Abstract
Aims
Previous study has reported that triglycerides-glucose (TyG) index, a product of triglycerides and fasting plasma glucose (FPG), might be useful in the prediction of incident type 2 diabetes (T2D). We evaluated the ability of the TyG index compared to FPG and OGTT as possible diabetes predictor in nondiabetic first-degree relatives (FDRs) of patients with T2D.
Methods
A total of 1,488 FDRs without diabetes of consecutive patients with T2D 30–70 years old (361 men and 1,127 women) were examined and followed for a mean (SD) of 6.9 (1.7) years for diabetes incidence. We examined the incidence of diabetes across quartiles of the TyG index and plotted a receiver operating characteristic (ROC) curve to assess discrimination. At baseline and through follow-up, participants underwent a standard 75-g two-hour oral glucose tolerance test.
Results
During 10,124 person-years of follow-up, 41 men and 154 women developed T2D. Those in the top quartile of TyG index were 3.4 times more likely to develop T2D than those in the bottom quartile (odds ratio 3.36; 95 % CI 1.83, 6.19). On ROC curve analysis, a higher area under the ROC was found for FPG (76.2; 95 % CI 71.9, 80.6), 1-hPG (81.0, 95 % CI 77.2, 84.9) and 2-hPG (76.5; 95 % CI 72.3, 80.8) than for TyG index (65.1; 95 % CI 60.5, 69.7).
Conclusions
TyG index is predicted T2D in high-risk individuals in Iran but FPG, 1-hPG and 2-hPG appeared to be more robust predictor of T2D in our study population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2D) is an important and growing public health problem worldwide [1]. Based on a recent report by the International Diabetes Federation (IDF), Middle East and North Africa region currently has the highest regional prevalence of T2D, and by 2030 it is expected to have the second largest proportional increase after Africa [2]. Based on available data of adults aged 20–70 years, the IDF Atlas of Diabetes [1] estimated a point prevalence of 9.33 % for T2D in 2011, and it has also predicted that this figure would rise to 13.15 % by 2030. T2D prevalence in Iran is significantly higher than its neighboring countries Pakistan (6.7 % for 2007) [3] and Turkey (7.2 %, for 1997–1998) [4]. On the other hand, our figures often fall below the prevalence rates observed in Arab communities [5]. Available reports of T2D prevalence among Middle Eastern countries vary substantially, ranging from 2.8 % in Israel [6] to 29 % in Bahrain [7]. In a 2009 systematic review, a prevalence rate of 10.5 % (95 % CI 8.6, 12.7 %) in the region was recorded [8].
Thus, with a strong evidence base for lifestyle interventions to prevent diabetes [9, 10], there is great interest in identifying individuals at high risk of developing diabetes with the aim of prevention and proper intervention. A simple, reliable, noninvasive, effective and widely applicable tool to rapidly identify asymptomatic individuals in whom oral glucose tolerance tests (OGTT) should be measured to rule out T2D would be practical for use by the general public and in primary health care. During the past few decades, various risk factors and predictive models have been suggested to identify persons at high risk for the future development of T2D [11–13]. It is well established that the level of fasting plasma glucose (FPG) and triglycerides is associated with increased risk of T2D [14–16]. The triglycerides-glucose (TyG) index, the product of FPG and triglycerides, is a novel index that has been suggested as a surrogate of insulin resistance (IR) in healthy subjects [17–19]. It was well correlated with the homeostasis model assessment of IR (HOMA-IR) and total glucose metabolism rate during hyperinsulinemic-euglycemic clamp studies [18]. The assessment of IR is of interest because of its key role in T2D and is already present 1–2 decades before the diagnosis [20, 21], surrogate indices of IR might aid in the prediction of incidence T2D. It has been tested in a Korean population with encouraging results [22], but it may not be universally applicable among all ethnic groups and populations. No study to date has examined T2D incidence with TyG index in Iran. Moreover, nothing is known about the relationship of TyG and T2D in FDR of patients with T2D who are at higher risk of diabetes.
The objective of this study, therefore, was to evaluate the ability of TyG index compared with FPG and oral glucose tolerance test (OGTT) to predict the incidence of T2D in an Iranian population without diabetes.
Patients and methods
Data collection
This study was conducted within the framework of the Isfahan Diabetes Prevention Study (IDPS), an ongoing cohort in central Iran to assess the various potential risk factors for diabetes in subjects with family history of T2D (one of the main risk factors for diabetes). The recruitment methods and examination procedures of the IDPS have been described before [23]. Our study sample comprised 3,409 (895 men and 2,514 women) first-degree relatives (FDR) of consecutive patients with T2D. All patients were attendees at clinics at Isfahan Endocrine and Metabolism Research Center, which is affiliated to Isfahan University of Medical Sciences, Iran. The study was conducted between the years 2003 and 2005. All participants were from Isfahan city and adjoining areas. They completed laboratory tests including a standard 75-g 2-h OGTT, a questionnaire on their health status and on various potential risk factors for diabetes. Participants received follow-up tests according to Standard of Medical Care in Diabetes [24] to update information on demographic, anthropometric and lifestyle factors and on newly diagnosed diabetes. Accordingly, if OGTT was normal at baseline, repeat testing was carried out at least at 3-year intervals. Otherwise, repeat testing was usually carried out annually. The study was conducted in accordance with the guidelines proposed in the World Medical Association Declaration of Helsinki [25] and has been approved by Institutional Review Board of Isfahan University of Medical Sciences, Iran, and an informed consent form was signed by each participant.
Follow-up and ascertainment of diabetes
Cases of diabetes were identified from baseline and follow-up OGTT according to American Diabetes Association criteria [26]. Pregnant women and people with type 1 diabetes were excluded. Among 3,409 persons who participated at baseline, 308 were excluded because of diagnosis of diabetes at baseline, 1,564 had no follow-up, and 49 had missing data, leaving 1,488 participants with a mean age 43.1 (6.6) (range 30–70) years for the present analysis, all of whom had at least one subsequent review during a mean [standard deviation (SD)] follow-up period of 6.9 (1.7) (range 3–10) years. Attendees at the follow-up visit did not differ significantly from non-attendees regarding most baseline characteristics: age, height, weight, body mass index (BMI), waist circumference (WC), hip circumference (HC), waist-hip ratio (WHR) and levels of HbA1c, cholesterol, low-density lipoprotein cholesterol (LDLC), triglycerides, systolic and diastolic blood pressure (BP) and obesity. However, non-attendees had slightly lower FPG (94.7 mg/dl vs. 95.7 mg/dl, P < 0.05) and plasma glucose (PG) at 30 min (138.7 mg/dl vs. 145.6 mg/dl, (P < 0.001), 60 min (141.1 mg/dl vs. 151.0 mg/dl, P < 0.001), 120 min (111.2 mg/dl vs. 120.6 mg/dl, P < 0.001) and a slightly lower TyG index (8.78 vs. 8.84, P < 0.001), but higher levels of high-density lipoprotein cholesterol (HDLC) (46.4 mg/dl vs. 45.0 mg/dl, P < 0.01).
Procedures
Information on age, gender, body size, HbA1c, cholesterol, LDLC, HDLC, triglycerides and BP, family and personal medical history was collected at baseline and through follow-up. The same methodology was used for baseline and follow-up studies. The participants included siblings and children. Participants reported to clinics in the morning after an overnight fast. They were asked to abstain from vigorous exercise in the evening and in the morning of the investigations. Smokers were encouraged to abstain from smoking in the morning of their visit. First, on arrival at the clinic, the information provided by the participants in the questionnaire on family history was verified. Then, with the subjects in light clothing and without shoes, height, weight, WC and HC were measured using standard apparatus. Weight was measured to the nearest 0.1 kg on a calibrated beam scale. Height, WC and HC were measured to the nearest 0.5 cm with a measuring tape. The waist was measured midway between the lower rib margin and the iliac crest at the end of gentle expiration in the standing position. HC was measured over the greater trochanters directly over the underwear. Resting BP was measured at each examination by physician after the participants had been seated for 10 min with a mercury column sphygmomanometer and appropriately sized cuffs, using standard techniques. The systolic BP and diastolic BP were recorded at the first appearance (phase I) and the disappearance (phase V) of Korotkoff sounds. FPG was measured with the glucose oxidase method. Participants with FPG ≥ 200 mg/dl or pharmacological treatment were considered as persons with diabetes. If FPG was ≥126 mg/dl and <200 mg/dl, a second FPG was measured on another day. If the second FPG was also ≥126 mg/dl, participants were considered as persons with diabetes. Those with FPG < 126 mg/dl underwent a standard OGTT (75 g glucose, 2 h) at baseline and follow-up visits. Venous blood was sampled 0, 30, 60 and 120 min after oral glucose administration. Plasma samples were centrifuged and analyzed the same day.
Glycosylated hemoglobin (HbA1c) (measured by ion-exchange chromatography), total cholesterol, triglycerides, HDLC and LDLC (calculated with the Friedewald equation [27] provided total triglycerides did not exceed 400 mg/dl) were recorded. All blood sampling procedures were performed in the central laboratory of the Isfahan Endocrine and Metabolism Research Center using enzyme-linked method.
Definitions
Based on the OGTT results, participants were categorized as having either normal glucose tolerance (NGT, FPG below 100 mg/dl and the 2-h plasma glucose (2hPG) < 140 mg/dl), impaired fasting glucose (IFG, FPG in the range of 100–125 mg/dl and the 2hPG was <140 mg/dl), impaired glucose tolerance (IGT, FPG < 126 mg/dl, but with 2hPG concentration ≥140 and <200 mg/dl) or diabetes (FPG ≥ 200 mg/dl or pharmacological treatment, FPG ≥ 126 and/or 2hPG ≥ 200 mg/dl) [28]. TyG index was computed for each participant as the natural logarithm (Ln) of [fasting triglycerides (mg/dl) × FPG (mg/dl)/2] [18]. Metabolic syndrome was considered present when at least three of the following characteristics were observed: central obesity, defined using ethnic-specific cut points of waist (waist circumference ≥ 102 cm in men and ≥88 cm in women); triglycerides ≥ 150 mg/dl; HDLC < 40 mg/dl in men and <50 mg/dl in women; BP ≥ 130/85 mmHg or on antihypertensive medication, or raised plasma glucose, defined as FPG ≥ 100 mg/dl [29].
Determination of diabetes incidence
Incidence was expressed as the number of cases of T2D per 1,000 person-years of follow-up beginning on the date of completion of the baseline examination in 2003–2005 and continuing until the occurrence of diabetes, the date of the last completed follow-up, death or end of follow-up on September 31, 2011, whichever came first.
Analysis
Statistical methods included the Student’s t test (for normally distributed variables), the Mann–Whitney U test (for not normally distributed variables), one-way ANOVA (for normally distributed variables), Kruskal–Wallis test (for not normally distributed variables), the Chi-squared test, Pearson correlation (for normally distributed variables), Spearman’s rank correlation (for not normally distributed variables) and binary logistic regression. Differences between more than two groups were estimated using one-way ANOVA with Bonferroni post hoc test. Pearson correlation analysis or Spearman’s rank correlation was used to examine the linear relationships between the TyG index and other variables. Univariate and multivariate binary logistic regression equations were fitted to identify predictors of new-onset diabetes using the SPSS version 18 for Windows [SPSS Inc., Chicago, IL, USA). Diastolic BP was not included in multivariate analysis to avoid co-linearity between systolic and diastolic BP. The TyG index was re-coded into quartiles and compared the risk of developing diabetes in each quartile with the lowest category of risk (reference group). The ability of TyG index, triglyceride, FPG, 1-hPG, 2-hPG and HbA1c values to predict the incidence diabetes was examined with receiver operating characteristic (ROC) curves and their respective areas under the curve, in which sensitivity was plotted as a function of 1-specificity. The area under the ROC curve is a global summary statistic of the discriminative value of a model, describing the probability that the score will be higher in an individual developing than in an individual not developing T2D. Areas under the ROC curves were compared by the algorithm developed by DeLong et al. [30]. Age-adjusted means were calculated and compared using general linear models. All tests for statistical significance were two-tailed, and all were done assuming a type I error probability of <0.05.
Results
Baseline characteristics of the 1,294 (87.0 %) participants without and 195 (13.0 %) with diabetes are shown in Table 1. As expected, those who developed diabetes were older and had higher mean BMI, WC, HC, follow-up, FPG and PG at 30, 60 and 120 min, higher HbA1c, triglyceride, cholesterol, LDLC and TyG index at baseline, and a higher proportion of overweight, metabolic syndrome, IFG and IGT. The mean (SD) age was 44.5 (7.0) years for those with and 42.9 (6.5) years for those without diabetes. The mean (SD) TyG index was 9.1 (0.5) for those with and 8.8 (0.5) for those without diabetes.
The baseline characteristics of the study participants by TyG index quartile are shown in Table 2. In comparison with variables at baseline, all variables were more likely to increase. HDLC, NGT and follow-up duration were more likely to decrease across all four subject groups.
During 10,124 (2,448 men and 7,676 women) person-years of follow-up, 195 (13.0 %) (41 men and 154 women) incident cases of T2D occurred. The overall incidence of subsequent T2D was 19.2 (95 % CI 16.6, 21.9) per 1,000 person-years. Incidence rates were slightly higher in women (20.1; 95 % CI 16.9, 23.2 per 1,000 person-years) than men (16.7; 95 % CI 12.0, 22.6), but the difference was not statistically significant. The TyG index was associated with T2D incidence. The incidence of T2D was 30.9 per 1,000 person-years (95 % CI 24.5, 38.5) for participants in the highest quartile of TyG index, and 7.9 per 1,000 person-years (95 % CI 4.9, 12.0) for the lowest quartile. The risk of T2D increased with increasing quartiles of TyG index. Compared with participants in the lowest quartile, the risk of T2D was 4.3 times higher in those in the highest quartile at baseline (odds ratio (OR) 4.29; 95 % CI 2.58, 7.13) and 2.7 times higher in those in the third quartile (OR 2.7; 95 % CI 1.60, 4.61) and 1.9 times higher in those in the second quartile (OR 1.85; 95 % CI 1.06, 3.22) in unadjusted models. Controlling for age and gender did not appreciably alter the OR compared to the unadjusted model. Further controlling for BMI, WC, BP and HbA1c attenuated associations. Further controlling for HDLC, LDLC and cholesterol slightly increased the ORs (Table 3).
The TyG index showed significant correlations (P < 0.001) with age (r = 0.157), weight (r = 0.202), BMI (r = 0.157), WC (r = 0.295), HC (r = 0.107), systolic BP (r = 0.204), diastolic BP (r = 0.188), levels of FPG (r = 0.339), 30 min PG (r = 0.259), 60 min PG (r = 0.303), 120 min PG (r = 0.219), HbA1c (r = 0.101), triglyceride (r = 0.882), cholesterol (r = 0.380), HDLC (r = −0.210), LDLC (r = 0.084).
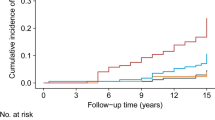
The ROC curves for the incidence of T2D for TyG index, triglyceride, FPG, 1-hPG, 2-hPG and HbA1c are shown in Fig. 1. The areas under the ROC curves (95 % CI) were 0.651 (0.605, 0.695) for TyG index, 0.762 (0.719, 0.806) for FPG, 0.811 (0.772, 0.849) for 1-hPG, 0.765 (0.723, 0.808) for 2-hPG, 0.655 (0.610, 0.701) for HbA1c and 0.591 (0.542, 0.639) for triglyceride. All parameters were significant predictors for future risk of T2D (P < 0.001). The areas under the curve were higher for 1-hPG, 2-hPG and FPG than for TyG index. The area for HbA1c was almost similar for TyG index. However, it is apparent that in this population of FDR of patients with T2D, the 1-hPG, 2-hPG and FPG were better than TyG index in predicting future risk of T2D (p < 0.001; areas under the ROC curves were compared with TyG index by the algorithm developed by DeLong et al. [30]).
Receiver operating characteristic curves for triglyceride and glucose (TyG) index, fasting plasma glucose (FPG), HbA1c, 1-h plasma glucose (1-hPG), 2-h plasma glucose (2-hPG) and triglyceride (Tg) to predict type 2 diabetes in first-degree relatives without diabetes of patients with type 2 diabetes. Sensitivity represents true-positive results and 1-specificity, the false-positive results. The estimates of the area under the ROC curves and their 95 % confidence intervals (CI) are shown. * P < 0.001; areas under the ROC curves were compared with TyG index by the algorithm developed by DeLong et al. [30]
When we re-analyzed the data with exclusion of subjects with prediabetes, the results did not appreciably alter compared to the analyses that include participants with IFG and IGT at baseline. This suggests that TyG index may uniformly predict T2D in both normal and individuals with IFG/IGT.
Discussion
The present study showed that elevated TyG index is a strong predictor of incident T2D independent of several known or suspected risk factors for T2D, in a large cohort of FDR of patients with T2D in Iran. Among participant without T2D at baseline, those in the top quartile of TyG index were 3.4 times more likely to develop T2D than those in the bottom quartile after full adjustment. Although TyG index could be an alternative test to predict T2D, 1-hPG, 2-hPG and FPG appeared to be more robust predictor of T2D in our study population. However, the association between TyG index and T2D was relatively similar to that observed with HbA1c and fasting triglyceride level. This observation was confirmed by the results from ROC curve analysis. Additionally, 1-hPG, 2-hPG and FPG outperformed HbA1c, TyG index and fasting triglyceride in predicting T2D. Fasting glucose, 1-hPG and 2-hPG had been also advocated in literature as valuable T2D prediction tools [31–34].
Only one Korean cohort study has assessed the risk of diabetes based on the TyG index [22], and some other studies assessed TyG index as surrogate for IR [17, 18, 35, 36]. In the Chungju Metabolic Disease Cohort Study of 5,354 nondiabetic individuals followed for median 4.6 years, Lee et al. [22] showed that TyG index was a strong predictor of T2D after full adjustment. Our data are in agreement with this study. In contrast to this study, we showed 1-hPG, 2-hPG and FPG appeared to be more robust predictor of T2D then TyG index. While they reported that TyG index is better than the triglyceride/HDLC ratio or the HOMA-IR, they did not measure the effect of parameters of OGTT on development of T2D. However, the baseline characteristics, such as age, BMI, waist and HC, BP, ethnicity, the prevalence of family history of diabetes, differ somewhat between the study by Lee et al. [22] and current analysis. In the Montreal-Ottawa New Emerging Team (MONET) population database, Bastard et al. [35] reported that TyG and insulin sensitivity/resistance indices were only relatively modestly related to hyperinsulinemia-euglycemic clamp and both fasting- and OGTT-derived surrogate indices appear to be more accurate in estimating insulin sensitivity/resistance. Guerrero-Romero et al. [17, 18] proposed the TyG index as a surrogate of IR and reported that the TyG index has high sensitivity and specificity to recognize IR. In a Brazilian study, Vasques et al. [36] reported that the TyG index correlated with adiposity, metabolic and atherosclerosis markers related to IR and presented a moderate degree of agreement with hyperinsulinemic-euglycemic clamp test. Abbasi and Reaven [19] showed that the TyG index was weakly correlated with insulin-stimulated glucose uptake measured as the steady-state plasma glucose concentration during insulin suppression testing. They also conclude that although the TyG index was not better than other indices, such as the triglyceride/HDLC ratio and HOMA-IR, for assessing IR, the TyG index might be of value for screening because of its low cost, being derived from routine clinical measurement.
Our study has several strengths and limitations. The strengths include use of a sample consisting of both men and women, diagnosis of diabetes based on standard OGTT and information on potential determinants of glucose intolerance. Selection and information bias were unlikely because of the prospective design. At follow-up, non-attendees in the entire population did not differ from attendees according to major risk factors for progression to diabetes, although a difference too small to explain the high progression rate to diabetes in our study was seen in the mean levels of HDL and PG. Our database is one of the few that followed FDR of patients with T2D, thereby enabling us to simultaneously control the genetic factors that may predict glucose tolerance status. Our study was limited to a cohort of individuals who are at increased risk of developing T2D, because they had a FDR with the disease. This group of individuals will only increase further with time, as the prevalence of diabetes is expected to increase worldwide [1]. In term of our definition of incidence T2D, some selection bias may be present as participants who attended for screening may have been more likely to be tested and consequently diagnosed as having T2D. Thus, participants with T2D who had low risk score may have been missed through lack of testing. Another limitation is lack of information on the use of lipid-lowering agents which might have influenced the results. Finally, given the variability of triglycerides levels according to ethnicity, further research is needed to evaluate the TyG index in different populations.
In conclusion, these data provide further evidence that TyG index is predicted T2D, but 1-hPG, 2-hPG and FPG appeared to be more robust predictor of T2D in our study population.
References
Whiting DR, Guariguata L, Weil C, Shaw J (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94:311–321
Guariguata L (2012) By the numbers: new estimates from the IDF diabetes atlas update for 2012. Diabetes Res Clin Pract 98:524–525
Shera S, Jawad F, Maqsood A (2007) Prevalence of diabetes in Pakistan. Diabetes Res Clin Pract 76:219–222
Satman I, Yilmaz T, Sengul A, Salman S, Salman F, Uygur S et al (2002) Population-based study of diabetes and risk characteristics in Turkey: results of the Turkish diabetes epidemiology study (TURDEP). Diabetes Care 25:1551–1556
Ashraf H, Rashidi A, Noshad S, Khalilzadeh O, Esteghamati A (2011) Epidemiology and risk factors of the cardiometabolic syndrome in the Middle East. Expert Rev Cardiovasc Ther 9:309–320
Chodick G, Heymann AD, Shalev V, Kookia E (2003) The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol 18:1143–1146
Al-Mahroos F, McKeigue PM (1998) High prevalence of diabetes in Bahrainis. Associations with ethnicity and raised plasma cholesterol. Diabetes Care 21:936–942
Motlagh B, O’Donnell M, Yusuf S (2009) Prevalence of cardiovascular risk factors in the Middle East: a systematic review. Eur J Cardiovasc Prev Rehabil 16:268–280
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al (2002) Diabetes prevention program research group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Abbasi A, Peelen LM, Corpeleijn E, van der Schouw YT, Stolk RP, Spijkerman AM et al (2012) Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study. BMJ 345:e5900
Buijsse B, Simmons RK, Griffin SJ, Schulze MB (2011) Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev 33:46–62
Noble D, Mathur R, Dent T, Meads C, Greenhalgh T (2011) Risk models and scores for type 2 diabetes: systematic review. BMJ 343:d7163
Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T et al (2005) Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 353:1454–1462
Rhee SY, Woo JT (2011) The prediabetic period: review of clinical aspects. Diabetes Metab J 35:107–116
Janghorbani M, Amini M (2011) Normal fasting plasma glucose and risk of prediabetes and type 2 diabetes: the Isfahan Diabetes Prevention Study. Rev Diabet Stud 8:490
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F (2008) The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 6:299–304
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernandez Gonzalez SO et al (2010) The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 95:3347–3351
Abbasi F, Reaven GM (2011) Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism 60:1673–1676
Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR (1990) Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 113:909–915
DeFronzo RA (2009) From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795
Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK et al (2014) Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One 9(2):e90430
Amini M, Janghorbani M (2007) Diabetes and impaired glucose regulation in first degree relatives of patients with type 2 diabetes in Isfahan, Iran: prevalence and risk factors. Rev Diabet Stud 4(169):76
American Diabetes Association (2008) Executive summary: Standard of Medical Care in Diabetes-2008. Diabetes Care 31:S5–S11
World Medical Association (2009) Declaration of Helsinki, Ethical principles for medical research involving human subjects. J Indian Med Assoc 107:403–405
American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1):S62–S69
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (2003) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care suppl 1:S5–S20
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Alyass A, Almgren P, Akerlund M, Dushoff J, Isomaa B, Nilsson P et al (2015) Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia 58:87–97
Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D et al (2007) Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 78:305–312
Janghorbani M, Amini M (2009) Comparison of fasting glucose with post-load glucose values and glycated hemoglobin for prediction of type 2 diabetes: the Isfahan diabetes prevention study. Rev Diabet Stud 6:117–123
Janghorbani M, Amini M (2012) Incidence of type 2 diabetes by HbA1c and OGTT: the Isfahan Diabetes Prevention Study. Acta Diabetol 49(Suppl 1):S73–S79
Bastard JP, Lavoie ME, Messier V, Prud’homme D, Rabasa-Lhoret R (2012) Evaluation of two new surrogate indices including parameters not using insulin to assess insulin sensitivity/resistance in non-diabetic postmenopausal women: a MONET group study. Diabetes Metab 38:258–263
Vasques AC, Novaes FS, de Oliveira MDS, Souza JR, Yamanaka A, Pareja JC et al (2011) TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract 93:e98–e100
Acknowledgments
The authors are grateful to Mr. Majid Abyar for computer technical assistance. This study was supported partially by a grant from the Isfahan University of Medical Sciences, Iran. This research was performed as a part of the academic activity of the university.
Conflict of interest
Janghorbani M, Almasi SZ and Amini M declare that they have no conflict of interest.
Ethical standard
This study was approved by the Institutional Ethics Committee of the Isfahan University of Medical Sciences, Iran.
Human and Animal Rights disclosure
All procedures followed were in accordance with the ethical standards of the Institutional Review Board of Isfahan University of Medical Sciences, Iran and with the Helsinki Declaration of 1975, as revised in 2009 [25].
Informed consent disclosure
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Porta.
Rights and permissions
About this article
Cite this article
Janghorbani, M., Almasi, S.Z. & Amini, M. The product of triglycerides and glucose in comparison with fasting plasma glucose did not improve diabetes prediction. Acta Diabetol 52, 781–788 (2015). https://doi.org/10.1007/s00592-014-0709-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0709-5