Abstract
Purpose
To investigate the suitability of the transpedicular approach (TPA) in a sheep model of IVD regenerative strategies
Methods
24 IVD from four sheep were used. TPA and biopsies of the Nucleus pulposus (NP) were performed in 18 IVD (6 IVD control). Seven discographies were performed to assess the feasibility of injecting contrast agent. MRI, micro-CT scan, and histological analyses were performed and the accuracy of the TPA was evaluated. The effects on the vertebra and endplates were analyzed.
Results
83% of our biopsies or injections were located in the NP. Osseous fragments in IVD were observed in 50%. We observed two cases (11%) of rostral endplate fracture and five cases (27%) of breaching of the cortical pedicle and encroachment into the spinal canal. Two cases of perivertebral venous embolism and two of backflow through the canal of the TPA inside the vertebra were noted. Significant damage occurred to the bone structure of the vertebra and to the rostral endplate on which the IVD had been inserted.
Conclusions
TPA induces damage to the endplates, and it may lead to neurological impairment and leakage of injected materials into the systemic circulation. These adverse effects must be fully considered before proceeding with TPA for IVD regenerative strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Low back pain (LBP) is a major public health issue and its socio-economic cost is increasing as the population ages [1]. LBP is frequently associated (40% of cases) with intervertebral disc degenerative disease (DDD) [2]. Currently, LBP is mainly managed by pharmacological treatments and, as treatment of last resort, by surgical interventions (spine fusion or disc prosthesis) that are reserved for advanced and debilitating cases of LBP. Whereas pain relief can generally be achieved with these surgical treatments [3, 4], they are subject to several limitations, including the risk of promoting the degeneration of adjacent IVDs. Moreover, they remain invasive, despite improvements in the development of minimally invasive routes [5].
Faced with the limitations of these therapies, and considering the recent improvement in knowledge regarding the processes of IVD degeneration, regenerative medicine offers new prospects for the treatment of DDD [6, 7]. It is currently widely acknowledged that this degenerative process initiates in the Nucleus pulposus (NP), the central region of the IVD, and becomes manifest as a result of a drastic reduction in cell numbers and matrix hydration [8]. Consequently, cell supplementation-based regenerative strategies of the NP have gained a degree of prominence in the past several years. These strategies usually aim to exploit the regenerative properties of mesenchymal stem cells (MSC). Based on their ability to produce the essential components of the NP extracellular matrix, and to release trophic factors, MSC have exhibited clear efficacy in a range of pre-clinical studies [9,10,11,12,13].
Despite this large body of evidence indicating that MSC are a promising source of cells for NP cell therapy, a great variety of issues will need to be addressed before this therapeutic option can enter into routine clinical practice. Among these issues, the surgical route to safely inject MSC into the NP, and the long-term efficacy of such injections, need to be carefully addressed. In addition, pre-clinical and clinical trials to date have suffered from several limitations, including disparity of the animal models used and the limited number of patients that were enrolled [14,15,16,17]. However, considering the great promise held by MSC, and the unmet medical need of DDD, it seems pertinent to further investigate the use of MSC, with or without injectable biomaterials, to further develop IVD regenerative approaches.
In this regard, the technical limitations linked to the in situ injection of cells due to the particular IVD environment should be taken into account. Indeed, the lateral surgical approach of the NP through the Annulus fibrosus (AF) has been a key stage of this procedure in multiple studies [18]. However, it is now well established that this route, based on needle puncture of the AF, could lead to further degeneration and IVD herniation [19]. Of note, a prospective human study found that discographies through the AF led to accelerated degeneration [20]. Interestingly, and in line with this clinical study, needle insertion into the AF is considered to be an excellent experimentally induced model of IVD degeneration [21,22,23]. Furthermore, the risk of extrusion of the injected material through the AF injury is an additional issue of concern [24]. Thus, maintaining the integrity of the AF is now seen as a critical aspect that has to be taken into account to maintain IVD homeostasis. In this context, alternative routes to the trans-AF approach are needed. Among the various possibilities in this regard, preliminary results for a promising new approach have been reported with an ovine model. This route is based on a transpedicular approach (TPA) to target the NP without inducing any AF lesions [25]. The procedure consists of targeting the NP after having introduced a wire in the caudal vertebra through the pedicle and the inferior endplate to the NP. First described in 1987 [26], this TPA is commonly used to achieve osteosynthesis [27] and cimentoplasty [28], thus already making it a promising approach for future human research studies.
The purpose of the present study was to determine the feasibility and the accuracy, as well as to describe specific complications of the TPA for injection into the NP in an ovine model. The outcomes for this TPA-mediated intradiscal injection were also documented, and the potential use of this technique in pre-clinical research is discussed.
Materials and methods
Ethical aspects, animals, and treatment conditions
All the animal experiments described in this study were approved by the French Ministry of Agriculture and by the ethics committee of the Pays de La Loire Region (Ethical number APAFIS 8401), and they were performed in the accredited Centre of Research and Pre-clinical Investigations at the National Veterinary School of Nantes. Four healthy sheep (1 year; 60–80 kg, Vendée breed, GAEC HEAS farm, Ligné F-44850) were used. Six lumbar IVD (L1–L2, L2–L3, L3–L4, L4–L5, L5–L6, and L6–L7) were used for the experiments (n = 24) (Table 1). TPA to reach the NP was performed in 18 of the IVD, whereas 6 of the IVD were used as controls.
Surgical procedure
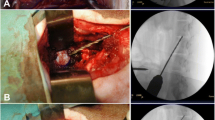
In a first set of experiments (i.e., the ex vivo condition), the lumbar motion segment was taken from two sheep cadavers after removal of all of the muscles and soft tissues. Under fluoroscopic guidance, TPA was performed using a 2-mm Jamshidi® bone marrow biopsy needle employing a hollow trocar and a cannula. The route was the same as described previously by Vadalà et al. [25]. The correct position of the needle was assessed by fluoroscopy (OEC9800plus, General Electric GE Healthcare, USA) based on anatomical markers (Fig. 1). In a second set of experiments (i.e., in vivo, cadaver), the whole cadaver of the third sheep was used to inject within the NP via a percutaneous TPA. In a final set of experiments (i.e., in vivo, no cadaver), TPA was performed in the fourth sheep under general anesthesia. The sedation was initially achieved by intramuscular injection of 15 μg/kg of medetomidine. The sheep was positioned in lateral decubitus position. Anesthesia was induced by intravenous injection of 4 mg/kg of ketamine and 0.2 mg/kg of diazepam, and maintained by inhalation of 1–3% isoflurane and intravenous injection of 2–4 mg/kg of propofol. Analgesia required intravenous injection of 0.5 mg/kg of morphine, via continuous rate infusion of 50–100 μg/kg/min of lidocaine and 0.6–1.2 μg/kg/min of ketamine. The TPA was carried out percutaneously using the same protocol. Following the experimental procedures, the sheep was sacrificed under general anesthesia by intravenous injection of 140 mg/kg of pentobarbital.
Anatomic landmarks of the transpedicular approach (TPA). Entry point of the needle was drilled in the caudal vertebra at the intersection of a horizontal line passing through the axis of the transverse process and a vertical line passing to the lateral border of the superior articular facet as previously described by Vadalà and al. The needle was then positioned respecting an angle of approximately 45° in frontal, sagittal, and transversal plans
Feasibility study: discography
To assess the feasibility to inject into the IVD via the TPA, discographies were performed. The cannula of the Jamshidi® needle was removed and a 16G bone biopsy needle was introduced through the trocar so as to cross the rostral endplate of the vertebra and to then enter the NP. Approximately, four biopsies were needed to decrease the high swelling pressure of non-degenerative IVD, as described by Vadalà et al. [29]. Biopsies of the NP without injections were carried out in the 10 other IVD. Five discographies were performed by injection of 100 μL of iohexol (Omnipaque®, GE Healthcare, USA) and two discographies using 100 μL of gadolinium-based (Dotarem®, Guerbet, France) contrast agents through the biopsy needle.
MRI procedure
Pre- and post-operative MRI of the entire lumbar sheep spines were performed using a 1.5 Tesla MRI scanner (Magnetom Essenza®, Siemens Medical Solutions, Erlangen, Germany) with a standard spine coil to obtain T2-weighted images (TR 3000 ms; TE, 86 ms with the following parameters: matrix, 512 × 358; field of view, 350 × 350 mm; and slice thickness, 2.5 mm with no interslice gap) and T1-weighted images (TR 459 ms; TE, 11 ms with the following parameters: matrix, 512 × 358; field of view, 350 × 350 mm; and slice thickness of 3 mm with no interslice gap). The pre-operative MRI revealed no degenerated IVD in the four sheep spines. The location of the biopsy and injection targets was assessed in the NP or in the AF using post-operative axial T2 MR images of IVD. Following the MRI investigation, all IVD were harvested and isolated in a bone-disc-bone segment by cutting the lumbar vertebrae to the axis of the spine with a bandsaw (Fig. 1). The caudal vertebra was sectioned below its transversal process in order to preserve the canal of the TPA.
Micro-computed tomography (µCT) scan procedure
To observe the effects of the TPA on the bones, the bone–disc–bone segments were analyzed by a micro-computed tomography (µCT) scan (Inveon, Siemens, Germany), with the following parameters: low magnification, time of exposure: 320 ms, 80 kV, 500 μA, and slice thickness 110 μm. Three-dimensional µCT reconstructions were made to control the correct position of the transpedicular tunnel into the vertebral body and pedicle.
Image analysis
All the imaging data were analyzed with the Osirix software 3.9© (Osirix Foundation, Geneva, Switzerland).
Histological analysis
IVD from all of the segments were carefully dissected, frozen, and sectioned using a cryostat (CryoStar NX70®, Thermo Fisher Scientific, Waltham, MA, USA). Tissues were prepared into 3-μm sections orthogonal to the axis of the spine. Van Kossa’s staining was conducted on IVD to detect the presence of calcium deposits. To observe the effects of TPA on the endplates, the caudal vertebrae were embedded in resin (Technovit®, Kulzer, Germany) and cut into 5-μm sections in the axis of the transpedicular canal. Some sections were made with a polycut (SM2500®, Leica, Nanterre, France) and then stained with Safranin O and Movat’s pentachrome. Safranin O and Movat’s pentachrome staining were performed to study the endplate articular cartilage and the vertebrae bony matrix, respectively. Stained sections were observed with a slide scanner, and all the image data were analyzed with NDP view software® (Hamamatsu, Japan).
Scanning electron microscopy (SEM)
For SEM observations, sections were made with a microtome (Isomet 1000®, Buehler, Esslingen, Germany), polished (Metasery 2000®, Buehler), and metal coated (Desk III®, Denton Vacuum, Moorestown, NJ, USA) before they were examined with a scanning electron microscope (LEO 1450 VP, Carl Zeiss, Jena, Germany) at a magnification of 50×.
Results
Route and injection feasibility
The route procedure described by Vadalà et al. [25] was followed (Fig. 1), and anatomical landmarks on the ovine spine were well identifiable. Six and eighteen IVD were used for the control and the TPA condition, respectively. Ex vivo experiments on 12 IVD were performed in a first set of experiments. The selective biopsy or injection of iodine contrast agent was confirmed by X-ray data on the center of the NP, and these confirmed the feasibility of targeting the NP (Fig. 2a). These data were substantiated by µCT scans obtained after injection of iodine contrast agent (Fig. 2b).
Route accuracy
Regarding the accuracy of the procedure, T2-weighted signal intensities of ovine spine were obtained by MRI (Fig. 3). Pre-operative images in sagittal and axial acquisitions confirmed the vertebra and IVD integrity (Fig. 3a–c). Following the biopsy or injection, success with the procedure, in terms of targeting of the NP, was obtained in 83% of the cases (15/18 IVD) (Fig. 3d). The three others procedures, for which targeting of the AF was observed, were considered to be failures. Over the course of the procedure, only one difficulty was encountered while performing the TPA. Indeed, the sheep abdomen partially interfered with frontal fluoroscopy, and this made precise targeting of the NP difficult.
Injection complications
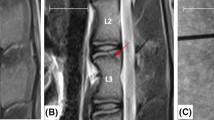
As indicated above, an accuracy level of 83% was achieved. However, in two percutaneous discographies, a backflow of injected gadolinium-based contrast agent was noted when compared with pre- (Fig. 4a) and post-operative sagittal T1-weighted signals (Fig. 4b). This backflow was localized in the canal of the TPA and inside the vertebra.
Injection complication after transpedicular approach (TPA). T1-weighted magnetic resonance images before (a) and after (b) percutaneous L1–L2 and L5–L6 discographies via TPA. Red arrows indicate backflows of injected gadolinium-based contrast agent in the canal of the TPA and inside the vertebra (in vivo, cadaver condition)
Effects on osseous and endplate tissues
Micro-CT analysis of IVD revealed significant adverse effects on the vertebra and its rostral endplate that were a consequence of the TPA (Fig. 5). Osseous fragments were found with 9 of the 18 TPA procedures (i.e. in 50%) (Fig. 5a). Five cases (27%) of a cortical pedicle breach and encroachment into the spinal canal were noted (Fig. 5b). No cerebrospinal fluid leaks were observed during the injections. Two cases (11%) of rostral endplate fracture were recorded (Fig. 5c). Perivertebral venous embolisms of iodine contrast agent were also noticed in two discographies that were performed in the fourth sheep (Fig. 5d). Van Kossa’s staining confirmed the presence of osseous fragments carried by the TPA to within the IVD, and located into the NP or the AF according to whether the procedure was deemed to be a success or a failure (Fig. 6). The canal of the TPA trough the vertebra, and its rostral endplate, was analyzed by histology (Fig. 7). Significant damages to the bone structure of the vertebra and to the rostral endplate on which the IVD was inserted were noted. Osseous fragments were found throughout the canal of the TPA, thus confirming our previous results. The rostral endplate was also fractured, with a mobile fragment located within the IVD.
Bony aftermaths and perivertebral venous embolism after transpedicular approach. Micro-computed tomography scan analysis of the transpedicular approach (in vivo, no cadaver condition). Red arrows indicates the presence of osseous fragments within the intervertebral disc (a), cortical breach of pedicle and encroachment into the spinal canal (b), rostral endplate fracture (c), and perivertebral venous embolism of injected iodine contrast agent (d)
Osseous fragments carried by transpedicular approach (TPA). Van Kossa’s histological staining of sheep intervertebral discs after TPA (a, c) showing failure of the procedure with a targeting of the annulus fibrosus and the presence of calcium deposits indicated by red arrows as compared with a control (b, d)
Consequences on osseous and endplate tissues of transpedicular approach. Scanning electron microscopy analysis (a) and histological study with safranin O (b) and Movat’s pentachrome (c) stainings of transpedicular approach showing significant damages to the vertebra and to its rostral endplate. Red arrows indicate osseous fragments carried by TPA within the IVD
Discussion
As previously described [25], TPA allows for discographies to be performed in sheep IVDs. It can, therefore, be deemed to be a suitable route for injection into the NP, while preserving the integrity of the AF, particularly in terms of IVD regenerative strategies in an ovine model. TPA was clearly feasible (success rate about 83%) from a surgical point of view. The accuracy of the TPA encountered in our study is hence quite satisfactory, despite the significant learning curve for this technique and problems with visualizing the needle under frontal fluoroscopy. To further improve this feasibility, the use of assisted CT guidance to perform TPA could be a good option, although this still needs to be properly evaluated [30].
Despite biopsies, the high swelling pressure in healthy IVDs during injections tends to lead to backflow of injected contrast agent through the transpedicular canal and vertebra. The leakage of intradiscal injected products, particularly when injecting a cell therapy product, could be a major issue, as it has been shown that theses leaks may lead to osteophyte formation [13]. On one hand, with the aim of circumventing this difficulty, performance of a complete nucleotomy prior to injection into the IVD has been proposed [29]. However, this practice is questionable from a clinical perspective when the ultimate goal is to regenerate the NP. On the other hand, one can assume that a degenerated and dehydrated IVD may exhibit lower swelling pressure, as compared to a healthy one [31]. We are hence inclined to hypothesize that in degenerated IVD, the injection would be easier and probably feasible without a total nucleotomy. However, in the absence of experimental results obtained with an aged animal model of degenerated IVD, it remains difficult to fully rule out the possibility that a decompressive partial nucleotomy is required.
In addition to the difficulties related to the injection (leakage and swelling pressure), effects of TPA on bone also have to be taken into consideration. In our study, we found osseous fragments that were carried into the IVD by the TPA in half of the cases. These bone fragments could induce calcification of the IVD, thereby increasing the risk of vertebral fusion. An in vivo longitudinal follow-up study of TPA in an ovine model has, however, not confirmed this hypothesis [30]. Nevertheless, the nucleotomies that were performed in Vadalà’s study [30] could have limited the bone lesions and thus led to underestimation of the risk of induced spine fusion. In addition to the intradiscal migration of bone fragments, we have also observed several cases of endplate fractures after TPA. These could result in spondyloarthritis, as described previously [30].
To date, not a single study has investigated or explored the risk of vertebral fractures associated with TPA-induced bone lesions. Indeed, TPA could induce severe lesions, especially in osteoporotic patients whose vertebral bones are fragile structures. To address this clinical issue, further investigations need to be considered in appropriate ovine models of osteoporosis, such as the ovariectomized ewes [32].
In our study, encroachment into the spinal canal was also noted. These results, and the cerebrospinal fluid leaks observed after TPA in a previous study [30], indicate that this procedure may risk neurological impairment. These data hence highlight the need to use assisted CT guidance to accurately target the NP through the pedicle without encroachment into the spinal canal.
Another limitation of TPA is the anatomical proximity between the vertebral endplate and the vascular system. This could result in a risk of perivertebral venous embolism of the injected products. Although such a side effect has not yet been carefully described with TPA for treatment of IVDs by regenerative medicine, it is, however, a well-documented effect in the literature. Thus, the risk of a pulmonary cement embolism has been reported to range from 3.5 to 23% after percutaneous vertebroplasty via TPA [33]. In terms of intradiscal injection of MSC, their migration into the bloodstream could be a significant occurrence via this route. This hypothetical risk of migration could induce various complications (tumor formation, immune reactions, and undesirable bone formation). These risks have already been documented for the use of MSC with other medical conditions. There is hence a degree of skepticism regarding the safety of their use in humans, and in particular in a high-pressure region, such as the IVD, where leakage of the implant may occur [9]. Whilst MSC have seen a degree of success in clinical trials of bone and cartilage repair, and they have so far been considered to be safe and their tumorigenic and immunogenic potential nonetheless need to be kept in mind [34, 35]. Unwanted ossification is also a potential negative consequence that has been described after intradiscal injection of MSC, with the formation of osteophytes within the damaged area in rabbit IVD in particular [13]. We have shown that TPA induced a significant damage to the bone structure of the vertebra and to the rostral endplate. Although, a transpedicular tunnel does not appear to affect the biomechanical properties of the IVD [36], its adverse effects on endplates should not be taken lightly. Vertebral endplates are considered to be essential supportive and nutritive structures for IVDs, particularly as they are a vascularized tissue. It has been shown that endplate violation is responsible for an impairment in IVD nutrition that could contribute to acceleration of the degenerative cascade [37]. Consistent with the nutritive role of endplates, their alteration has already been successfully used as an experimentally induced model of IVD degeneration [22].
Viewed together, our data strongly suggest that TPA involves many risks and effects on bone that raise doubts regarding its suitability as a clinically relevant injection route for the treatment of IVD by regenerative medicine. Nevertheless, we wish to avoid over interpreting our results. Indeed, unlike Vadalà et al. [30], in our study, we did not conduct longitudinal follow-ups. This study by Vadalà et al. has shown promising long-term results with TPA in an ovine model, reporting very low perio- and post-operative complications [30]. This difference with the results from our study may be attributed to endplate repair. Indeed, Vadalà et al. repaired the endplates by sealing the edge of the tunnel using a press-fit porous polyurethane cylinder [30]. This technique could have contributed to being able to avoid leakage of the injected biomaterials through the transpedicular tunnel and the associated risks of perivertebral venous embolism while limiting the degenerative cascade induced by endplate damage. Finally, as mentioned above, our operating conditions may be improved so as to increase the accuracy of the TPA, and to overcome the effects of the high swelling pressure in healthy ovine IVDs.
Additional data regarding TPA as an appropriate injection route for the treatment of IVD by regenerative medicine should be obtained. Notably, longitudinal studies with long-term follow-up should be performed so as to confirm our results and those reported in the literature. The effects on bone that we observed after TPA should be carefully investigated in such future studies. Data are still lacking in regard to the effects of TPA on the vertebra and the rostral endplate, which appear to be particularly prone to damage with this route. As endplates are an essential nutritive support for IVD, the potential of damaging them could pose a significant obstacle to the development of TPA [37] due to the induced risk of spondylarthrosis [30] and degeneration of the IVD.
While awaiting the findings from further investigations, for the time being the lateral surgical approach of the NP through the AF appears to be a less invasive route in terms of effects on the vertebrae and the rostral endplate. Furthermore, there is a degree of controversy in the literature regarding the necessity to preserve the AF for regenerative strategies. Indeed, a recent study [38] has shown that the risk of induced DDD after injections through the AF is not constant, as it depends on the ratio between the gauge of the needle and the height of the IVD.
Conclusion
TPA provides a suitable alternative to the conventional dorso-lateral approach through the AF. Nevertheless, TPA can cause significant damage to the endplates and it may lead to neurological impairment. Furthermore, leakages of injected products into the systemic circulation were observed in our study. These effects require that the conditions of injection after the TPA are improved. An in vivo study with a longitudinal follow-up is also needed before proceeding any further with the use of TPA for IVD regenerative strategies.
References
Martin BI, Deyo RA, Mirza SK et al (2008) Expenditures and health status among adults with back and neck problems. JAMA 299:656–664. doi:10.1001/jama.299.6.656
Luoma K, Riihimäki H, Luukkonen R et al (2000) Low back pain in relation to lumbar disc degeneration. Spine 25:487–492
Deyo RA, Mirza SK (2006) Trends and variations in the use of spine surgery. Clin Orthop Relat Res 443:139–146. doi:10.1097/01.blo.0000198726.62514.75
Resnick DK, Watters WC (2007) Lumbar disc arthroplasty: a critical review. Clin Neurosurg 54:83–87
Van den Eerenbeemt KD, Ostelo RW, van Royen BJ et al (2010) Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J 19:1262–1280. doi:10.1007/s00586-010-1445-3
Colombier P, Camus A, Lescaudron L et al (2014) Intervertebral disc regeneration: a great challenge for tissue engineers. Trends Biotechnol 32:433–435. doi:10.1016/j.tibtech.2014.05.006
Sakai D (2008) Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J 17(suppl 4):452–458. doi:10.1007/s00586-008-0743-5
Colombier P, Clouet J, Hamel O et al (2014) The lumbar intervertebral disc: from embryonic development to degeneration. Jt Bone Spine 81:125–129. doi:10.1016/j.jbspin.2013.07.012
Acosta FL, Metz L, Adkisson HD et al (2011) Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A 17:3045–3055. doi:10.1089/ten.tea.2011.0229
Sakai D, Mochida J, Iwashina T et al (2006) Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 27:335–345. doi:10.1016/j.biomaterials.2005.06.038
Henriksson HB, Svanvik T, Jonsson M et al (2009) Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine 34:141–148. doi:10.1097/BRS.0b013e31818f8c20
Xin H, Zhang C, Wang D et al (2013) Tissue-engineered allograft intervertebral disc transplantation for the treatment of degenerative disc disease: experimental study in a beagle model. Tissue Eng Part A 19:143–151. doi:10.1089/ten.TEA.2012.0255
Vadalà G, Sowa G, Hubert M et al (2012) Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 6:348–355. doi:10.1002/term.433
Yoshikawa T, Ueda Y, Miyazaki K et al (2010) Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine 35:E475–E480. doi:10.1097/BRS.0b013e3181cd2cf4
Orozco L, Soler R, Morera C et al (2011) Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92:822–828. doi:10.1097/TP.0b013e3182298a15
Pettine KA, Murphy MB, Suzuki RK, Sand TT (2015) Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 33:146–156. doi:10.1002/stem.1845
Noriega DC, Ardura F, Hernández-Ramajo R et al (2016) Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. doi:10.1097/TP.0000000000001484
Oehme D, Goldschlager T, Rosenfeld J et al (2012) Lateral surgical approach to lumbar intervertebral discs in an ovine model. Sci World J 2012:873726. doi:10.1100/2012/873726
Iatridis JC, Nicoll SB, Michalek AJ et al (2013) Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J 13:243–262. doi:10.1016/j.spinee.2012.12.002
Carragee EJ, Don AS, Hurwitz EL et al (2009) 2009 ISSLS Prize Winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine 34:2338–2345. doi:10.1097/BRS.0b013e3181ab5432
Sato K, Nagata K, Ariyoshi M et al (2000) Intradiscal pressure after intradiscal injection of hypertonic saline: an experimental study. Eur Spine J 9:213–217
Masuda K, Aota Y, Muehleman C et al (2005) A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14
Elliott DM, Yerramalli CS, Beckstein JC et al (2008) The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine 33:588–596. doi:10.1097/BRS.0b013e318166e0a2
Lipson SJ, Muir H (1981) Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum 24:12–21
Vadalà G, Russo F, Pattappa G et al (2013) The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine 38:E319–E324. doi:10.1097/BRS.0b013e318285bc4a
Galibert P, Deramond H, Rosat P, Le Gars D (1987) Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 33:166–168
Ringel F, Stoffel M, Stüer C, Meyer B (2006) Minimally invasive transmuscular pedicle screw fixation of the thoracic and lumbar spine. Neurosurgery 59:ONS361–ONS366. doi:10.1227/01.NEU.0000223505.07815.74 (discussion ONS366–367)
Taylor RS, Fritzell P, Taylor RJ (2007) Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J 16:1085–1100. doi:10.1007/s00586-007-0308-z
Vadalà G, Russo F, Pattappa G et al (2015) A nucleotomy model with intact annulus fibrosus to test intervertebral disc regeneration strategies. Tissue Eng Part C Methods 21:1117–1124. doi:10.1089/ten.TEC.2015.0086
Vadalà G, De Strobel F, Bernardini M et al (2013) The transpedicular approach for the study of intervertebral disc regeneration strategies: in vivo characterization. Eur Spine J 22(suppl 6):S972–S978. doi:10.1007/s00586-013-3007-y
Wognum S, Huyghe JM, Baaijens FPT (2006) Influence of osmotic pressure changes on the opening of existing cracks in 2 intervertebral disc models. Spine 31:1783–1788. doi:10.1097/01.brs.0000227267.42924.bb
Verron E, Gauthier O, Janvier P et al (2010) In vivo bone augmentation in an osteoporotic environment using bisphosphonate-loaded calcium deficient apatite. Biomaterials 31:7776–7784. doi:10.1016/j.biomaterials.2010.06.047
Krueger A, Bliemel C, Zettl R, Ruchholtz S (2009) Management of pulmonary cement embolism after percutaneous vertebroplasty and kyphoplasty: a systematic review of the literature. Eur Spine J 18:1257–1265. doi:10.1007/s00586-009-1073-y
Zhu W, Xu W, Jiang R et al (2006) Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 80:267–274. doi:10.1016/j.yexmp.2005.07.004
Breitbach M, Bostani T, Roell W et al (2007) Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110:1362–1369. doi:10.1182/blood-2006-12-063412
Russo F, Hartman RA, Bell KM et al (2016) Biomechanical Evaluation of Transpedicular Nucleotomy with Intact Annulus Fibrosus. Spine. doi:10.1097/BRS.0000000000001762
Urban JPG, Smith S, Fairbank JCT (2004) Nutrition of the intervertebral disc. Spine 29:2700–2709
Fusellier M, Colombier P, Lesoeur J et al (2016) Longitudinal comparison of enzyme- and laser-treated intervertebral disc by MRI, X-ray, and histological analyses reveals discrepancies in the progression of disc degeneration: a Rabbit Study. Biomed Res Int 2016:5498271. doi:10.1155/2016/5498271
Acknowledgements
This study was supported by Grants from the Société Française de Neurochirurgie, Société Française de Chirurgie du Rachis, the Agence de la Biomédecine, the Institut National de la Santé et de la Recherche Médicale (INSERM), the Région des Pays de la Loire, ANR générique 2014 (REMEDIV Project), the Fondation pour la Recherche Médicale FRM Bioingénierie (DBS20131128442), the Région des pays de la Loire Research Program “Longévité Mobilité Autonomie” (LMA), and the University Hospital of Angers. The authors gratefully acknowledge the technical assistance that they received from personnel of the CRIP (i.e., Patrice Roy, Dominique Rouleau, Christian Raphael, Stéphane Madec, Ingrid Leborgne, and Olga Topie), of the INSERM U1066-MINT (i.e., Laurent Lemaire and Laurence Sindji), of the SCIAM (Service Commun d’imagerie et d’analyses microscopiques (i.e., Rodolphe Perrot and Romain Mallet), and the SC3M platform (Service Commun de Microscopie électronique, Microcaractérisation et Morpho-histologie-imagerie fonctionnelle from the INSERM UMS016-SFR François Bonamy) as well as Sophie Domingues (Longdom publishing) for editing the manuscript.
Author information
Authors and Affiliations
Contributions
LLF, MF, JG, JC, PM, and CMM conceived of the study. MF and JC coordinated the study. MF performed the MRI and the micro-scan. MF also performed the data analysis and drafted the manuscript. LLF and JC participated in the surgery and the image analysis. LLF, BH, and JL performed the histological stainings and they performed the analysis of the histological data with JC. OG participated in the design of the study, particularly with the animal management. PM, and CMM, helped with writing of the manuscript. LLF, MF, JG, and JC participated in writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that they have no potential conflict of interest.
Human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were adhered to. All procedures involving animals performed in this study were in accordance with the “3R” rule (Replacement, Reduction, and Refinement), the ethics standards of the institution or the practice at which the study was performed.
Additional information
Luc Le Fournier and Marion Fusellier are co-first authors, and Jérôme Guicheux and Johann Clouet are co-last authors.
Rights and permissions
About this article
Cite this article
Le Fournier, L., Fusellier, M., Halgand, B. et al. The transpedicular surgical approach for the development of intervertebral disc targeting regenerative strategies in an ovine model. Eur Spine J 26, 2072–2083 (2017). https://doi.org/10.1007/s00586-017-5199-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5199-z