Abstract
Purpose
To investigate the vitreous and serum levels of angiopoietin-like protein 8 (ANGPTL-8) and vascular endothelial growth factor (VEGF) in patients with proliferative diabetic retinopathy (PDR). The serum levels of these factors were also analyzed in patients with diabetes and no diabetic retinopathy (NDR) and with non-proliferative diabetic retinopathy (NPDR), to detect the possible correlation between the ANGPTL-8 levels and hyperlipidemia.
Methods
Vitreous samples were obtained from 28 patients with PDR and from 12 patients without diabetes and with idiopathic macular hole (IMH). Serum samples were also obtained from 26 patients with NDR and 22 patients with NPDR. ANGPTL-8 levels and other factors were determined using an enzyme-linked immunosorbent assay.
Results
The ANGPTL-8 and VEGF levels in the vitreous and serum of the patients with PDR were higher than those in the patients with IMH, and were significantly correlated. The vitreous and serum ANGPTL-8 levels were more correlated with the triglyceride and low-density lipoprotein cholesterol levels than with the high-density lipoprotein cholesterol or total cholesterol levels in the patients with PDR.
Conclusions
The vitreous and serum ANGPTL-8 levels were both upregulated in patients with PDR. There was an association between the elevation in the ANGPTL-8 levels and angiogenic and hyperlipidemic factors in the patients with PDR. These results suggest that ANGPTL-8 is a potential new diagnostic marker and therapeutic target for PDR treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is a microvascular complication that affects approximately 30% of patients with diabetes and is the leading cause of preventable blindness in patients of working age [1,2,3]; the identification of effective treatments and prevention measures has long been the objective of numerous studies [4, 5]. Several studies have reported the important role of vascular endothelial growth factor (VEGF) in angiogenesis and inflammation due to dysglycemia in DR progression [6,7,8]. However, the pathogenesis and underlying mechanism of DR, especially proliferative diabetic retinopathy (PDR), are currently unknown.
Increasing evidence from recent studies has suggested that dyslipidemia is closely involved in the progression of PDR [9]; novel adipocytokines, such as angiopoietin-like proteins (ANGPTLs), have been of particular interest [10, 11]. ANGPTL-8, a novel ANGPTL family member, plays a role in both dyslipidemia and dysglycemia [9]. Several recent studies have reported that circulating ANGPTL-8 levels are increased in patients with type 1 and type 2 diabetes mellitus (T1/2DM) [12,13,14,15]. Paradoxically, a single-center study has shown that serum ANGPTL-8 levels decreased significantly in T2DM and patients with obesity [16]. Some researchers have attributed these conflicting results to the enzyme-linked immunosorbent assay (ELISA) kits used in these studies [17]. Nonetheless, ANGPTL-8 has been suggested as a potential biomarker of T2DM. However, there have been few studies on the potential crosstalk between ANGPTL-8 and VEGF, especially considering that the expression levels of ANGPTL-8 and VEGF are both elevated in patients with diabetes.
Recent studies have reported that levels of ANGPTL-8 correlate with low-density lipoprotein cholesterol (LDL-C) [18,19,20]; furthermore, a substantial amount of data has shown that LDL-C mediates or correlates with VEGF levels [21,22,23,24]. Based on all these findings, we examined whether ANGPTL-8 together with the proangiogenic factor, VEGF, play roles in the progression of PDR. If a correlation between ANGPTL-8 and VEGF levels exists, then ANGPTL-8 could possibly be used as a therapeutic target for PDR. In this study, we identified a correlation between ANGPTL-8 and VEGF in the vitreous fluid and serum of patients with PDR. To our knowledge, this is the first study that has reported a novel dyslipidemic role for ANGPTL-8 in dysglycemia-related PDR.
Materials and methods
Participants
The research followed the tenets of the Declaration of Helsinki (as revised in Brazil 2013) and was approved by the First Affiliated Hospital of Soochow University. The nature and possible consequences of the study were explained to all participants, and written informed consent was obtained before participation. After biochemical and physiological tests (medical/health history, vitals, body mass index [BMI], fasting glucose [FG], glycosylated hemoglobin [HbA1c], serum lipid profiles, liver enzymes, and electrocardiography) and careful retinal examination or fluorescein angiography followed by pupillary dilation with tropicamide, 88 patients were divided into the following four groups: the idiopathic macular hole (IMH) group (12 eyes of 12 patients with IMH), nondiabetic retinopathy (NDR) group (26 eyes of 26 patients with T2DM and no diabetic retinopathy), non-proliferative diabetic retinopathy (NPDR) group (22 eyes of 22 patients with NPDR), and PDR group (28 eyes of 28 patients with PDR). The IMH group served as controls, and none of the patients with IMH had DM. The PDR group and IMH group consisted of patients admitted to undergo vitreous surgery. The patients were excluded if they had received treatment for ocular diseases. Weight was measured in light outdoor clothing without shoes, and BMI was calculated by dividing weight (kilograms) by height squared (meters squared). The demographic details of the patients are shown in Table 1.
Vitreous collection and analysis
The vitreous samples were collected from the PDR group and IMH group that underwent vitreous surgery (23-gauge, Alcon Instruments, USA) for the treatment of retinal disorders. In the present study, the patients who had undergone previous laser treatment or a repeat vitrectomy before their current surgery were excluded. After patient consent was obtained, the vitreous fluid (0.3–0.5 ml) was aspirated through a vitreous cutter under the simultaneous inflation of the vitreous cavity with air through an infusion cannula; the fluid was frozen rapidly at −80 °C until the assay was performed. The vitreous ANGPTL-8 measurements were performed using a commercially available ELISA kit (Aviscera Bioscience, CA, USA) according to the manufacturer’s protocol. Similarly, the vitreous VEGF measurements were performed using a Quantikine VEGF assay kit (R&D Systems, Inc., Minneapolis, MN, USA).
Serum collection and analysis
Serum samples were collected from all 88 participants. Venous blood was collected from an antecubital vein in a 5-ml K3EDTA Vacutainer using minimum hemostasis after the patients fasted overnight for at least 10 h. The blood was well mixed by gently inverting the vacutainer 10 times. Once centrifuged, the serum was separated and frozen at −80 °C until analysis. The HbA1c measurements were performed by high-pressure liquid chromatography on a Bio-Rad Variant II instrument. Triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were analyzed using an automatic biochemical analyzer (FUJI DRI-CHEM 4000i, Fuji, Japan).
Statistical analysis
The results are expressed as the means ± standard deviation (SD). Group means were compared by one-way ANOVA using GraphPad Prism 4.0 software (GraphPad, San Diego, CA, USA) and the statistical software program SPSS version 17.0 for Windows (SPSS, Chicago, IL, USA). Pearson correlation tests were also performed. For all comparisons, a value of P < 0.05 was considered statistically significant.
Results
Table 1 shows the clinical data of all participants in the four groups studied, including the PDR group, IMH group, NDR group, and NPDR group. There was no significant difference in age, BMI, serum level of TC, or medical/health history among the four groups (Table 1). The FG level in the PDR group was 11.26 ± 1.41 mmol/l, which was significantly higher than that in the IMH group (5.45 ± 0.32 mmol/l, P < 0.01; Table 1). The HbA1c level in the PDR group was 8.55 ± 0.48%, which was significantly higher than that in the IMH group (5.36 ± 0.31%, P < 0.01; Table 1). In addition, the serum LDL-C level in the PDR group was 2.72 ± 0.41 mmol/l, which was higher than that in the IMH group (2.19 ± 0.65 mmol/l, P < 0.01; Table 1). In contrast, the serum HDL-C level in the PDR group was 1.08 ± 0.33 mmol/l, which was lower than that in the IMH group (1.36 ± 0.25 mmol/l, P < 0.01; Table 1). Furthermore, the serum TG level was significantly higher in the PDR group (2.77 ± 0.69 mmol/l) than that in the IMH group (1.43 ± 0.56 mmol/l, P < 0.01; Table 1).
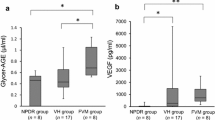
To determine whether the level of ANGPTL-8 was altered in patients with PDR compared with the patients with IMH, the expression levels of ANGPTL-8 in the serum and vitreous samples were measured using ELISA. Serum ANGPTL-8 was detectable in all four groups (Fig. 1b). The mean serum ANGPTL-8 level in the PDR group (1.60 ± 0.19 ng/ml) was significantly higher than that in the IMH group (1.02 ± 0.21 ng/ml, P < 0.01; Table 1), the NDR group (1.12 ± 0.23 ng/ml, P < 0.01; Table 1), and the NPDR group (1.17 ± 0.11 ng/ml, P < 0.01; Table 1). Moreover, the mean vitreous ANGPTL-8 level in patients with PDR (2.10 ± 0.43 ng/ml) was significantly higher than that in the patients with IMH (0.60 ± 0.12 ng/ml, P < 0.001; Table 2; Fig. 1a). Accordingly, we sought to determine whether there was a relationship among the elevated biomarkers. A significant correlation was found between serum and vitreous ANGPTL-8 levels (R = 0.73, P < 0.001; Fig. 2a).
Correlations between ANGPTL-8 with VEGF in PDR patients. Pearson’s correlation tests showed the positively significant correlations a between vitreous ANGPTL-8 and serum ANGPTL-8 (R = 0.73, P < 0.001), b between vitreous ANGPTL-8 and vitreous VEGF (R = 0.85, P < 0.001), and c between serum ANGPTL-8 and serum VEGF (R = 0.72, P < 0.001) in PDR patients
Previous studies over the past few decades have suggested that VEGF is the most quintessential and representative biomarker of angiogenesis, and the most frequent clinical sign induced by PDR. In this study, we evaluated possible correlations between increasing levels of vitreous ANGPTL-8 and vitreous VEGF. In the PDR group, 14 eyes had vitreous hemorrhages (VH), six eyes had tractional retinal detachments (TRD), and eight eyes had VH and TRD simultaneously (Table 2). The mean VEGF level in the vitreous of the patients with PDR (1,551.00 ± 420.50 pg/ml) was significantly higher than that in the patients with IMH (31.2 pg/ml, P < 0.001; Table 2). In addition, the mean serum VEGF level in the PDR group (112.60 ± 19.81 pg/ml) was significantly higher than that in the IMH group (46.55 ± 8.74 pg/ml, P < 0.001; Table 1), in the NDR group (49.28 ± 13.91 pg/ml, P < 0.001; Table 1), and in the NPDR group (64.25 ± 12.53 pg/ml, P < 0.001; Table 1). Consistent with our hypothesis, there was a significantly positive correlation between the vitreous ANGPTL-8 and VEGF levels (R = 0.85, P < 0.001; Fig. 2b). There was also a significant positive correlation between the serum ANGPTL-8 and VEGF levels (R = 0.72, P < 0.001; Fig. 2c).
The correlation between BMI and the serum ANGPTL-8 level (R = 0.37, P = 0.055; Fig. 3b) was less than that between BMI and the vitreous ANGPTL-8 level (R = 0.50, P = 0.007; Fig. 3a) in the patients with PDR. In contrast, the correlation between HbA1c and serum ANGPTL-8 levels (R = 0.66, P < 0.001; Fig. 3d) was greater than that between the HbA1c and vitreous ANGPTL-8 levels (R = 0.59, P < 0.001; Fig. 3c) in patients with PDR.
Correlations between ANGPTL-8 with BMI and HbA1c in PDR patients. Pearson’s correlation tests showed the positive correlations a between vitreous ANGPTL-8 and BMI (R = 0.50, P = 0.007), b between serum ANGPTL-8 and BMI (R = 0.37, P = 0.055), c between vitreous ANGPTL-8 and HbA1c (R = 0.59, P < 0.001), and d between serum ANGPTL-8 and HbA1c (R = 0.66, P < 0.001) in PDR patients
Consistent with the observation that PDR is usually accompanied by hyperlipidemia, there was also a correlation between the ANGPTL-8 level and the serum lipid profile in the patients with PDR. The positive correlation between the serum TG and ANGPTL-8 (R = 0.63, P < 0.001; Fig. 4b) was less than that between the serum TG and vitreous ANGPTL-8 (R = 0.73, P < 0.001; Fig. 4a) in the patients with PDR. In contrast, the positive correlation between the serum LDL-C and serum ANGPTL-8 levels (R = 0.69, P < 0.001; Fig. 4d) was greater than that between the serum LDL-C and vitreous ANGPTL-8 levels (R = 0.59, P < 0.01; Fig. 4c) in patients with PDR. However, negative correlations were found between the serum HDL-C and ANGPTL-8 levels (R = −0.44, P = 0.020; Fig. 4f) and between the serum HDL-C and vitreous ANGPTL-8 levels (R = −0.41, P = 0.028; Fig. 4e) in the patients with PDR. A lower positive correlation between the serum TC and serum ANGPTL-8 levels was also confirmed (R = 0.43, P = 0.023; Fig. 4h); however, the correlation between the serum TC and vitreous ANGPTL-8 levels was not significant in the patients with PDR (R = 0.33, P = 0.091; Fig. 4g).
Correlation between ANGPTL-8 with serum lipids in PDR patients. Pearson’s correlation tests showed the significant correlations a between vitreous ANGPTL-8 and serum TG (R = 0.73, P < 0.001), b between serum ANGPTL-8 and serum TG (R = 0.63, P < 0.001), c) between vitreous ANGPTL-8 and serum LDL-C (R = 0.59, P < 0.001), d between serum ANGPTL-8 and serum LDL-C (R = 0.69, P < 0.001), e between vitreous ANGPTL-8 and serum HDL-C (R = −0.41, P = 0.028), f between serum ANGPTL-8 and serum HDL-C (R = −0.44, P = 0.020), g between vitreous ANGPTL-8 and serum TC (R = 0.33, P = 0.091), and h between serum ANGPTL-8 and serum TC (R = 0.43, P = 0.023) in PDR patients
To explore whether ANGPTL-8 is a more representative adipocytokine than regular lipid molecules in the disease state of PDR, we analyzed the correlation between VEGF and lipid molecules, such as TG, LDL-C, HDL-C, and TC. We found that there were positive correlations only between the vitreous VEGF and serum TG levels (R = 0.64, P = 0.002; Fig. 5a) and between the vitreous VEGF and serum LDL-C levels (R = 0.67, P < 0.001; Fig. 5b). However, there was no significant correlation between the VEGF levels and other lipid molecules (P > 0.05; Fig. 5).
Correlation between VEGF with serum lipids in PDR patients. Pearson’s correlation tests showed the significant correlations a between vitreous VEGF and serum TG (R = 0.64, P = 0.002), b between serum VEGF and serum TG (R = 0. 36, P = 0.057), c between vitreous VEGF and serum LDL-C (R = 0.67, P < 0.001), d between serum VEGF and serum LDL-C (R = 0.31, P = 0.111), e between vitreous VEGF and serum HDL-C (R = −0.32, P = 0.093), f between serum VEGF and serum HDL-C (R = −0.19, P = 0.340), g between vitreous VEGF and serum TC (R = 0.14, P = 0.463), and h between serum VEGF and serum TC (R = 0.27, P = 0.165) in PDR patients
Discussion
Recent studies have reported that dyslipidemia may contribute to DR susceptibility [9]. ANGPTL-8, also known as lipasin [25, 26], refeeding-induced in fat and liver (RIFL) [27], and betatrophin [28], is an important adipocytokine in lipid metabolism that contributes to both dyslipidemia and dysglycemia [29,30,31]. However, the mechanism by which dyslipidemia alters retinal homeostasis is unknown; multiple pathways may be involved in the progression of DR. [1, 32].
In this study, we first determined the ANGPTL-8 levels in the human vitreous and serum samples, and showed that the vitreous and serum levels of ANGPTL-8 were increased in the patients with PDR compared with those in the patients with IMH. The most destructive damage during PDR is thought to be closely associated with VEGF overexpression, and we showed a positive correlation between the VEGF and ANGPTL-8 levels in the vitreous and serum of the patients with PDR. To our knowledge, this is the first report to link ANGPTL-8, a novel adipocytokine, with VEGF in patients with PDR. Nonetheless, the mechanism underlying the correlation between ANGPTL-8 and VEGF remains unclear.
One possible explanation for the crosstalk between these two proteins in the vitreous fluid is that leakage of ANGPTL-8 from the blood to the vitreous body may occur as a result of the destruction of the blood–retinal barrier. However, we cannot exclude the possibility that ANGPTL-8 is produced by cells of the vascular endothelium and other cells within the ischemic retina or fibrovascular epiretinal membranes.
Recent studies have reported that ANGPTL-8 levels are correlated with LDL-C [18,19,20]. Observational and preclinical studies have reported that elevated LDL-C and TG levels could be used to predict the progression of DR. [33,34,35] Furthermore, Dandapat A et al. have found that small concentrations of oxygenized LDL promote capillary tube formation by inducing the expression of VEGF via lectin-like oxygenized LDL receptor 1 mediated activation of the NADPH-oxidase/MAPKs/NF-κB pathway [36]. Thus, we speculate that the LDL/MAPKs/NF-κB pathway may be a potential alternative target for future in-vivo and in-vitro research focusing on the correlation between ANGPTL-8 and VEGF, especially given the clinical application of anti-VEGF drugs (e.g., ranibizumab and conbercept) [37].
Although the mechanism underlying the increased ANGPTL-8 levels in patients with PDR is currently unknown, we have determined that the increased ANGPTL-8 levels significantly correlated with increased TG and LDL-C levels, more than with TC and HDL-C levels, in patients with PDR. Moreover, despite the fact that other lipid molecules were simultaneously increased in patients with PDR, analogous correlations were only found between the vitreous VEGF levels and increased TG and LDL-C levels; this finding suggests, at least partly, a greater role for ANGPTL-8 than regular lipid molecules in hyperlipidemia accompanied by PDR in patients. Later, a more significant correlation between the vitreous ANGPTL-8 levels and BMI, as opposed to that between the serum ANGPTL-8 levels and BMI, was found in the patients with PDR. It is widely known that most patients with PDR suffer from hyperlipidemia, which suggests that routine and dietary habits may have contributed, at least in part, to the increase in ANGPTL-8 levels.
We also observed higher serum ANGPTL-8 levels in older patients with IMH, but not in the older patients with PDR. Espes D et al. reported that the ANGPTL-8 levels positively correlated (P = 0.01) with age in patients without diabetes [15]. It would therefore be meaningful in future studies to determine possible correlations between ANGPTL-8 levels and age in different groups.
The main limitation of this study was that it was a single-center, small-sample, cross-sectional study; a multicenter, large-sample, prospective study is needed to confirm these results. Additionally, the molecular mechanism(s) and underlying pathway(s) between ANGPTL-8 and VEGF require further in-vivo and in-vitro studies. Finally, the potential feedback between ANGPTL-8 and dyslipidemia in patients with PDR requires a more comprehensive investigation.
ANGPTL-8, which is representative of lipid metabolism, may contribute to glucose homeostasis and facilitate future targeted synergistic therapies for hyperglycemia and hyperlipidemia [29,30,31]. After all, PDR is much more than simple chronic hyperglycemia in one eye and may involve novel factors, which suggests that future studies are needed to determine how to control ANGPTL-8 regulation of VEGF expression in hyperglycemia homeostasis. Due to the importance of VEGF in the treatment of PDR, the current results suggest the importance of future studies regarding the roles of ANGPTL-8 in diabetic retinal complications.
References
Lu QY, Chen W, Lu L, Zheng Z, Xu X (2014) Involvement of RhoA/ROCK1 signaling pathway in hyperglycemia-induced microvascular endothelial dysfunction in diabetic retinopathy. Int J Clin Exp Pathol 7:7268–7277
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376:124–136. doi:10.1016/S0140-6736(09)62124-3
Jialal I, Rajamani U, Siegel D (2014) The role of dyslipidemia in diabetic retinopathy: a brighter focus? J Diabetes Complicat 28:753–754. doi:10.1016/j.jdiacomp.2014.07.002
Zheng Z, Chen H, Ke G, Fan Y, Zou H, Sun X, Gu Q, Xu X, Ho PC (2009) Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes 58:954–964. doi:10.2337/db07-1524
Chen W, Cao H, Lu QY, Wang N, Zhao SZ, Xu X, Zheng Z (2014) Urinary 6-sulfatoxymelatonin level in diabetic retinopathy patients with type 2 diabetes. Int J Clin Exp Pathol 7:4317–4322
Grant MB, Afzal A, Spoerri P, Pan H, Shaw LC, Mames RN (2004) The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs 13:1275–1293. doi:10.1517/13543784.13.10.1275
Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A (2013) Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab 17:61–72. doi:10.1016/j.cmet.2012.12.010
Nakao S, Arima M, Ishikawa K, Kohno R, Kawahara S, Miyazaki M, Yoshida S, Enaida H, Hafezi-Moghadam A, Kono T, Ishibashi T (2012) Intravitreal anti-VEGF therapy blocks inflammatory cell infiltration and re-entry into the circulation in retinal angiogenesis. Invest Ophthalmol Vis Sci 53:4323–4328. doi:10.1167/iovs.11-9119
Wang Y, Quagliarini F, Gusarova V, Gromada J, Valenzuela DM, Cohen JC, Hobbs HH (2013) Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc Natl Acad Sci U S A 110:16109–16114. doi:10.1073/pnas.1315292110
Santulli G (2014) Angiopoietin-like proteins: a comprehensive look. Front Endocrinol (Lausanne) 5:4. doi:10.3389/fendo.2014.00004
Xin X, Rodrigues M, Umapathi M, Kashiwabuchi F, Ma T, Babapoor-Farrokhran S, Wang S, Hu J, Bhutto I, Welsbie DS, Duh EJ, Handa JT, Eberhart CG, Lutty G, Semenza GL, Montaner S, Sodhi A (2013) Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci U S A 110:E3425–E3434. doi:10.1073/pnas.1217091110
Espes D, Lau J, Carlsson PO (2014) Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia 57:50–53. doi:10.1007/s00125-013-3071-1
Hu H, Sun W, Yu S, Hong X, Qian W, Tang B, Wang D, Yang L, Wang J, Mao C, Zhou L, Yuan G (2014) Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care 37:2718–2722. doi:10.2337/dc14-0602
Fu Z, Berhane F, Fite A, Seyoum B, Abou-Samra AB, Zhang R (2014) Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci Rep 4:5013. doi:10.1038/srep05013
Espes D, Martinell M, Carlsson PO (2014) Increased circulating betatrophin concentrations in patients with type 2 diabetes. Int J Endocrinol 2014:323407. doi:10.1155/2014/323407
Gomez-Ambrosi J, Pascual E, Catalan V, Rodriguez A, Ramirez B, Silva C, Gil MJ, Salvador J, Fruhbeck G (2014) Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J Clin Endocrinol Metab 99:E2004–E2009. doi:10.1210/jc.2014-1568
Fu Z, Abou-Samra AB, Zhang R (2014) An explanation for recent discrepancies in levels of human circulating betatrophin. Diabetologia 57:2232–2234. doi:10.1007/s00125-014-3346-1
Hanson RL, Leti F, Tsinajinnie D, Kobes S, Puppala S, Curran JE, Almasy L, Lehman DM, Blangero J, Duggirala R, DiStefano JK (2016) The Arg59Trp variant in ANGPTL8 (betatrophin) is associated with total and HDL-cholesterol in American Indians and Mexican Americans and differentially affects cleavage of ANGPTL3. Mol Genet Metab 118:128–137. doi:10.1016/j.ymgme.2016.04.007
Quagliarini F, Wang Y, Kozlitina J, Grishin NV, Hyde R, Boerwinkle E, Valenzuela DM, Murphy AJ, Cohen JC, Hobbs HH (2012) Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci U S A 109:19751–19756. doi:10.1073/pnas.1217552109
Abu-Farha M, Abubaker J, Al-Khairi I, Cherian P, Noronha F, Hu FB, Behbehani K, Elkum N (2015) Higher plasma betatrophin/ANGPTL8 level in type 2 diabetes subjects does not correlate with blood glucose or insulin resistance. Sci Rep 5:10949. doi:10.1038/srep10949
Fu D, Yu JY, Connell AR, Yang S, Hookham MB, McLeese R, Lyons TJ (2016) Beneficial effects of berberine on oxidized LDL-induced cytotoxicity to human retinal muller cells. Invest Ophthalmol Vis Sci 57:3369–3379. doi:10.1167/iovs.16-19291
Kanata S, Akagi M, Nishimura S, Hayakawa S, Yoshida K, Sawamura T, Munakata H, Hamanishi C (2006) Oxidized LDL binding to LOX-1 upregulates VEGF expression in cultured bovine chondrocytes through activation of PPAR-gamma. Biochem Biophys Res Commun 348:1003–1010. doi:10.1016/j.bbrc.2006.07.133
Rudolf M, Winkler B, Aherrahou Z, Doehring LC, Kaczmarek P, Schmidt-Erfurth U (2005) Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch’s membrane of LDL receptor knockout mice. Br J Ophthalmol 89:1627–1630. doi:10.1136/bjo.2005.071183
Yin L, Wu X, Gong Y, Shi Y, Qiu Y, Zhang H, Liu X, Gu Q (2011) OX-LDL up-regulates the vascular endothelial growth factor-to-pigment epithelium-derived factor ratio in human retinal pigment epithelial cells. Curr Eye Res 36:379–385. doi:10.3109/02713683.2010.537427
Zhang R (2012) Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun 424:786–792. doi:10.1016/j.bbrc.2012.07.038
Fu Z, Yao F, Abou-Samra AB, Zhang R (2013) Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem Biophys Res Commun 430:1126–1131. doi:10.1016/j.bbrc.2012.12.025
Ren G, Kim JY, Smas CM (2012) Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am J Physiol Endocrinol Metab 303:E334–E351. doi:10.1152/ajpendo.00084.2012
Iroz A, Couty JP, Postic C (2015) Hepatokines: unlocking the multi-organ network in metabolic diseases. Diabetologia. doi:10.1007/s00125-015-3634-4
Seymour PA, Serup P (2013) Bulking up on beta cells. N Engl J Med 369:777–779. doi:10.1056/NEJMcibr1307038
Crunkhorn S (2013) Metabolic disorders: Betatrophin boosts beta-cells. Nat Rev Drug Discov 12:504. doi:10.1038/nrd4058
Kugelberg E (2013) Diabetes: Betatrophin—inducing beta-cell expansion to treat diabetes mellitus? Nat Rev Endocrinol 9:379. doi:10.1038/nrendo.2013.98
Lu Q, Lu L, Chen W, Chen H, Xu X, Zheng Z (2015) RhoA/mDia-1/profilin-1 signaling targets microvascular endothelial dysfunction in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 253:669–680. doi:10.1007/s00417-015-2985-3
Ferris FL 3rd, Chew EY, Hoogwerf BJ (1996) Serum lipids and diabetic retinopathy. Early Treatment Diabetic Retinopathy Study Research Group. Diabetes Care 19:1291–1293
Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL 3rd, Knatterud GL (1998) Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study report #18. Invest Ophthalmol Vis Sci 39:233–252
Orchard TJ, Dorman JS, Maser RE, Becker DJ, Ellis D, LaPorte RE, Kuller LH, Wolfson SK Jr, Drash AL (1990) Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care 13:741–747
Dandapat A, Hu C, Sun L, Mehta JL (2007) Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1-dependent redox-sensitive pathway. Arterioscler Thromb Vasc Biol 27:2435–2442. doi:10.1161/ATVBAHA.107.152272
Simunovic MP, Maberley DA (2015) Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: a systematic review and meta-analysis. Retina 35:1931–1942. doi:10.1097/IAE.0000000000000723
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers/animals were followed during this research. Our research adhered to the tenets of the Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Lu, Q., Lu, L., Chen, W. et al. Expression of angiopoietin-like protein 8 correlates with VEGF in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 255, 1515–1523 (2017). https://doi.org/10.1007/s00417-017-3676-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3676-z