Abstract
The proinflammatory lysophosphatidic acid (LPA) is a potent activator of several transcriptional factors and signaling pathways and a potent modulator of genes involved in inflammation, angiogenesis and fibrosis. This study was conducted to measure the levels of LPA and LPA-producing enzymes, autotaxin (ATX) and acylglycerol kinase (AGK) in the vitreous fluid from patients with proliferative diabetic retinopathy (PDR) and to correlate their levels with clinical disease activity and the level of vascular endothelial growth factor (VEGF). In addition, we examined the expression of ATX, AGK and VEGF receptor-2 (VEGFR-2) in the retinas of diabetic rats. Vitreous samples from 42 PDR and 35 nondiabetic patients were studied by enzyme-linked immunosorbent assay. Vitreous samples and retinas of rats were examined by Western blot analysis. VEGF, LPA and AGK levels in vitreous samples from PDR patients were significantly higher than those in control patients without diabetes (p < 0.001 for all comparisons). ATX levels in PDR with active neovascularization and inactive PDR were significantly lower than those in nondiabetic patients (p = 0.045). Mean VEGF and AGK levels in PDR with active neovascularization were significantly higher than those in inactive PDR and nondiabetic patients (p < 0.001 for both comparisons). A significant correlation was observed between levels of VEGF and levels of AGK in PDR patients (r = 0.954; p < 0.001). Western blot analysis revealed a significant increase in the expression of AGK and VEGFR-2 in vitreous samples and the retinas of diabetic rats compared to nondiabetic controls, whereas ATX was significantly downregulated. Our findings suggest that ATX–AGK–LPA signaling axis might be an important player in the development and progression of diabetic retinopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation, ischemia-induced angiogenesis and expansion of extracellular matrix (ECM) in association with the outgrowth of fibrovascular membranes at the vitreoretinal interface is the pathological hallmark in proliferative diabetic retinopathy (PDR). Development of PDR is a complex process where proteases, growth factors, cytokines, and chemokines are released from retinal cells and interact with each other to promote neovascularization and fibrosis [1–5]. Among those bio-molecules, lysophosphatidic acid (LPA) stands out as a unique potent bioactive phospholipid growth factor. LPA mediates diverse biological activities including platelet aggregation, smooth muscle contraction, cell proliferation, migration and survival, wound healing, tumor cell invasion and metastasis, angiogenesis, fibrosis, suppression of apoptosis and cytokine and chemokine secretion. LPA acts through the activation of six specific G protein-coupled receptors, named LPA1-6 [6–8].
The proinflammatory LPA, which is elevated in multiple inflammatory diseases [9, 10], is a potent activator of several transcription factors and signaling pathways which are known to be involved in the pathophysiology of diabetic retinopathy such as nuclear factor-κβ (NF-κβ), AP-1, hypoxia-inducible factor-1α (HIF-1α), Rho A/Rho kinase, mitogen-activated protein (MAP) kinases, and phosphatidylinositol 3-kinase (PI3K) [9, 11–14]. LPA, therefore, is a potent modulator of gene expression. LPA target genes include those involved in inflammation, angiogenesis, and fibrosis which are the pathological hallmarks of PDR [1–5] such as monocyte chemoattractant protein-1 (MPC-1) [11], interleukin-8 (IL-8) [11], intercellular adhesion molecule-1 (ICAM-1) [15], vascular cell adhesion molecule-1 (VCAM-1) [15], stromal cell-derived factor-1α (SDF-1α) [13], cyclooxygenase-2 and prostaglandin E2 production [10], vascular endothelial growth factor (VEGF) [12, 13, 16], matrix metalloproteinase (MMP)-9 [17], and connective tissue growth factor (CTGF) [18]. These findings suggest that LPA may contribute to PDR development and progression through regulation of the expression of its target genes, resulting in an inflammatory, angiogenic and fibrogenic microenvironment.
The major source of serum and plasma LPA seems to come from the lysophospholipase D activity of autotaxin (ATX/NPP2). ATX was initially isolated as a tumor motility- and metastatic-stimulating protein. ATX is a member of the ectonucleotide pyrophosphatase and phosphodiesterase family of enzymes that can catalyze hydrolysis of lysophosphatidylcholine into LPA. ATX is abundantly expressed in various biological fluids such as blood, plasma, serum, cerebrospinal fluid and seminal fluid. Expression of ATX is ubiquitous, but relatively high levels of ATX are expressed in brain, kidney and lymphoid organs. Extremely high levels of ATX are found in endothelial cells of high endothelial venules in lymphoid organs where it has a role in promoting the lymphocyte–endothelial cell interaction [6–8]. Another potential pathway for LPA synthesis is phosphorylation of monoacylglycerols by a specific lipid kinase, acylglycerol kinase (AGK) [14, 19, 20].
Given the key roles of LPA in inflammation, angiogenesis and fibrosis, we hypothesized that LPA and the LPA-producing enzymes ATX and AGK may contribute to PDR development and progression. To test this hypothesis, we measured the levels of LPA, ATX and AGK in the vitreous fluid from a series of patients with PDR and patients without diabetes and correlated their levels with clinical disease activity and the levels of the angiogenic factor VEGF. In addition, we investigated the expression of ATX and AGK, and vascular endothelial growth factor receptor-2 (VEGFR-2) in the retinas of diabetic rats.
Materials and methods
Vitreous samples
Undiluted vitreous fluid samples (0.3–0.6 ml) were obtained from 42 patients with PDR and 35 nondiabetic patients with rhegmatogenous retinal detachment (RD) during pars plana vitrectomy. The indications for vitrectomy in patients with PDR were traction RD and/or nonclearing vitreous hemorrhage. In patients with PDR, the severity of retinal neovascular activity was graded clinically at the time of vitrectomy using previously published criteria [21]. Neovascularization was considered active if there were visible perfused new vessels on the retina or optic disk present within tractional epiretinal membranes. Neovascularization was considered inactive (involuted) if only nonvascularized, white fibrotic epiretinal membranes were present. Active PDR was present in 20 patients and inactive PDR was present in 22 patients. The diabetic patients were 33 males and 9 females whose ages ranged from 22 to 81 years with a mean of 54.9 ± 13.6 years. Thirty patients had insulin-dependent diabetes mellitus, and 12 patients had noninsulin-dependent diabetes mellitus. The control group consisted of 35 patients who had undergone vitrectomy for the treatment of RD with no proliferative vitreoretinopathy. Controls were free from systemic disease and were 26 males and 9 females whose ages ranged from 18 to 85 years with a mean of 48.1 ± 17.7 years. Vitreous samples were collected undiluted by manual suction into a syringe through the aspiration line of vitrectomy, before opening the infusion line. The samples were centrifuged (500 rpm for 10 min, 4 °C), and the supernatants were aliquoted and frozen at −80 °C until assay. The study was conducted according to the tenets of the Declaration of Helsinki, and informed consent was obtained from all patients. The study was approved by the Research Centre, College of Medicine, King Saud University.
Animals
Diabetes was induced in 20 rats (male Sprague–Dawley, 200–220 g) with streptozotocin (55 mg/kg body weight). Rats were considered diabetic if their blood glucose was greater than 250 mg/dl. Age-matched 20 normal rats served as controls. After 10–12 weeks, the animals were killed by pentobarbital overdose, and the retinas were removed, snap-frozen in liquid nitrogen, and stored at −80 °C to analyze by using Western blot analysis. All experiments were performed in accordance with the Association of Research in Vision and Ophthalmology on treatment of Animals in Research and the King Saud University’s Animal Care and Use Committee Guidelines.
Enzyme-linked immunosorbent assay kits
Enzyme-linked immunosorbent assay (ELISA) kits for human ATX (Quantikine Autotaxin, Cat No: DENP20) and VEGF (Quantikine Human Vascular Endothelial Growth Factor, Cat No: DVE00) were purchased from R&D Systems, Minneapolis, MN. Human AGK (Acylglycerol Kinase, Cat No: E96236Hu) and LPA (Lysophosphatidic acid, Cat No: K-2800) were purchased from USCNK Life Sciences Inc, Wuhan, China and Echelon Biosciences Inc., Salt Lake City, USA, respectively. The detection limit of each ELISA kit for ATX, VEGF, and AGK was 93, 9.0, and 81 pg/ml, respectively. The ELISA plate readings were done using FLUOstar Omega-Miroplate reader from BMG Labtech, Offenburg, Germany.
Measurement of human ATX, VEGF, and AGK
The quantifications of the level of ATX, VEGF, and AGK in the vitreous fluid were determined using specific ELISAs according to the manufacturer’s instruction. For each ELISA, the undiluted standard served as the highest concentration and calibrator diluents serves as the blank. Depending upon the detection range for each ELISA, the supernatant vitreous obtained were either used directly or diluted with calibrator diluents (supplied with ELISA kit).
Vitreous sample were 20- and 2-fold diluted for ATX and VEGF with calibrator diluent supplied with ELISA kit. Diluted vitreous samples of 100 and 50 μl for ATX and VEGF, respectively, were added into each of the ELISA plate for the analysis, whereas 100 μl undiluted vitreous sample for AGK quantification was added into each ELISA well. Following sample incubation (2 h) into the wells of ELISA plates, antibodies against ATX, VEGF, and AGK conjugated to horseradish peroxidase were added to each well of the ELISA plate. After incubation and washing, substrate mix solution was added for color development. The reaction was completed by the addition of 2 N sulfuric acid, and optical density (OD) was read at 450 nm in a microplate reader. Each assay was performed in duplicate. Using the 4-parameter fit logistic (4-PL) curve equation, the concentration for each sample was calculated after multiplying with the dilution factors to get the actual reading for each sample.
Measurement of human LPA
For the measurement of LPA from vitreous fluid, the assay was performed as manufacturer’s recommendation. After blocking the 96-well plate, an amount of 100 μl of undiluted vitreous and anti-LPA antibody solution (sample to antibody at ratio of 4:1) were added in the respective wells. After incubating the plate for 1 h and washing out the unbound antigens, the secondary antibody conjugated to horseradish peroxidase was added to each well of the ELISA plate at room temperature for 1 h. After washing, substrate solution was added for color development. The reaction was completed by the addition of stop solution 2 N sulfuric acid and OD was read at 450 nm in microplate reader. Each assay was performed in duplicate. Using the 4-parameter fit logistic (4-PL) curve equation, the concentration for each sample was calculated.
Western blot analysis
To determine the ATX, AGK, and VEGFR2 protein levels, in the retinas of nondiabetic and diabetic rats, retinal tissues were homogenized in lysis buffer (30 mM Tris–HCL; pH 7.5; 5 mM EDTA; 1 % Triton X-100; 250 mM sucrose; 1 mM Sodium Vanadate and protease inhibitor cocktail). The lysate was centrifuged at 14,000×g for 15 min (4 °C) and supernatants decanted and the protein concentrations estimated using the Bio-Rad protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA). Protein samples were boiled in Laemmli’s sample buffer for 10 min, and equal amounts of protein (25–40 μg) were separated on 8–10 % SDS-polyacrylamide gels (SDS-PAGE) and transferred onto nitrocellulose membranes. To determine the expression levels of ATX and AGK in the vitreous samples, equal volume of samples were boiled in Laemmli’s sample buffer (1:1, v/v, reducing condition) for 10 min. Equal volume of lysed solution (15 μl) was loaded and separated on 8–10 % SDS-PAGE and transferred onto nitrocellulose membranes. After protein transferring, the membrane was blocked (1.5 h, room temperature) with 5 % nonfat milk made in Tris-buffered saline containing 0.1 % Tween-20 (TBS-T). For immunodetection of ATX and AGK and VEGFR2, the membrane was incubated overnight at 4 °C with rabbit polyclonal anti-ATX (1:300; Cat No: sc-66813, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), goat polyclonal anti-AGK (1 μg/ml; Cat No: ab77266, Abcam, UK), and goat polyclonal anti-VEGFR2 (0.1 μg/ml; Cat No: AF357, R&D System Minneapolis, MN), respectively. After overnight incubation with primary antibody, the membrane was washed three times with TBS-T (5 min each), and for ATX, the membrane was incubated at room temperature for 1.5 h with anti-rabbit secondary horseradish peroxidase-conjugated antibody (1:2,000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and for AGK and VEGFR2 with anti-goat secondary horseradish peroxidase-conjugated antibody (1:5,000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Membrane was again washed four times with TBS-T (5 min each), and the immunoreactivity of bands was visualized on a Chemi-DocXRS+ systems (Bio-Rad Laboratories Inc.) using enhanced chemiluminescence, and Western blotting luminol reagents (1:1 v/v, Cat No: SC-2048, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). By densitometric analysis using Image-Lab 2.0.1 software (open source, Bio-Rad Laboratories Inc.), the protein bands were quantified. For internal control, membranes were stripped and incubated with a mouse monoclonal anti-β-actin antibody (1:2,000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and all remaining steps were followed as detailed above.
Statistical analysis
The Mann–Whitney test was used to compare means from two independent groups. The χ2 test was used to compare proportions when analyzing data for two categorical variables. Nonmeasurable levels of any continuous variable were treated as zero during the statistical analyses. Pearson correlation coefficients were computed to investigate correlations between variables. One-way ANOVA and post-ANOVA pairwise comparisons of means were conducted using the Kruskal–Wallis test. For three groups, the critical Z value for post-ANOVA pairwise mean comparisons was Z = 2.39 at a 5 % level of significance. A p value less than 0.05 indicated statistical significance. SPSS version 12.0 and program 3S and LR from Bio-Medical Data Processing version 2007 (BMDP 2007) Statistical Software (Cork Technology Pack, Model Farm Road, Cord, Ireland) were used for the statistical analyses.
Results
Levels of VEGF, ATX, AGK, and LPA in vitreous samples
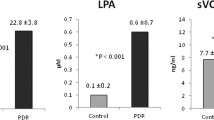
VEGF was measured in 25 out of the 27 (92.6 %) vitreous samples from patients with PDR assayed for VEGF, and in 4 out of 22 (18.2 %) samples from control patients without diabetes. The rate of measurable values of VEGF in vitreous samples from patients with PDR (92.6 %) was significantly higher than the rate of measurable values in vitreous samples from control patients (18.2 %) (p < 0.001; χ2 test). When all patients were considered, mean VEGF level in vitreous samples from PDR patients was significantly higher than those in control patients without diabetes (p < 0.001; Mann–Whitney test) (Table 1).
LPA was measured in all vitreous samples from patients with PDR (n = 42) and control patients without diabetes (n = 35). LPA mean level in vitreous samples from PDR patients was significantly higher than that in control patients without diabetes (p < 0.001; Mann–Whitney test) (Table 1).
ATX was measured in all vitreous samples from patients with PDR (n = 30) and control patients without diabetes (n = 22). The measured ATX levels in PDR patients were lower than those in control patients without diabetes, but the difference was not statistically significant (p = 0.065; Mann–Whitney test) (Table 1).
AGK was measured in 22 out of the 27 (81.5 %) vitreous samples from patients with PDR assayed for AGK, and in 4 out of 22 (18.2 %) samples from control patients without diabetes. The rate of measurable values of AGK in vitreous samples from patients with PDR (81.5 %) was significantly higher than the rate of measurable values in vitreous samples from control patients (18.2 %) (p < 0.001; χ2 test). AGK mean level in vitreous samples from PDR patients was significantly higher than that in control patients without diabetes (p < 0.001; Mann–Whitney test) (Table 1).
Western blot analysis was also used to quantify the expression levels of ATX and AGK in vitreous samples from patients with PDR (n = 16) and control patients without diabetes (n = 16). Densitometric analysis of the bands demonstrated a significant decrease in ATX (p = 0.029) and a significant increase in AGK (p < 0.001) expressions in vitreous samples from PDR patients compared to control patients (Fig. 1).
Relationship between VEGF, LPA, ATX and AGK levels, and activity of PDR
Comparison of the mean levels among active PDR patients, inactive PDR patients, and control patients without diabetes was conducted using the Kruskal–Wallis test, and the results are shown in Table 1. Mean levels differed significantly between the 3 groups for VEGF (p < 0.001), LPA (p < 0.001), ATX (p = 0.045), and AGK (p < 0.001). Post-ANOVA pairwise comparisons of means indicated that mean VEGF level was significantly higher in patients with active PDR than in patients with inactive PDR (Z = 2.61). Mean levels for patients with active PDR or inactive PDR were significantly higher than those for control patients without diabetes (Z = 5.62; Z = 3.20, respectively). For LPA, the mean levels for patients with active or inactive PDR were significantly higher than those for control patients without diabetes (Z = 5.74; Z = 4.35, respectively). For ATX, the mean level for patients with inactive PDR was significantly lower than that in control patients without diabetes (Z = 2.43). For AGK, mean levels was significantly higher in patients with active PDR than in patients with inactive PDR (Z = 2.70). Mean levels for patients with active PDR or inactive PDR were significantly higher than those for control patients without diabetes (Z = 5.49; Z = 2.96, respectively).
Correlations
There were significant correlations between vitreous fluid levels of VEGF and AGK in all PDR patients (r = 0.954; p < 0.001), active PDR patients (r = 0.947; p < 0.001), and inactive PDR patients (r = 0.805; p < 0.001). There was a significant correlation between vitreous fluid levels of LPA and ATX in only patients with active PDR (r = 0.595; p = 0.032).
Effect of diabetes on retinal expression of VEGFR-2, ATX, and AGK in experimental rats
The body weights of the diabetic rates were significantly lower, and their blood glucose values were significantly higher compared with age-matched normal control rates (234.4 ± 38.6 vs 361.6 ± 16.0 g (p < 0.001) and 523.1 ± 71.1 vs 109.8 ± 6.9 mg/dl (p < 0.001), respectively). We quantified the expression of VEGFR-2, ATX, and AGK in rat retinas by Western blot analysis. Densitometric analysis of the bands revealed a significant increase in VEGFR-2 (p = 0.003; Mann–Whitney test) and AGK (p < 0.001) and a significant decrease in ATX (p = 0.001) in diabetic retinas (n = 20) compared to nondiabetic controls (n = 20) (Fig. 2).
Discussion
Our study is the first to analyze expression levels of LPA and the LPA-producing enzymes, ATX and AGK, in the vitreous fluid from patients with PDR and in the retinas of diabetic rats in order to clarify the role of LPA in the development and progression of diabetic retinopathy. The main findings were as follows: (1) LPA and AGK were significantly upregulated in the vitreous fluid from patients with PDR, whereas ATX was significantly downregulated; (2) AGK levels in the vitreous from PDR patients with active neovascularization were significantly higher than those in patients with quiescent PDR; (3) There was a strong positive significant correlation between the levels of AGK and VEGF in the vitreous from all PDR patients, active PDR patients, and patients with quiescent PDR; (4) There was a positive significant correlation between the vitreous levels of LPA and ATX in patients with active PDR; and (5) AGK and VEGFR-2 were also upregulated in the retinas of diabetic rats, whereas ATX was downregulated. Our findings suggest that the ATX–AGK–LPA signaling axis might be an important player in the development and progression of diabetic retinopathy.
Strong evidence indicates that chronic, low-grade inflammation is implicated in the pathogenesis of diabetic retinopathy. Diabetic retinal vascular leakage, capillary nonperfusion, and endothelial cell damage are associated with leukocyte recruitment and adhesion to the retinal vasculature, findings that correlate with the increased expression of retinal ICAM-1 and the leukocyte integrin CD18. Inhibition of ICAM-1 activity suppresses both retinal leukostasis and vascular leakage [22, 23]. The causal relationship between inflammation and angiogenesis is now widely accepted [4, 24]. An emerging issue in diabetic retinopathy research is the focus on the mechanistic link between activation of subclinical inflammation and angiogenesis. Several studies reported elevated levels of biomarkers of inflammation and endothelial dysfunction in the vitreous from patients with PDR [1, 4]. In the present study, we report that the proinflammatory lipid mediator LPA was significantly upregulated in the vitreous fluid from patients with PDR. These results suggest that LPA may contribute to PDR development and progression by upregulating the expression of its target genes, resulting in a more inflammatory, angiogenic, and fibrogenic microenvironment. Our results are consistent with previous reports that demonstrated elevated levels of LPA in other inflammatory diseases. High concentrations of LPA have been detected in the bronchoalveolar lavage fluid of animal models of allergic asthma [9], and in the synovial fluid obtained from rheumatoid arthritis patients [10]. Previous studies reported that LPA induces inflammatory responses in endothelial cells, such as overexpression of the chemokines IL-8 and MCP-1 [11] and of the cell adhesion molecules ICAM-1 and VCALM-1 [15]. The LPA1 receptor has been shown to be essential for LPA-mediated activation of ICAM-1 mRNA and expression of cell-surface protein as well as the adhesion of macrophages to LPA-activated endothelial cells [15]. In a previous report, we demonstrated that LPA1 receptor was expressed by vascular endothelial cells and stromal cells in PDR fibrovascular epiretinal membranes [25]. This proinflammatory phenotype was mediated by the activation of NF-κβ, Rho kinase, p38 MAP kinase, and c-Jun N-terminal kinase (JNK) pathways [11, 15]. Recently, LPA has been recognized as an angiogenic factor. LPA promotes endothelial cell migration and differentiation in vitro and promotes barrier function in such cultures [26]. Recently, Rivera-Lopez et al. [27] demonstrated that LPA is a direct angiogenic factor in vivo and that the angiogenic response was quantitatively equivalent to that of VEGF. The angiogenic response obtained with LPA was due to the activation of LPA1 and/or LPA3 receptors. However, LPA-induced vessels were larger than those induced by VEGF, suggesting that LPA-induced vessels are more mature than VEGF-induced vessels.
The identification of angiogenesis regulatory factors is essential to understanding the complex process of new blood vessel formation in PDR [28]. LPA induces activation of the transcription factor HIF-1α that triggers the activation of a large number of genes encoding proteins that regulate angiogenesis, such as VEGF [12]. VEGF, an endothelial cell mitogen that also enhances vascular permeability, is thought to be the major angiogenesis factor in PDR [29]. VEGF binds with high affinity and activates two tyrosine kinase receptors VEGFR-1 and VEGFR-2. These receptors regulate physiological as well as pathological angiogenesis. VEGFR-2 has strong tyrosine kinase activity and is the major positive signal transducer for pathological angiogenesis including cancer and diabetic retinopathy as well as microvascular permeability [30]. LPA’s effects on VEGF have attracted much attention given VEGF’s role in angiogenesis and vascular hyperpermeability. LPA stimulates the expression of VEGF and VEGFR-2 to promote tumor angiogenesis [12, 13, 16, 31]. These findings can be correlated with our current data that show a strong significant correlation between the vitreous levels of the LPA-producing enzyme AGK and that of VEGF. In addition, recent studies showed significant involvement of LPA and LPA1 receptor in the development of pulmonary, liver, renal, and dermal fibrosis. LPA/LPA1 signaling activation was found to be associated with increased vascular leakage, increased fibroblast recruitment and proliferation, stimulated macrophage recruitment, and expression of the pro-fibrotic cytokine CTGF [32–35]. In a previous study, we demonstrated that the LPA-producing enzymes ATX and AGK and LPA1 receptor were specifically localized in myofibroblasts in proliferative vitreoretinopathy epiretinal membranes [25]. These findings suggest that LPA/LPA1 signaling may be a new therapeutic target in fibrotic disease. Jeon et al. [13] demonstrated that LPA induces expression of α-smooth muscle actin (α-SMA), a marker for myofibroblasts, in human adipose tissue-derived mesenchymal stem cells and that LPA1 receptor plays a key role in the LPA-induced α-SMA expression. LPA also induces the expression of many genes in quiescent fibroblasts that encode secreted factors, including chemokines, cytokines, growth factors, pro-angiogenic factors, and pro-fibrotic factors, involved in inflammation, leukocyte recruitment, angiogenesis, tissue remodeling, and wound healing [36].
In the present study, AGK was upregulated in the vitreous from patients with PDR and in the retinas of diabetic rats, whereas ATX was downregulated. In particular, levels of AGK were significantly higher in vitreous from patients with active PDR compared with quiescent PDR and correlated with VEGF levels. These results suggest that AGK plays a pivotal role in both the development and progression of PDR and that AGK, but not ATX, might be responsible for the increased synthesis and secretion of LPA in PDR. Recently, upregulation of AGK expression in prostate cancer was demonstrated. Increased expression of AGK increased formation and secretion of LPA. This increase resulted in concomitant transactivation of the epidermal growth factor receptor (EGF-R) and sustained activation of extracellular signal-regulated kinase (ERK) 1/2, culminating in enhanced cell proliferation and increased migratory responses [19, 20, 37]. In addition, overexpression of lentiviral AGK in human bronchial epithelial cells increased intracellular LPA production, enhanced LPA-mediated IL-8 secretion, and stimulated tyrosine phosphorylation of EGF-R. Downregulation of native AGK by AGK small interfering RNA decreased intracellular LPA levels and attenuated LPA-induced p38 MAPK, JNK, and NF-κβ activation, tyrosine phosphorylation of EGF-R, and IL-8 secretion [14].
In vivo studies demonstrated that the potent tumor motility-stimulating protein ATX is also an angiogenic factor. Purified ATX increased the vessel density of subcutaneously implanted Matrigel plugs. Furthermore, the angiogenic response was comparable to that elicited by VEGF [38]. In addition, a pathologic positive feedback loop between VEGF, ATX, and its product LPA was demonstrated. VEGF via VEGFR-2 stimulated expression of ATX and, consequently, LPA production as well as LPA1 receptor signaling in human endothelial cells [39]. ATX, in turn, increased VEGF expression via its product LPA [12, 13]. Downregulation of ATX expression significantly decreased VEGFR-2 and abolished endothelial cell migration to LPA, ATX, and VEGF [39]. However, to our surprise, ATX was significantly downregulated in the vitreous fluid from patients with PDR and in the retinas of diabetic rats. Our results are consistent with a previous report showing nonsignificant reduction of adipose tissue-ATX mRNA levels in mice 4 weeks after streptozotocin-induced diabetes [40]. These findings suggest that AGK, but not ATX, might be responsible for the increased synthesis and secretion of LPA in PDR, or alternatively, other pathways might be involved. Recent in vitro and in vivo studies demonstrated that ATX is also critically involved in the regression of newly formed blood vessels suggesting a dual role for ATX in angiogenic disorders [41]. In addition, recent studies demonstrated anti-inflammatory and antioxidant activities for ATX. This protective effect of ATX was partially reduced in the presence of an LPA-receptor antagonist [42]. Our findings suggest that hyperglycemia reduces ATX production in diabetic retinas and in the vitreous from patients with PDR and that downregulation of ATX expression might be a novel pathway that contributes to the persistence of inflammation and progression of neovascularization in PDR. However, this hypothesis, as well as the importance of ATX in the pathogenesis of diabetic retinopathy warrants further investigation.
In conclusion, our previous study [25], together with the present results, suggest that the LPA-rich microenvironment is advantageous for the progression of PDR and that the ATX–AGK–LPA/LPA1 signaling axis might be an important player in the development and progression of diabetic retinopathy. Further studies are needed to understand the mechanisms by which this signaling axis might promote the development of diabetic retinopathy. Our findings suggest that this signaling axis could be a novel target in diabetic retinopathy. Multiple approaches targeting specific components of this signaling axis are in preclinical development [43].
References
Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J (2006) Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw 17:155–165
Abu El-Asrar AM, Van den Steen PE, Al-Amro SA, Missotten L, Opdenakker G, Geboes K (2007) Expression of angiogenic and fibrogenic factors in proliferative vitreoretinal disorders. Int Ophthalmol 27:11–22
Descamps FJ, Martens E, Kangave D, Struyf S, Geboes K, Van Damme J, Opdenakker G, Abu El-Asrar AM (2006) The activated form of gelatinase B/matrix metalloproteinase-9 is associated with diabetic vitreous hemorrhage. Exp Eye Res 83:401–407
Abu El-Asrar AM, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S, Al-Shabrawey M (2001) High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis 17:1829–1838
Abu El-Asrar AM, Missotten L, Geboes K (2007) Expression of hypoxia-inducible factor-1 alpha and the protein products of its target genes in diabetic fibrovascular epiretinal membranes. Br J Ophthalmol 91:822–826
Okudaira S, Yukiura H, Aoki J (2010) Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie 92:698–706
Houben AJS, Moolenaar WH (2011) Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev 30:557–565
Nakanaga K, Hama K, Aoki J (2010) Autotaxin—an LPA producing enzyme with diverse functions. J Biochem 148:13–24
Zhao Y, Natarajan V (2009) Lysophosphatidic acid signaling in airway epithelium: role in airway inflammation and remodeling. Cell Signal 21:367–377
Nochi H, Tomura H, Tobo M, Tanaka N, Sato K, Shinozaki T, Kobayashi T, Takagishi K, Ohta H, Okajima F, Tamoto K (2008) Stimulatory role of lysophosphatidic acid in cyclooxygenase-2 induction by synovial fluid of patients with rheumatoid arthritis in fibroblast-like synovial cells. J Immunol 181:5111–5119
Shimada H, Rajagopalan LE (2010) Rho-kinase mediates lysophosphatidic acid-induced IL-8 and MCP-1 production via p38 and JNK pathways in human endothelial cells. FEBS Lett 584:2827–2832
Lee J, Park SY, Lee EK, Park CG, Chung HC, Rha SY, Kim YK, Bae GU, Kim BK, Han JW, Lee HW (2006) Activation of hypoxia-inducible factor-1α is necessary for lysophosphatidic acid-induced vascular endothelial growth factor expression. Clin Cancer Res 12:6351–6358
Jeon ES, Heo SC, Lee IH, Choi YJ, Park JH, Choi KU, Park DY, Suh DS, Yoon MS, Kim JH (2010) Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1α from human mesenchymal stem cells. Exp Mol Med 42:280–293
Kalari S, Zhao Y, Spannhake EW, Berdyshev EV, Natarajan V (2009) Role of acylglycerol kinase in LPA-induced IL-8 secretion and transactivation of epidermal growth factor-receptor in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 296:L328–L336
Shimada H, Rajagopalan LE (2010) Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-κβ p65. J Biol Chem 285:12536–12542
Dutta S, Wang F-Q, Wu H-S, Mukherjee TJ, Fishman DA (2011) The NF-κβ pathway mediates lysophosphatidic acid (LPA)-induced VEGF signaling and cell invasion in epithelial ovarian cancer (EOC). Gynecol Oncol 123:129–137
Park SY, Jeong KJ, Panupinthu N, Yu S, Lee J, Han JW, Kim JM, Lee JS, Kang J, Park CG, Mills GB, Lee HY (2011) Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene 30:1351–1359
Heusinger-Ribeiro J, Eberlein M, Wahab NA, Goppelt-Struebe M (2001) Expression of connective tissue growth factor in human renal fibroblasts: regulating roles of Rho A and cAMP. J Am Soc Nephrol 12:1853–1861
Bektas M, Payne SG, Liu H, Goparaju S, Milstien S, Spiegel S (2005) A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol 169:801–811
Spiegel S, Milstien S (2005) Critical role of acylglycerol kinase in epidermal growth factor-induced mitogenesis of prostate cancer cells. Biochem Soc Trans 33:1362–1365
Aiello LP, Avery RL, Arrig PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL (1994) Vascular endothelial growth factor in ocular fluid in patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP (2004) A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 18:1450–1452
Miyamoto K, Khosrof S, Brusell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP (1999) Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA 96:10836–10841
Van Beijnum JR, Buurman WA, Griffioen AW (2008) Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11:91–99
Abu El-Asrar AM, Missotten L, Geboes K (2011) Expression of autotaxin and acylglycerol kinase in proliferative vitreoretinal epiretinal membranes. Acta Ophthalmol. doi:10.1111/j.1755-3768.2011.02303.x
Lee H, Goetzl EJ, An S (2000) Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am J Physiol Cell Physiol 278:C612–C618
Rivera-Lopez CM, Tucker AL, Lynch KR (2008) Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis 11:301–310
Abu El-Asrar AM, Nawaz MI, Kangave D, Siddiquei MM, Ola MS, Opdenakker G (2011) Angiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathy. Acta Diabetol. doi:10.1007/s00592-011-0330-9
Spranger J, Pfeiffer AP (2001) New concepts in pathogenesis and treatment of diabetic retinopathy. Exp Clin Endocrinol Diabetes 109:S438–S450
Shibuya M (2006) Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 39:469–478
Song Y, Wu J, Oyesanya RA, Lee Z, Mukherjee A, Fang X (2009) Sp-1 and c-Myc mediate lysophosphatidic acid-induced expression of vascular endothelial growth factor in ovarian cancer cells via a hypoxia-inducible factor-1-independent mechanism. Clin Cancer Res 15:492–501
Pradère JP, Gonzalez J, Klein J, Valet P, Grēs S, Salant D, Bascands JL, Saulnier-Blache JS, Schanstra JP (2008) Lysophosphatidic acid and renal fibrosis. Biochim Biophys Acta 1781:582–587
Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD (2008) The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14:45–54
Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC, Fagan P, Baccei CS, Santini AM, Hutchinson JH, Seiders TJ, Parr TA, Prasit P, Evans JF, Lorrain DS (2010) A novel, orally active LPA (1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol 160:1699–1713
Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM (2011) Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum 63:1405–1415
Stortelers C, Kerkhoven R, Moolenaar WH (2008) Multiple actions of lysophosphatidic acid on fibroblasts revealed by transcriptional profiling. BMC Genomics 9:387–402
Nouh MAA, Wu X-X, Okazoe H, Tsunemori H, Haba R, Abou-Zeid AM, Saleem MD, Inui M, Sugimoto M, Aoki J, Kakehi Y (2009) Expression of autotaxin and acylglycerol kinase in prostate cancer: association with cancer development and progression. Cancer Sci 100:1631–1638
Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, Stracke ML (2001) Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res 61:6938–6944
Ptaszynska MM, Pendrak ML, Stracke ML, Roberts DD (2010) Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor-induced endothelial cell migration. Mol Cancer Res 8:309–321
Boucher J, Quilliot D, Pradère JP, Simon MF, Grēs S, Guigné C, Prévot D, Ferry G, Boutin JA, Carpéné C, Valet P, Saulnier-Blache JS (2005) Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetelogia 48:569–577
Im E, Motiejunaite R, Aranda J, Park EY, Federico L, Kim T, Clair T, Stracke ML, Smyth S, Kazlauskas A (2010) Phospholipase Cγ activation drives increased production of autotaxin in endothelial cells and lysophosphatidic acid-dependent regression. Mol Cell Biol 30:2401–2410
Awada R, Rondeau P, Grès S, Saulnier-Blache JS, Lefebvre d’ Hellencourt C, Bourdon E (2012) Autotaxin protects microglial cells against oxidative stress. Free Radical Biol Med 52:516–526
Panupinthu N, Lee HY, Mills GB (2010) Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br J Cancer 102:941–946
Acknowledgments
The authors thank Ms. Connie B. Unisa-Marfil for secretarial work. This work was supported by Dr. Nasser Al-Rasheed Research Chair in Ophthalmology (Abu El-Asrar AM).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Renato Lauro.
Rights and permissions
About this article
Cite this article
Abu El-Asrar, A.M., Mohammad, G., Nawaz, M.I. et al. Expression of lysophosphatidic acid, autotaxin and acylglycerol kinase as biomarkers in diabetic retinopathy. Acta Diabetol 50, 363–371 (2013). https://doi.org/10.1007/s00592-012-0422-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-012-0422-1