.17[0.04–0.30]). The ROC (AUC[95 % CI]) for HbA1c and 1-h PG were 0.65[0.57–0.73] and 0.79[0.72–0.85], respectively. Importantly, 1-h PG ≥ 155 mg/dl (8.6 mmol/l) showed good sensitivity (74.3 % [62.4–84.0]) and specificity 69.7 % [61.5–77.1]) with a LR of 2.45. The ability of 1-h PG to discriminate prediabetes was better than that of HbA1c (∆AUC: −0.14; Z value: 2.5683; P = 0.01022).
Conclusion
In a real-world clinical practice setting, the 1-h PG ≥ 155 mg/dl (8.6 mmol/l) is superior for detecting high-risk individuals compared with HbA1c. Furthermore, HbA1c is a less precise correlate of insulin sensitivity and β-cell function than the 1-h PG and correlates poorly with the 2-h PG during the OGTT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Screening and early detection for hyperglycemia are a significant public health priority. According to a recent report by Centers for Disease Control and Prevention (CDC), the lifetime probability of developing diabetes is 40 % [1] for the average American. Furthermore, nearly 90 % of the 86 million Americans with prediabetes are not aware of their condition [2]. Using a validated computer simulation model [3], Herman et al. [4] demonstrated a 29 % relative risk reduction (RRR) in cardiovascular disease (CVD) outcomes and 17 % RRR in all-cause mortality after 5 years among screened individuals undergoing routine care compared with a 3-year delay in diagnosis and treatment. The study further demonstrates that the beneficial effects mostly accrued from early diagnosis and identification of high-risk individuals.
As β-cell function is impaired with progressive dysglycemia, [5] it becomes increasingly more challenging to lower plasma glucose levels [6]. Furthermore, the vascular risk associated with diabetes begins well before the diagnostic glucose thresholds for prediabetes are reached [7, 8]. Therefore, identification of effective and routinely available screening tools are of importance for detecting high-risk individuals amenable to intervention.
Fasting plasma glucose (FPG) and the 2-h oral glucose tolerance test (OGTT) are recommended for screening of prediabetes and T2DM [9]. However, most primary care physicians do not formally screen for dysglycemic conditions with these tests. A survey conducted among primary care physicians showed that only 7 % of providers prescribed an OGTT to identify individuals with prediabetes [10]. Recently, the harmonization of assay methods and the availability of reference material have allowed the measurement of glycated hemoglobin (HbA1c) as a third test to diagnose glucose intolerance [11]. Subsequently, the International Expert Committee (IEC) and the American Diabetes Association (ADA) recommended HbA1c for diagnosing diabetes [11, 12]. However, the utility of HbA1c as a screening tool has not been well accepted given the discordance between HbA1c and OGTT [13, 14] in addition to which several other factors such as age, hemoglobin glycation, or ethnicity [14, 15] may influence its reliability. Furthermore, single measurements of FPG and 2-h PG perform better than HbA1c in detecting β-cell dysfunction and insulin resistance [16]. As diabetes is clinically defined by elevated post-glucose (PG) and not by glycation of proteins, there is a concern that classification of T2DM by HbA1c may lead to a paradigm shift in the pathophysiology that defines the condition [17].
During the 2-h OGTT, the 1-h PG load value has also been measured to determine β-cell function. However, the clinical relevance of this measurement is not well defined, and there are no formally accepted thresholds for defining high-risk individuals. Recently, data obtained from population-based studies [18, 19] have consistently shown that the 1-h PG ≥ 155 mg/dl (8.6 mmol/l) load during the OGTT may be a better predictor of incident T2DM and associated complications than fasting or 2-h levels [20, 21]. Concurrently, normal glucose-tolerant (NGT) individuals with elevated 1-h PG ≥ 155 mg/dl (8.6 mmol/l) have also been shown to be predisposed to early fatty liver disease [22], inflammation [23], subclinical atherosclerosis [24, 25], decline in kidney function [26], and early mortality [18]. Furthermore, NGT individuals with elevated 1-h PG ≥ 155 mg/dl (8.6 mmol/l) were found to be more insulin resistant and have worse β-cell function [19] and an atherogenic profile similar to those with prediabetes [16, 27]. There have been no comparison studies assessing HbA1c and 1-h PG in prediabetes.
Therefore, the objectives of this study were to assess the association of HbA1c and 1-h PG with conventional glycemic parameters (FPG, 2-h PG) as well as surrogate insulin indices [Matsuda’s insulin sensitivity (ISI) and oral disposition index (DIo)] and to assess the performance of HbA1c defined by ADA (5.7–6.4 %) and IEC (6.0–6.4 %) guidelines and the 1-h PG ≥ 8.7 mmol/l in identifying prediabetes based on OGTT as the reference test.
Methods
Participants
Between June 1, 2010 and September 30, 2015, 253 high-risk participants seen at the New York University Langone Diabetes and Endocrine Associates underwent screening for dysglycemia (prediabetes or diabetes) and were included for this analysis. Individuals were eligible if there was no prior diagnosis of T2DM and if they had clinical risk factors for diabetes, including a positive family history of diabetes and/or gestational diabetes and were overweight or obese (BMI > 27.0 kg/m2). Individuals with the following conditions, known to potentially interfere with the interpretation of glycemic status such as anemia, chronic renal or liver disease, and/or presence of hemoglobin variants, were excluded. A 75-g OGTT was conducted following an overnight fast with samples collected for fasting, 1 and 2-h for plasma glucose, insulin and HbA1c determinations.
This cross-sectional analysis evaluated the diagnostic accuracy of the HbA1c and 1-h PG for identifying prediabetes with an OGTT. A 2-h OGTT was done with venous plasma sampling in the fasting state, and at 1-h and 2-h after 75-g glucose consumption. Our findings were presented according to Standards for Reporting of Diagnostic Accuracy (STARD) initiative guidelines [28]. This study was approved by the New York University School of Medicine Institutional Review Board.
Glucose, HbA1c, and insulin measurements
Glucose samples were obtained using sodium fluoride/oxalate preservative. Chemical analyses were performed at the New York Langone Clinical Laboratory using an automated analyzer with appropriate quality controls. Plasma glucose was measured using glucose oxidase method run on Ortho Clinical Diagnostics VITROS 5600 analyzer® (VITROS Chemistry Systems; Ortho Clinical Diagnostics), Rochester, NY). HbA1c was measured using a fully automated glycosylated hemoglobin analyzer (Tosoh Bioscience Inc., Tokyo, Japan). The HbA1c assay was National Glycohemoglobin Standardization Program certified and standardized to the Diabetes Control and Complications Trial (DCCT) assay method. Insulin concentrations were estimated by the one-step chemiluminescent immunoassay (the ARCHITECT® insulin assay, Abbott Laboratories).
Calculations
The insulin sensitivity was calculated by Matsuda’s insulin sensitivity index [29] and β-cell function by oral disposition index [30] (total-AUCI0–120/G0–120) as previously described.
Classification of glucose tolerance
Glucose tolerance was classified by ADA criteria [12] on the basis of glucose levels in a single 75-g OGTT. Individuals were designated as NGT (FPG < 100 mg/dl; 5.6 mmol/l, with 2-h PG < 140 mg/dl; 7.8 mmol/l), prediabetes (IFG: FPG between: 100–125 mg/dl; ≥5.6–≤ 6.9 mmol/l with 2 h PG: 200 mg/dl; <11.1 mmol/l and/or IGT with FPG < 126 mg/dl; 7.0 mmol/l and 2-h PG ≥ 140–199 mg/dl; 7.8– <11.1), and diabetes (FPG ≥ 126 mg/dl; 7.0 mmol/l or 2-h PG ≥ 200 mg/dl; 11.1 mmol/l). The additional IEC criteria [11] identified individuals as normal (HbA1c < 6 %(<42 mmol/mol)), high-risk for diabetes (HbA1c: 6.0–6.4 %; (42–<48 mmol/mol)), and diabetes (HbA1c ≥ 6.5 % (≥48 mmol/mol)), whereas ADA criteria identified individuals as normal (HbA1c < 5.7 %(<39 mmol/mol)), high-risk (HbA1c 5.7–6.4 % (39–48 mmol/mol)), and diabetes (HbA1c ≥ 6.5 % (≥48 mol/mol)) [12]. For this analysis, we have excluded individuals with diabetes.
Statistical analysis
Descriptive statistics were presented according to the diagnosis of prediabetes. Normally distributed variables were expressed as mean ± SD or a median [inter-quartile range] for skewed variables. Partial correlation analyses controlled for age and sex were used to determine the relationships between HbA1c, 1-h PG with fasting, 2-h PG and indices of insulin sensitivity, and β-cell function. The levels of agreement between diagnostic methods were ascertained using Cohen’s kappa coefficient (Κ). Receiver operating characteristic (ROC) curve was used to analyze the performance of the HbA1c and 1-h PG test in identifying prediabetes considering OGTT as reference diagnostic criteria. The diagnostic properties of different HbA1c thresholds were contrasted by determining sensitivity, specificity, and likelihood ratios (LR). For this analysis, we tested the HbA1c diagnostic criteria advocated by the ADA (5.7–6.4 %; (>39–<48 mmol/mol)) and IEC criteria (6.0–6.4 %; (>42–<48 mmol/mol)) against conventional OGTT criteria. In addition, we also tested the utility of 1-h PG ≥ 155 mg/dl (8.6 mmol/l). Data analysis was conducted with IBM SPSS statistics version 23.0 (IBM Corp., Armonk, NY, USA) for Windows. For all analysis, a P value (two-tailed) <0.05 was considered to indicate statistical significance.
Results
Prediabetes—concordance analysis
Of the 253 individuals at high-risk for developing diabetes, we have included 212 for the present analysis (see Fig. 1). The mean age [min–max] was 55.7 [21.0–79.0] years, and mean body mass index [BMI min–max] was 28.2 [15.7–49.7] kg/m2. Seventy percent were female. The prevalence of prediabetes according to ADA-OGTT [12], ADA-HbA1c (5.7–6.4 %) [12], and IEC (6.0–6.4 %) [11] criteria were 70 (33.0 %), 181 (85.7 %) and 98 (46.2 %), respectively. Ninety-five (44.8 %) individuals were at high-risk for developing diabetes according to 1-h PG ≥ 155 mg/dl (8.6 mmol/l). Figure 2 shows the concordance between the OGTT, the HbA1c, and 1-h PG criteria. The level of agreement between 1-h PG ≥ 155 mg/dl (8.6 mmol/l) and the OGTT (Κ: 0.40 [95 % CI 0.28–0.53]) diagnostic test was stronger than that of ADA/HbA1c criteria (5.7–6.4 %) [Κ 0. 0.10 (95 % CI 0.03–0.16)] and IEC criteria HbA1c (6.0–6.4 %) [Κ 0.17 (95 % CI 0.04–0.30)]. FPG, 2-h PG, and surrogate insulin measures according to the different diagnostic criteria are shown in Table 1. Overall, participants with prediabetes had a defect in glycemic metabolism and decreased insulin sensitivity and β-cell function.
Study design (according to standards for reporting of diagnostic accuracy (STARD) guidelines. a Concordance of prediabetes (by OGTT criteria) versus HbA1c by ADA definition (5.7–6.4 %). b Concordance of prediabetes (by OGTT criteria) versus HbA1c by IEC definition (6.0–6.4 %). c Concordance of prediabetes (by OGTT criteria) versus 1-h PG > 155 mg/dl (8.6 mmol/l)
Correlations of HbA1c and 1-h PG with glycemic and surrogate insulin measures
The categorization of high-risk individuals stratified based on 1-h PG ≥ 155 mg/dl (8.6 mmol/l) is an equivalent marker as OGTT for identifying individuals with decreased insulin sensitivity (%∆: OGTT −31.0; 1-h PG −35.0) and deteriorated β-cell function (%∆: OGTT −40.7; 1-h PG:−38.6). The HbA1c criteria defined by ADA and IEC were moderately associated with changes in ISI and DIo. The correlation coefficients between OGTT indices (FPG, 2-h PG), HOMA-IR, and β-cell function with HbA1c and 1-h PG are shown in Table 2. 1-h PG showed stronger associations with 2-h PG, ISI, and β-cell function than HbA1c (P < 0.05 for all). The association of FPG was similar between the two tests. We have also compared the 1-h PG and 2-h PG during the OGTT with the ISI and β-cell function. The 1-h PG was strongly associated with ISI and β-cell function compared with the 2-h PG. However, the comparison between the two tests was nonsignificant (data not shown).
Measures of diagnostic accuracy of HbA1c and 1-h PG
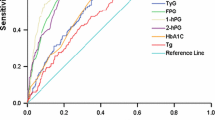
Figure 3 shows the discriminatory power of HbA1c and 1-h PG in identifying individuals with prediabetes. The ROC for HbA1c and 1-h PG were 0.65 [95 % CI 0.57–0.73] and 0.79 [95 % CI 0.72–0.85], respectively. The HbA1c value of ≥5.7–6.4 % defining the prediabetes threshold (ADA criteria) demonstrated high specificity of 94.3 % [95 % CI 86.0–98.4 %] but very poor sensitivity [19.0 % (95 % CI 12.9–26.5 %)] with a lower likelihood ratio of 1.16. The IEC guidelines of HbA1c: 6.0–6.4 % demonstrated a moderate sensitivity (58.6 % [95 % CI 46.2–70.2 %]) and specificity (59.9 % [95 % CI 51.3–68.0 %]) with a likelihood ratio of 1.46. Conversely, 1-h PG ≥ 155 mg/dl (8.6 mmol/l) showed a good sensitivity (74.3 % [95 % CI 62.4–84.0 %]) and specificity 69.7 % [95 % CI 61.5–77.1 %]) with a likelihood ratio of 2.45. The ability of 1-h PG to discriminate prediabetes was better than that of HbA1c (∆AUC: -0.14; Z value: −2.57; P = 0.01) with high specificity and sensitivity. The IEC guidelines of HbA1c ≥6.0 % were superior for identifying individuals with prediabetes than current ADA HbA1c ≥5.7 % guidelines.
Receiver operating characteristic curve for HbA1c and 1-h PG to discriminate individuals with prediabetes. Legend: The figure shows the ROC curve for HbA1c and 1-h PG to discriminate individuals with prediabetes (IFG or/and IGT). OGTT-based criteria for prediabetes was compared with HbA1c and 1-h PG levels. The 1-h PG is superior for identifying prediabetic individuals [AUC: 0.79 (95 % CI 0.72–0.85)] than HbA1c [AUC: 0.65 (95 % CI 0.57–0.73)]
Discussion
There are two main findings in this report. First, HbA1c is a less precise correlate of insulin sensitivity, β-cell function, and 2-h PG than single determinations of 1-h PG ≥ 155 mg/dl (8.6 mmol/l). Previously, the Veterans Administration Genetic Epidemiology Study [31] showed that the association of insulin sensitivity and β-cell function with HbA1c was nonlinear in Mexican Americans. A cross-sectional study among Chinese individuals showed that OGTT-based glycemic measurements (FPG, 2-h PG) were better in identifying individuals with impaired β-cell dysfunction [32]. These results demonstrate that the 1-h PG provides a better tool to identify subjects with β-cell dysfunction compared with HbA1c. Since β-cell function is the principal factor responsible for the development of T2DM, these results underscore the need for an OGTT for identifying abnormalities. A recent cross-sectional study by Fiorentino et al. [33] demonstrated that individuals with 1-h PG ≥ 155 mg/dl (8.6 mmol/l) during an OGTT have increased risk of T2DM compared with IFG. Furthermore, a large population-based genome-wide study among Caucasians showed that in men without T2DM insulin sensitivity and insulin secretion was determined to be <2 % variance in HbA1c [34]. The same study also demonstrated that the variance in HbA1c was largely determined by non-glycemic factors such as age, gender, BMI, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, current smoking status, and alcohol consumption. A study including 855 participants from three ethnic groups (non-Hispanic whites, African-Americans, and Hispanics) showed that HbA1c levels between 5.7 and 6.4 % is a less precise marker to identify insulin sensitivity and β-cell function than FPG and 2-h PG. Hence, HbA1c gives substantially less insight into the pathophysiology of glycemic dysregulation.
The second main finding was that 1-h PG ≥ 155 mg/dl (8.6 mmol/l) had better discriminatory power to identify at-risk individuals than the presently advocated ADA and IEC diagnostic cutoff criteria for HbA1c. To our knowledge this is the first discriminatory comparator study which assessed the effectiveness of HbA1c (ADA and IEC guidelines) and 1-h PG ≥ 155 mg/dl (8.6 mmol/l) and found that the 1-h PG was a better screening tool for identifying high-risk individuals than HbA1c (Z: 2.57; P = 0.01). The findings that HbA1c criteria demonstrated poor accuracy in identifying high-risk individuals are consistent with other population-based studies including screening for diabetes and prediabetes, NHANES III [14], Japanese study [35], and KORA S4/F4 [13]. In addition, a 1-h PG ≥ 8.7 mmol/l showed better concordance with the OGTT (54.7 %) than ADA (36.4 %) and IEC (41.8 %) designated HbA1c values.
HbA1c threshold levels identified an increased proportion of individuals as having prediabetes [ADA: 181 (85.7 %); IEC 98 (46.2 %)] versus the OGTT [70 (33.0 %)]. A higher prevalence of prediabetes employing the HbA1c was previously reported in several population-based studies [36] including from China. However, other studies reported lower prevalence of prediabetes using HbA1c thresholds [37–39].
The small sample size and the predominance of females in this sample are limitations of the present study although the significance of these findings nonetheless supports the importance of the observations. The performance of OGTT was carried out only once, an approach, however, reflecting the realities of clinical practice. In summary, the 1-h PG ≥ 155 mg/dl (8.6 mmol/l) is superior for detecting high-risk individuals compared with ADA (5.7–6.4 %) and IEC (6.0–6.4 %) guidelines. Furthermore, HbA1c is a less precise correlate of β-cell function than the 1-h PG and correlates poorly with the 2-h PG during the OGTT. These data demonstrate that the 1-h PG ≥ 155 mg/dl (8.6 mmol/l) identifies those at risk for developing diabetes better than established HbA1c criteria. Further prospective studies are needed for assessing the utility of 1-h PG as a screening tool for preventive measures and also to identify standardized upper and lower boundaries. It is plausible, based on our findings [18, 19] as well from others [26, 40, 41], that the 1-h PG ≥ 155 mg/dl (8.6 mmol/l) could serve as a screening test to identify high-risk individuals and potentially replace the traditional 2-h OGTT. This would not only make this test more acceptable in practice, but also improve the ability to identify high-risk individuals at an earlier time-point resulting in even greater prospects of preventing progression to diabetes than is currently feasible.
References
Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KMV, Thompson TJ (2014) Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol 2(11):867–874. doi:10.1016/S2213-8587(14)70161-5
Centers for Disease Control and Prevention (CDC) Awareness of prediabetes–United States, 2005–2010 (2013). MMWR Morbidity and mortality weekly report 62 (11):209–212
Barhak J, Isaman DJM, Ye W, Lee D (2010) Chronic disease modeling and simulation software. J Biomed Inform 43(5):791–799. doi:10.1016/j.jbi.2010.06.003
Herman WH, Ye W, Griffin SJ et al (2015) Early detection and treatment of type 2 diabetes reduce cardiovascular morbidity and mortality: a simulation of the results of the Anglo-Danish-Dutch study of intensive treatment in people with screen-detected diabetes in primary care (ADDITION-Europe). Diabetes Care: dc142459
Meier JJ, Bonadonna RC (2013) Role of reduced β-cell mass versus impaired β-cell function in the pathogenesis of type 2 diabetes. Diabetes Care 36(Suppl 2):S113–S119. doi:10.2337/dcS13-2008
Nichols GA, Alexander CM, Girman CJ, Kamal-Bahl SJ, Brown JB (2006) Treatment escalation and rise in HbA1c following successful initial metformin therapy. Diabetes Care 29(3):504–509
Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK (1990) Cardiovascular risk factors in confirmed prediabetic individuals: Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 263(21):2893–2898
Bergman M (2014) The early diabetes intervention program–Is early actually late? Diabetes/Metab Res Rev 30(8):654–658. doi:10.1002/dmrr.2563
Nathan DM, Davidson MB, DeFronzo RA et al (2007) Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30(3):753–759. doi:10.2337/dc07-9920
Gohdes D, Amundson H, Oser CS, Helgerson SD, Harwell TS (2009) How are we diagnosing cardiometabolic risk in primary care settings? Primary Care Diabetes 3(1):29–35. doi:10.1016/j.pcd.2008.12.002
Committee IE (2009) International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32(7):1327–1334
Association AD (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Supplement 1):S62–S69
Rathmann W, Kowall B, Tamayo T et al (2012) Hemoglobin A1c and glucose criteria identify different subjects as having type 2 diabetes in middle-aged and older populations: the KORA S4/F4 Study. Ann Med 44(2):170–177. doi:10.3109/07853890.2010.531759
Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS (2010) Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 33(10):2184–2189. doi:10.2337/dc10-0433
Cosson E, Chiheb S, Cussac-Pillegand C et al (2013) Haemoglobin glycation may partly explain the discordance between HbA1c measurement and oral glucose tolerance test to diagnose dysglycaemia in overweight/obese subjects. Diabetes Metab 39(2):118–125. doi:10.1016/j.diabet.2012.08.013
Marini MA, Succurro E, Frontoni S et al (2012) Insulin sensitivity, beta-cell function, and incretin effect in individuals with elevated 1-hour postload plasma glucose levels. Diabetes Care 35(4):868–872. doi:10.2337/dc11-2181
Bonora E, Tuomilehto J (2011) The pros and cons of diagnosing diabetes with A1C. Diabetes Care 34(Suppl 2):S184–S190. doi:10.2337/dc11-s216
Bergman M, Chetrit A, Roth J, Dankner R (2015) Dysglycemia and long-term mortality: observations from the Israel study of glucose intolerance, obesity and hypertension. Diabetes/Metab Res Rev 31(4):368–375. doi:10.1002/dmrr.2618
Jagannathan R, Sevick MA, Li H et al (2015) Elevated 1-hour plasma glucose levels are associated with dysglycemia, impaired beta-cell function, and insulin sensitivity: a pilot study from a real world health care setting. Endocrine. doi:10.1007/s12020-015-0746-z
Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L (2009) Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care 32(2):281–286. doi:10.2337/dc08-1264
Alyass A, Almgren P, Akerlund M et al (2015) Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia 58(1):87–97. doi:10.1007/s00125-014-3390-x
Sesti G, Hribal ML, Fiorentino TV, Sciacqua A, Perticone F (2014) Elevated 1 h postload plasma glucose levels identify adults with normal glucose tolerance but increased risk of non-alcoholic fatty liver disease. BMJ Open Diabetes Res Care 2(1):e000016. doi:10.1136/bmjdrc-2014-000016
Bardini G, Dicembrini I, Cresci B, Rotella CM (2010) Inflammation markers and metabolic characteristics of subjects with 1-h plasma glucose levels. Diabetes Care 33(2):411–413. doi:10.2337/dc09-1342
Succurro E, Marini MA, Arturi F et al (2009) Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 207(1):245–249. doi:10.1016/j.atherosclerosis.2009.04.006
Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M (2000) Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 23(12):1830–1834
Succurro E, Arturi F, Lugara M et al (2010) One-hour postload plasma glucose levels are associated with kidney dysfunction. Clin J Am Soc Nephrol CJASN 5(11):1922–1927. doi:10.2215/CJN.03240410
Bianchi C, Miccoli R, Trombetta M et al (2013) Elevated 1-hour postload plasma glucose levels identify subjects with normal glucose tolerance but impaired beta-cell function, insulin resistance, and worse cardiovascular risk profile: the GENFIEV study. Journal Clin Endocrinol Metab 98(5):2100–2105. doi:10.1210/jc.2012-3971
Bossuyt PM, Reitsma JB, Bruns DE et al (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative: standards for reporting of diagnostic accuracy. Clin Chem 49(1):1–6
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22(9):1462–1470
Ram J, Snehalatha C, Selvam S et al (2015) The oral disposition index is a strong predictor of incident diabetes in Asian Indian prediabetic men. Acta Diabetol. doi:10.1007/s00592-015-0718-z
Kanat M, Winnier D, Norton L et al (2011) The relationship between {beta}-cell function and glycated hemoglobin: results from the veterans administration genetic epidemiology study. Diabetes Care 34(4):1006–1010. doi:10.2337/dc10-1352
Li C, Yang H, Tong G, et al (2014) Correlations between A1c, fasting glucose, 2 h postload glucose, and β-cell function in the Chinese population. Acta Diabetol 51(4):601–608
Fiorentino TV, Marini MA, Andreozzi F et al (2015) One-hour postload hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. J Clin Endocrinol Metab 100(10):3744–3751
Fizelova M, Stancakova A, Lorenzo C et al (2015) Glycated hemoglobin levels are mostly dependent on nonglycemic parameters in 9398 Finnish men without diabetes. J Clin Endocrinol Metab 100(5):1989–1996. doi:10.1210/jc.2014-4121
Oka R, Aizawa T, Miyamoto S, Yoneda T, Yamagishi M (2015) One-hour plasma glucose as a predictor of the development of Type 2 diabetes in Japanese adults. Diabetic Med J Br Diabetic Assoc. doi:10.1111/dme.12994
Kahn R, Davidson MB (2014) The reality of type 2 diabetes prevention. Diabetes Care 37(4):943–949. doi:10.2337/dc13-1954
Christensen DL, Witte DR, Kaduka L et al (2010) Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 33(3):580–582
Silverman RA, Thakker U, Ellman T et al (2011) Hemoglobin A1c as a screen for previously undiagnosed prediabetes and diabetes in an acute-care setting. Diabetes Care 34(9):1908–1912. doi:10.2337/dc10-0996
Mohan V, Vijayachandrika V, Gokulakrishnan K et al (2010) A1C cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care 33(3):515–519. doi:10.2337/dc09-1694
Manco M, Panunzi S, Macfarlane DP et al (2010) Relationship between Insulin S, Cardiovascular Risk C, One-hour plasma glucose identifies insulin resistance and beta-cell dysfunction in individuals with normal glucose tolerance: cross-sectional data from the Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) study. Diabetes Care 33(9):2090–2097. doi:10.2337/dc09-2261
Priya M, Anjana RM, Chiwanga FS, Gokulakrishnan K, Deepa M, Mohan V (2013) 1-hour venous plasma glucose and incident prediabetes and diabetes in Asian indians. Diabetes Technol Ther 15(6):497–502. doi:10.1089/dia.2013.0025
Acknowledgments
This study was funded by CTSI Grant Number 1UL1RR029893 (NCRR, NIH, and the Schuman Foundation) and partly by NIH-K24-NR012226.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
This study was approved by the New York University School of Medicine Institutional Review Board.
Human and animal rights disclosure
All human rights were observed in keeping with Declaration of Helsinki 2008 (ICH GCP). There are no animal rights issues as this is a clinical study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Jagannathan, R., Sevick, M.A., Fink, D. et al. The 1-hour post-load glucose level is more effective than HbA1c for screening dysglycemia. Acta Diabetol 53, 543–550 (2016). https://doi.org/10.1007/s00592-015-0829-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0829-6