Abstract
Purpose
The purpose of this study is to establish a new method to reduce the radiation dose during puncture and cannulation in percutaneous endoscopic lumbar discectomy (PELD).
Methods
Sixty patients with lumbar disk herniation undergoing PELD were prospectively enrolled and randomly divided into an ultrasound (US) guidance group and an X-ray guidance group. The puncture, cannulation, and total operation times; number of fluoroscopy shots; and radiation dose were recorded in both groups. The factors influencing the operation were analyzed. The clinical effect of PELD was evaluated using the straight leg elevation test, visual analog scale (VAS) and Oswestry disability index (ODI). The researchers who collected and analyzed the data were blinded to the group assignments.
Results
The puncture, cannulation and operation times in the US group were comparable to those in the X-ray group. The patients in the US group received 2.13 ± 0.35 fluoroscopy shots and a radiation dose of 5.34 ± 0.63 (mSV), which were significantly lower than the values in the X-ray group (7.57 shots ± 2.99 shots and 18.25 mSV ± 10.52 mSV) (P < 0.001). In the US group, the puncture time was significantly longer at the L5–S1 level, in patients with a BMI greater than 28 kg/m2 and in patients with a high iliac crest. The US and X-ray groups had comparable VAS and ODI scores 1 h and 3 months after PELD, and the VAS scores were significantly lower after PELD (all P < 0.001). No complications were observed in either group.
Conclusions
US guidance is a new method that reduces the radiation dose required during puncture and cannulation in PELD.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transforaminal percutaneous endoscopic lumbar discectomy (PELD) has become an established and mainstream minimally invasive surgical technique used to treat lumbar disk herniation (LDH) and can be used to remove a pro-lapsed disk, free a compressed nerve, and simultaneously relieve the symptoms associated with these conditions [1,2,3]. X-ray image guidance facilitates puncture and cannulation, which are very risky procedures performed close to the nerve root [4], by enabling stepwise visualization in two sections. This approach is currently a commonly used image guidance method.
However, exposure to X-ray radiation may increase the incidence of malignant tumors, such as thyroid cancer, skin erythema, and leukemia [5]. In pregnant women and young patients with concerns about fertility, exposure to X-ray radiation may increase the risk of malformations in progeny, which is a cause of great concern when performing PELD in these populations [6]. High cumulative effective doses have also been found in patients with Crohn’s disease [7], cystic fibrosis [8], and end-stage kidney disease [9]. Given the demanding learning curve required to become proficient in the puncture process performed during PELD [10, 11], patients may experience longer puncture times and receive larger doses of radiation when treated by inexperienced surgeons. Surgeons who perform PELD may also receive large doses of X-rays [12], even when they use preventive strategies, such as minimizing the use and dose of fluoroscopy, wearing proper protective gear, and using novel spinal locators [13, 14]. Therefore, an image guidance system that does not require the use of X-rays to perform PELD is needed.
Ultrasound (US) is a real-time dynamic imaging tool that does not require ionizing radiation. This method has been used for the diagnosis of spinal and intervertebral disk diseases [15] and to guide facet joint injection [16], selective nerve root blocks [17], and lumbar transforaminal injections [18]. In this study, we used US as the guidance method to avoid radiation exposure during PELD. Thus, our aim was to establish a new US-guided puncture and cannulation protocol for PELD that can reduce the use of ionizing radiation, thus increasing the safety of this procedure.
Patients and methods
Trial design
This investigation was a parallel randomized clinical trial. A random number generator was used to generate the random allocation sequence. The ratio of the experimental group to the control group was 1:1. Only the surgeon and US physician who performed the US-guided PELD procedure were aware of the group assignments. The authors who generated the random allocation sequence, enrolled the participants, assigned the participants to groups and were involved in the PELD operation, were blinded to the data collection and analysis.
Patients
Institutional approval for these procedures was obtained by the ethics committee of our hospital in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants.
From January 2018 to May 2018, 60 patients diagnosed with LDH who failed to respond to conservative treatment for more than 6 weeks were prospectively enrolled in this study and randomly assigned to the US-guided group (30 patients) or X-ray-guided group (30 patients). PELD was performed in both groups, and data were collected at the Chinese PLA General Hospital.
The following inclusion criteria were applied: (1) radicular leg pain caused by soft LDH, (2) PELD performed at a single level, and (3) more than 2 months of regular conservative treatment with no effect.
The following exclusion criteria were applied: (1) segmental instability, (2) lumbar spinal stenosis, (3) calcified disk herniation, (4) recurrent LDH, (5) painless weakness, (6) cauda equina syndrome, (7) multiple PELD levels, (8) mental disorder or inability to hear, speak to or communicate with the surgeon, and (9) severe disease preventing the patient from maintaining a prone position for several hours. In addition, patients with a body mass index (BMI) greater than 32 kg/m2, lumbar muscle fascia calcifications or severe lumbar muscle atrophy, were excluded because acoustic attenuation causes poor US image quality.
The US-guided PELD puncture and cannulation technique
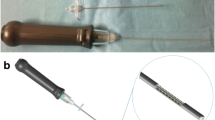
A portable Mindray M9 US System (Mindray, Shenzhen, China) with a C5-1S convex array transducer (central frequency, 5 Hz) was used in this study. The patients were asked to lie in a prone position on a radiolucent table with a pillow under their belly.
The procedure was performed under local anesthesia; one US doctor (a physician with more than 4 years of experience with interventional US and musculoskeletal US) held the probe, and one surgeon (an orthopedist with more than 5 years of experience with PELD) performed the puncture. The positions of the US doctor and surgeon and the positions of the US probe and surgeon’s hands during the puncture process are displayed in Fig. 1. In the control group, the same orthopedist performed the entire procedure independently.
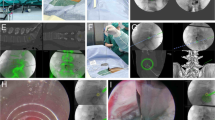
First, the targeted spinal level was located. We used a longitudinal section to display the transverse processes, which had a finger-like appearance. The lowest transverse process involved in these procedures was L5. Then, we rotated the probe 90° to display the transverse section of the transverse process and assessed the spinal level by detecting the transverse processes from L5 to L4, L3, L2, L1, and T12. L3 has the longest transverse process, and T12 has a connected rib.
Second, the puncture position was displayed. We found the transverse process and moved the probe upward, causing the soft tissue outside the intervertebral foramen to appear as a circular hyperechoic zone (HZ) after the disappearance of the superior margin of the transverse process, which is located below the facet joint (Fig. 2).
Ultrasound image and diagrammatic sketch of the lumbar and hyperechoic zones. aSP spinous process, FJ facet joint, HZ hyperechoic zone. The yellow arrow points to the HZ, which is the puncture target and is indicated by a yellow circle. The SP and FJ appear as hyperechogenicities on the ultrasound image. b The blue circle indicates the HZ, which is located under the FJ
Third, we drew an auxiliary line on the skin of the patient’s back at least 9–13 cm from the midline. We sterilized the skin with iodophor for better coupling with the probe. The HZ is located cephalad to the transverse process, which is located below the facet joint, and was used as the target. A needle was inserted from 12 to 3 o’clock (clockwise) of the HZ. The tip of the 18-gauge spinal needle first touched the facet joint and was then slipped inside the intervertebral foramen. G-arm X-ray (Whale, Massachusetts, United States) was used to test the needle position in the anteroposterior and lateral directions after confirming that the tip of the needle had been placed at the target point (Fig. 3).
Fourth, a guide wire was inserted through the spinal needle, and the spinal needle was removed. Then, a dilator was introduced along the guide wire. Finally, the endoscope outer sheath was inserted through the guide wire, and the position of the sheath was verified by G-arm X-ray (Fig. 4). Subsequently, transforaminal PELD was performed.
In the X-ray group, the whole puncture and cannulation process was guided only by G-arm X-ray.
Data collection
General information about the patients, including age, sexual status, height, weight, BMI and treatment level, was recorded.
The puncture, cannulation and total operation times were recorded in minutes. The number of fluoroscopy shots was recorded. The radiation dose received by the patients was recorded in milli-gray units by adding all shots in two views.
To evaluate the factors that could influence US-guided PELD, we divided the patients into different subgroups according to the height of the iliac crest and BMI. Among the L5–S1 cases, the patients were divided according to iliac crest height into those with a high crest, indicating that the iliac crest was higher than the L5 pedicle, and those with a low crest, indicating that the iliac crest was lower than the L5 pedicle.
Clinical efficacy was evaluated using the straight leg elevation test and by calculating the scores (1–10) on the visual analog scale (VAS) [19] for pain and the Oswestry disability index (ODI) [20] for functional status.
Statistical analysis
The sample size was calculated based on pretest data. All statistical analyses were performed by an independent statistician using IBM SPSS for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). The continuous variables were expressed as the mean ± SD. Differences in continuous variables between the US group and the control group were compared using an independent Student’s t test. A P value < 0.05 was considered to indicate a significant difference.
Results
Clinical information
The clinical data of the patients in the two groups are presented in Table 1. The analysis showed no significant differences between the groups.
Time and radiation doses in the US and X-ray groups
No significant differences in the puncture, cannulation and operation times were detected between the US and X-ray groups (Table 2).
The US group received 2.13 ± 0.35 fluoroscopy shots, which was significantly lower than the number received in the X-ray group (7.57 ± 2.99, P < 0.001). In addition, the US group received a radiation dose of 5.34 ± 0.63 mSV, whereas the X-ray group received a dose of 18.25 ± 10.52 mSV (P < 0.001).
Factors influencing the puncture and cannulation times in the US group
The puncture times for the patients treated at the L5–S1 level were significantly longer than those for the patients treated at the L4–5 and L3–4 levels. In addition, the puncture times for the patients with a BMI greater than 28 kg/m2 were significantly longer than those for the patients with a BMI less than 28 kg/m2. In cases treated at the L5–S1 level, the puncture times for the patients with a high iliac crest were significantly longer than those in the patients with a low iliac crest. Regarding the cannulation time, no significant differences were found among the patients according to different treatment levels, BMI values, or iliac crest heights. Table 3 shows the factors influencing the puncture and cannulation times in the US-guided PELD procedures.
Clinical effects of US- and X-ray-guided PELD
The US-guided PELD group had VAS scores of 1.53 ± 1.01 at 1 h and 1.03 ± 0.76 at 3 months after PELD. These scores were significantly lower than the VAS scores recorded before PELD (both P < 0.001). Similar results were observed in the X-ray-guided group (Table 4). In the US-guided PELD group, the ODI score was 7.47 ± 1.66 at 3 months after PELD, which was significantly lower than the ODI score recorded before surgery (P < 0.001). Similar results were observed in the X-ray-guided group (Table 4).
Complications
No postoperative complications, such as soft tissue and intervertebral disk infection or nerve injuries, were observed in any cases.
Discussion
Repeated fluoroscopic scanning is essential when performing puncture and cannulation during PELD. Fan et al. [21] showed that fluoroscopy was performed with (34.32 ± 4.78) shots in conventional PELD in one group of patients and (33.98 ± 2.69) shots in another group of patients [22]. For doctors, although protective measures can be taken, long-term accumulation of radiation exposure increases the cumulative radiation dose. Patients who undergo PELD receive minimal protection from radiation during this procedure. Additionally, the radiation dose is even higher when the procedure is performed by inexperienced surgeons. Therefore, pregnant women may forgo PELD surgery and instead endure pain during pregnancy. Young patients concerned about fertility may worry about the best time to become pregnant, which is a cause of substantial concern when determining when to perform PELD. The results of our study show that in most cases, the puncture and cannulation processes required during PELD can be precisely guided by US.
In addition to reduce the radiation dose, US guidance can provide a safe alternative for needle guidance and cannula insertion. This approach can offer real-time guidance while inserting the needle tip during the puncture process, thus decreasing the likelihood of causing injury to the intestines and blood vessels. Real-time US guidance also allows surgeons to more clearly visualize the distance between the working sheath and the foramen during the process of forcible insertion, allowing them to more precisely control their movements close to the foramen and nerve root.
No significant differences in the puncture, cannulation, and operation times were observed between the US- and X-ray-guided groups, demonstrating that US guidance achieves an efficiency similar to that of X-ray guidance.
US has been used for the diagnosis of spinal and intervertebral disk diseases [15] and to guide facet joint injection [16], selective nerve root blocks [17], and lumbar transforaminal injections [18]. In our study, US guidance was applied to PELD surgery. We used the freehand US guidance technique, which requires a US physician with extensive experience in both musculoskeletal and interventional US. The needle must puncture the tissue in a line strictly along the direction of the probe section; otherwise, the needle tip will not be clearly visible. The needle tip can be vibrated into a better position or the probe can be used to locate the needle tip. A needle should never be used for a deep puncture if the needle tip is not clearly visible. Although MRI is a good visualization tool for evaluating intervertebral disks and surrounding structures [23, 24] and real-time US–MRI fusion image virtual navigation has been demonstrated to be an effective and precise method for guiding spinal surgery, the navigation process used during these procedures is complicated and requires a special navigation system that cannot be combined with most US instruments [25]. Therefore, in our study, we used more accessible US guidance.
Few studies have explored the use of US guidance in PELD surgery. Wu et al. reported [26] that US-guided PELD can be performed in patients with a BMI less than 24 kg/m2. In our study, we successfully used US guidance for PELD in patients with a BMI as high as 30.5 kg/m2. Wu et al. used the lateral margin of the facet joints as the target point, whereas in our study, we used different localization methods. We used the HZ above the transverse process and at the ventral side of the articular process for guidance and the foramen as the target. We inserted the needle from 12 to 3 o’clock (clockwise) on the left side or from 12 to 9 o’clock (clockwise) on the right side of the HZ because this approach is more convenient and can be completed using only transverse section guidance. However, US guidance in PELD surgery also has limitations. In obese patients or patients with a hyperechoic fascia muscularis, muscular atrophy or calcification, due to the high attenuation of US, the anatomical landmarks surrounding the lumbar vertebrae may be unclear. In addition, treatment at L5–S1 or in patients with a high iliac crest is difficult because the tilt of the puncture direction prevents clear visualization of the HZ. US-guided PELD is difficult to perform in these cases.
In our study, the US-guided puncture and cannulation procedures were performed by two doctors as follows: a US physician with more than 4 years of experience with interventional US and musculoskeletal US held the probe, and an orthopedist with more than 5 years of experience performing PELD in more than 2000 cases carried out the puncture and cannulation procedure. This study demonstrates a useful strategy executed by two experienced doctors in this research field. We believe that as more orthopedists and US physicians learn and understand spinal US, this technology may become more established, and more doctors may learn to perform this procedure. In addition, with the additional development of more easily punctured guiding stents, the puncture and cannulation procedure may be completed by one person, and this technology is expected to become further established and therefore has great potential.
Our study has limitations. First, we did not study the learning curve of inexperienced doctors performing the US-guided puncture and cannulation procedures. Second, foraminal stenosis may impair puncture and cannulation in PELD [27], which was not assessed in our study.
Conclusion
US-guided PELD is a useful approach that significantly reduces radiation doses while producing treatment effects similar to those achieved with traditional X-ray guidance.
References
Cong L, Zhu Y, Tu G (2016) A meta-analysis of endo-scopic disectomy versus open disectomy for symptomatic lumbar disk herniation. Eur Spine 25:134–143
Rasouli MR, Rahimi-Movaghar V, Shokraneh F, Moradi-Lakeh M, Chou R (2014) Minimally invasive disectomy versus microdisectomy/open disectomy for symptomatic lumbar disc herniation. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010328.pub2
Hussein M, Abdeldayem A, Mattar MM (2014) Surgical technique and effectiveness of microendoscopic discectomy for large uncontained lumbar disc herniations: a prospective, randomized, controlled study with 8 years of follow-up. Eur Spine J 23(9):1992–1999
Choi KC, Lee DC, Shim HK, Shin SH, Park CK (2017) A strategy of percutaneous endoscopic lumbar disectomy for migrated disc herniation. World Neurosurg 99:259–266
Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M (2003) Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA 100:13761–13766
Di Martino A, Russo F, Denaro L, Denaro V (2017) How to treat lumbar disc herniation in pregnancy? A systematic review on current standards. Eur Spine J 26(Suppl 4):496–504
Desmond AN, O’Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F (2008) Crohn’s disease: factors associated with exposure to high levels of diagnostic radiation. Gut 57:1524–1529
O’Connell OJ, McWilliams S, McGarrigle A, O’Connor OJ, Shanahan F, Mullane D, Eustace J, Maher MM, Plant BJ (2012) Radiologic imaging in cystic fibrosis: cumulative effective dose and changing trends over 2 decades. Chest 141(6):1575–1583
Coyle J, Kinsella S, McCarthy S, MacWilliams S, McLaughlin P, Eustace J, Maher MM (2012) Cumulative ionizing radiation exposure in patients with end stage kidney disease: a 6-year retrospective analysis. Abdom Imaging 37:632–638
Ao S, Wu J, Zheng W, Zheng W, Zhou Y (2018) A Novel targeted foraminoplasty device improves the efficacy and safety of foraminoplasty in percutaneous endoscopic lumbar disectomy: preliminary clinical application of 70 cases. World Neurosurg 115:e263–e271
He J, Xiao S, Wu Z, Yuan Z (2016) Microendoscopic discectomy versus open discectomy for lumbar disc herniation: a meta-analysis. Eur Spine J 25(5):1373–1381
Mariscalco MW, Yamashita T, Steinmetz MP, Krishnaney AA, Lieberman IH, Mroz TE (2011) Radiation exposure to the surgeon during open lumbar microdisectomy and minimally invasive microdisectomy: a prospective, controlled trial. Spine (Phila Pa 1976) 36:255–260
Mendelsohn D, Strelzow J, Dea N, Ford NL, Batke J, Pennington A, Yang K, Ailon T, Boyd M, Dvorak M, Kwon B, Paquette S, Fisher C, Street J (2016) Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J 16:343–354
Srinivasan D, Than KD, Wang AC, La Marca F, Wang PI, Schermerhorn TC, Park P (2014) Radiation safety and spine surgery: systematic review of exposure limits and methods to minimize radiation exposure. World Neurosurg 82:1337–1343
Naish C, Mitchell R, Innes J, Halliwell M, McNally D (2003) Ultrasound imaging of the intervertebral disc. Spine (Phila Pa 1976) 28(2):107–113
Greher M, Kirchmair L, Enna B, Kovacs P, Gustorff B, Kapral S, Moriggl B (2004) Ultrasound-guided lumbar facet nerve block: accuracy of a new technique confirmed by computed tomography. Anesthesiology 101(5):1195–1200
Darrieutort-Laffite C, Hamel O, Glémarec J, Maugars Y, Le Goff B (2014) Ultrasonography of the lumbar spine: sonoanatomy and practical applications. Joint Bone Spine 81(2):130–136
Gofeld M, Bristow SJ, Chiu SC, McQueen CK, Bollag L (2012) Ultrasound-guided lumbar transforaminal injections: feasibility and validation study. Spine (Phila Pa 1976) 37(9):808–812
Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE (1985) Pain measurement: an overview. Pain 22(1):1–31
Fairbank JC, Pynsent PB (2000) The Oswestry disability index. Spine (Phila Pa 1976) 25(22):2940–2952
Fan G, Han R, Gu X, Zhang H, Guan X, Fan Y, Wang T, He S (2017) Navigation improves the learning curve of transforaminal percutaneous endoscopic lumbar disectomy. Int Orthop 41(2):323–332
Fan G, Gu X, Liu Y, Wu X, Zhang H, Gu G, Guan X, He S (2016) Lower learning difficulty and fluoroscopy reduction of transforaminal percutaneous endoscopic lumbar disectomy with an accurate preoperative location method. Pain Physician 19(8):E1123–E1134
Chung TS, Yang HE, Ahn SJ, Park JH (2015) Herniated lumbar disk: real-time MR imaging evaluation during continuous traction. Radiology 275(3):755–762
Carrino JA, Lurie JD, Tosteson AN, Tosteson TD, Carragee EJ, Kaiser J, Grove MR, Blood E, Pearson LH, Weinstein JN, Herzog R (2009) Lumbar spine: reliability of MR imaging findings. Radiology 250(1):161–170
Liu TJ, Shen F, Zhang C, Huang PT, Zhu YJ (2018) Real-time ultrasound-MRI fusion image virtual navigation for locating intraspinal tumour in a pregnant woman. Eur Spine J 27:436–439
Wu R, Liao X, Xia H (2017) Radiation exposure to the surgeon during ultrasound-assisted transforaminal percutaneous endoscopic lumbar disectomy: a prospective study. World Neurosurg 101:658–665
Wildermuth S, Zanetti M, Duewell S, Schmid MR, Romanowski B, Benini A, Böni T, Hodler J (1998) Lumbar spine: quantitative and qualitative assessment of positional (upright flexion and extension) MR imaging and myelography. Radiology 207:391–398
Acknowledgements
This study is supported by the clinical research support fund of PLA general hospital (No. 2018XXFC-18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, M., Yan, L., Li, S. et al. Ultrasound-guided transforaminal percutaneous endoscopic lumbar discectomy: a new guidance method that reduces radiation doses. Eur Spine J 28, 2543–2550 (2019). https://doi.org/10.1007/s00586-019-05980-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-05980-9