Abstract
Intervertebral disc degeneration is a primary cause of low back pain and has a high societal cost. Research on cell-based therapies for intervertebral disc disease is emerging, along with the interest in biological therapy to treat disc disease without reducing the mobility of the spinal motion segment. Results from animal models have shown promising results under limited conditions; however, future studies are needed to optimise efficacy, methodology, and safety. To advance research on cell-based therapy for intervertebral disc disease, a better understanding of the phenotype and differentiation of disc cells and of their microenvironment is essential. This article reviews current concepts in cell-based therapy for intervertebral disc disease, with updates on potential cell sources tested primarily using animal models, and discusses the hurdles to clinical application. Future perspectives for cell-based therapies for intervertebral disc disease are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue destruction and altered function of intervertebral discs (IVD) result in instability of the associated spinal motion segment, which largely accounts for low back pain, secondary spinal deformity, and neural compressive manifestations. Low back pain and related disease have a high societal cost, including direct medical costs, insurance, lost productivity, and disability benefits [7]. These costs are estimated to be £12 billion per year in the United Kingdom. In the United States, over $50 billion in annual health costs are accredited to low back pain directly or indirectly [7, 18].
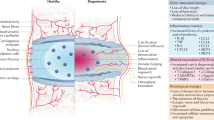
Although it has been shown that IVD degeneration is the cause of more than 20% of low back pain, the pathological mechanism by which the IVD degenerates is largely unknown. The disease process is thought to involve sequential events that lead to loss of cells and of disc matrix, which is composed of proteoglycans and collagen, and to altered biomechanics [3–5]. Current treatment of disease resulting from disc degeneration includes surgical procedures, such as discectomy and spinal fusion; however, these procedures do not preserve the function of the IVD. Moreover, they reduce the mobility of the associated spinal motion segment and increase the mechanical load and stresses on adjacent discs [26]. Mobility preserving techniques using artificial disc and nucleus implants have been tested clinically to preserve mobility in cases of severely damaged discs. Appropriate patient selection is needed to use these techniques effectively, and both these treatments and fusion surgeries have uncertain long-term results. Therefore, the demand for therapeutic inhibition of the early stages of the IVD degeneration process is increasing. Much of the recent research in the area of the IVD has focused on the biologic restoration of the IVD using growth factors, gene therapy, tissue engineering, and cells [1, 4] (Fig. 1).
Time-course pattern of normal ageing versus pathological disc degeneration. Biological therapies (drugs, protein injections, gene therapy, and cell therapy) may have potential to reverse the progression of degeneration by the appropriate timing of intervention. Chronic back pain may be associated to period where pathological disc degeneration progresses from moderate to severe. In the severe period, secondary spinal deformity, and neural compressive manifestations are manifested
Therapeutic scenario using cells for the treatment of disc disease
The IVD consists of the nucleus pulposus (NP), annulus fibrosus (AF), and the cartilage end plate (EP); three tissues that differ histologically, chemically, and physiologically. Previous histological studies have shown that one of the initial triggers of disc degeneration is a decrease in the number of viable cells in the NP [3, 5]. These cells synthesize matrix proteoglycan, which binds to water, resulting in a large swelling pressure. A decrease in the function and number of NP cells results in a decrease in this pressure, which leads to a disorientation of the lamellar structure in the inner AF [3]. In addition, biomechanical stress can lead to sequential annular tears. These events can occur in any order, and differ between individuals and events. NP and AF cells possess poor regenerative capacity compared to cells in other tissues.
In particular, the NP has a low cell density and is a site where cellular proliferation is limited. The NP contains different cell types, including notochordal and chondrocyte-like cells, depending on the age of the subject [5]. The microenvironment of NP and AF cells is subjected to high stress, and is characterised by low oxygen tension, low pH, and limited nutrition. It has been shown that NP cells are well adapted to this harsh microenvironment through utilisation of glycolysis and expression of hypoxia resistance factors [27]. The concept of cell supplementation by cell transplantation has emerged with the aim of either inhibiting or regenerating the loss of NP cells. In 1998, an animal model study was performed for the first time by Nishimura and Mochida demonstrated that the reinsertion of autologous fresh or cryopreserved NP tissue slowed degeneration in the rat IVD using degeneration model created by nucleus aspiration [22]. Since then, a number of studies have reported the efficacy of cell transplantation therapy using various animal models and donor cell types [2, 6, 11–13] (Fig. 2; Table 1).
Therapeutic scenario for the use of cells to treat disc disease. Pathological disc degeneration due to disc herniations, etc. will most likely progress after surgical intervention. The rescue of viable cells by supplementing various donor cells using in vitro culture techniques may help delay or regenerate the progressive degeneration process
Cell transplantation is a suitable approach for the IVD because of its unique structure, in which the NP is surrounded by the AF and the EP, which prevents cell migration and allows space for donor cells to adapt. The contained structure of the IVD is also thought to play an important role in limiting the immune response following cell transplantation. Cells from the NP have been reported to express Fas ligand (FasL), a specific protein seen in organs that are immune privileged [25, 33]. These anatomical and molecular data suggest that the NP region may tolerate cells from other areas of the body or even other individuals.
Review of potential cell sources tested in basic research
Autologous or allogeneic NP cells
Regeneration of a given tissue likely to be achieved by transplanting active cells that possess many characteristics of the native cells; however, the preparation of NP cells for reimplantation is problematic because autologous transplantation requires more cells than can be harvested from a single disc and harvest of cells from healthy discs may create iatrogenic degeneration in the donor disc. To obtain greater numbers of active NP cells, Okuma et al. [24] found that NP cell viability (primarily notochordal cells) could be improved by co-culture with AF cells. With low cellular yields and low proliferative activity of NP cells in the early phases of primary culture, further enhancement of the biological and metabolic viability of NP cells is desirable. A novel method to obtain further activation of NP cells was reported by Yamamoto et al. [35] using a direct cell-to-cell contact co-culture system with mesenchymal stem cells (MSCs). Besides differentiating into multiple cell types of mesenchymal origin, MSCs serve as feeder or nursing cells for other cells.
The ability of MSCs to enhance the biological and metabolic viability of NP cells (primarily notochordal cells) was evaluated using rabbit cell cultures. The results showed significantly better NP cell proliferation, DNA synthesis, proteoglycan synthesis, and cytokine and/or growth factor production in a co-culture system with direct cell-to-cell contact with MSCs, compared to a conventional co-culture system or using monolayer cultures of NP cells. Furthermore, concentrations of transforming growth factor (TGF)-β, insulin-like growth factor 1 (IGF-1), epidermal growth factor, and platelet-derived growth factor were significantly increased in the direct cell-to-cell contact co-culture group, which presumably led to enhanced NP cell proliferation [35]. With the positive results of this co-culture system, pre-clinical studies to test its effects on human NP cells are ongoing.
Allografting of NP cells and tissue has also proven to be effective in an animal model. Nomura et al. [23] transplanted allogeneic NP cells (primarily notochordal NP cells) and tissue in a rabbit disc degeneration model, and found that they effectively preserved the disc structure without a major immune response. This may provide more evidence for IVD as an immune-privileged organ. Furthermore, they have shown that transplanting NP tissue achieved better preservation of disc structure than implanting cells [23].
Autologous disc chondrocytes
Several groups have used the term “autologous disc chondrocytes” as cells taken from the IVD (in some studies including AF) possessing chondrocytic character. This may be separated from other works as they focus on utilising animal species that possess primarily chondrocytic NP cells, like humans.
Gruber et al. [11] implanted autologous disc cells in a sand rat, an animal that undergoes spontaneous disc degeneration. The cells were harvested from IVDs, expanded in monolayer culture, labelled, and then implanted into different disc sites of the donor animals. The cells were either placed on a bioresorbable scaffold or injected directly into the recipient disc. Labelled cells were seen in the discs of animals as long as 8 months after transplantation; clearly autologous disc cell implantation can successfully lead to cell survival and integration into a disc.
Ganey et al. [10] studied a canine model, in which disc degeneration was induced by partial discectomy. Six million autologous disc cells expanded from disc aspiration were transplanted to the NP region of injured discs without a carrier matrix. The discs in the dogs receiving transplants were significantly better maintained in terms of disc height and structure than in the controls. The effect lasted for about 12 months after transplantation. Furthermore, with success in the animal model, Meisel et al. have brought this procedure to a prospective, controlled, randomized, multicenter clinical study of safety and efficacy [9, 16, 20].
Other chondrocytes
Because human IVD cells share matrix-producing properties with chondrocytes, a study tested transplanting chondrocytes from other parts of the body. Gorensek et al. [12] transplanted chondrocytes from elastic cartilage into rabbit discs. They found that this method also reduced the loss of proteoglycans from injured IVDs compared to discs not receiving treatment assuming that transplanted cell producing proteoglycans. This approach raises the question of whether any type of chondrocyte, or any other cell that produces proteoglycans and type II collagen, could be successfully used as an alternative cell source. The lack of a definitive cell marker to distinguish IVD cells from other cells has made it difficult to provide an answer to this question. However, recent studies have shown that there are indeed phenotypic differences between NP cells, AF cells, and articular chondrocytes. A study by Mwale et al. [21] reported that the proteoglycan to collagen ratio is distinctly higher in the NP compared to cartilage in individuals of the same age, and that this difference can be used as a distinguishing cell phenotype.
Several molecules have also been suggested as candidates for an NP cell marker. Risbud et al. [27] have reported a difference in the expression of hypoxia inducible factor-1 (HIF-1) in NP cells compared to AF cells or chondrocytes. Fujita et al. [8] have suggested CD24 and Semba et al. [31] the sickle tail (Skt) gene as NP cell markers for rats and mice. From microarray analysis, Lee et al. [15] have reported that the expression of annexin A3, glypican 3, keratin 19, and pleiotrophin was significantly higher in NP cells compared to AF cells and articular chondrocytes. By utilising these markers, or other potential markers, it may be possible to determine the phenotype of the most appropriate cells for transplantation.
Stem cells
If a suitable cell source is unavailable, obvious candidates would be progenitor cells or stem cells. Sakai et al. [28–30] studied the potential of MSCs as an alternative cell source. They transplanted autologous MSCs tagged with the gene for green fluorescent protein (GFP) in rabbit disc degeneration model created by nucleus aspiration, and followed the GFP-labelled cells for a period of 48 weeks, tracking the effects using magnetic resonance imaging (MRI) and radiography. They also used immunohistochemistry for chondroitin sulphate, keratan sulphate, collagen types I, II, and IV, HIF-1α and β, HIF-2α and β, glucose transporters GLUT-1 and GLUT-3, and matrix metalloproteinase-2 (MMP-2), and applied reverse transcriptase-polymerase chain reaction (RT-PCR) to assess expression of the genes for aggrecan, versican, collagen types I and II, interleukin-1β (IL-1β) and IL-6, tumour necrosis factor-α (TNFα), MMP-9, and MMP-13. MRI and radiographic results confirmed the regenerative effects of the procedure. GFP-positive cells were detected in the nucleus throughout the time course at proportions rising from 21% ± 6% at 2 weeks to 55% ± 8% at 48 weeks, which demonstrated the survival and proliferation of MSCs. Immunohistochemistry showed positive staining for all proteoglycan epitopes and type II collagen in some of the GFP-positive cells. MSCs produced HIF-1α, MMP-2, and GLUT-3 with phenotypic activity comparable to NP cells. The RT-PCR demonstrated significant restoration of aggrecan, versican, and type II collagen gene expression, and significant suppression of TNF-α and IL-1β expression in the transplantation group. Thus, MSCs transplanted into degenerating discs in vivo can survive, proliferate, and differentiate into cells expressing the phenotype of NP cells with suppression of inflammatory genes [29] (Fig. 3).
Macroscopic evaluation and T2 weighted magnetic resonance images (MRI) of undamaged intervertebral disc (IVD)(normal control), IVD degeneration induced by nucleotomy (degeneration model) and mesenchymal stem cell (MSC) transplantation (stem cell transplanted). Note that the structure of the nucleus pulposus (NP) is better retained and MRI signal intensity has increased in the stem cell-transplanted IVD, suggesting positive effect of the procedure in delaying the progression of IVD dgeneration
Since the first report of MSC transplantation, several studies have confirmed the effectiveness of this procedure. Crevensten et al. [6] demonstrated that MSCs injected into a rat disc using hyaluronan gel as a scaffold maintained viability and proliferated. Using cell labelling, viable cells were detected over the 28 day study period, maintaining disc height. Zhang et al. [36] implanted allogeneic MSCs containing the marker gene LacZ from young rabbits into rabbit intervertebral discs to determine the potential of this cell-based approach. They reported that transplanted allogeneic MSCs could survive and increase the proteoglycan content within the disc, supporting their use as a potential treatment for intervertebral disc degeneration. Hiyama et al. confirmed the effectiveness of MSC transplantation in large animal models (chondrodystrophoid breed canine with nucleotomy) that are closer to humans. They also showed that transplanted MSCs expressed FasL after transplantation to the NP region, suggesting preservation of immune privilege in MSC transplantation [13]. These findings are to some extent similar to the results performed in the rabbit studies which used primarily notochordal NP cells.
The possibility of allogeneic MSC transplantation has also been suggested. Leung et al. [16] reported multiple advantages of MSC transplantation for disc disease, including that if the NP were an immune-privileged environment, then MSCs being less antigen presenting, would be able to escape alloantigen recognition.
Recently, MSCs from adipose tissue have been reported as another potential cell source. Using a goat ABC chondroitinase degeneration model, Hoogendoorn et al. [14] reported that adipose-derived MSCs can be beneficial for cell therapy of IVD disease from the standpoint that they are easily isolated compared to bone marrow MSCs. Like the case with bone marrow MSCs, co-culture induction study has also been reported using adipose-derived MSCs [17].
Hurdles to clinical application
Despite the growing variety of potential cell sources tested in large and small animal models, more investigation and discussion are needed before actually considering translation to the clinic. There are several points of concern. The first is the problem arising from the absence of an appropriate animal model that mimics IVD degeneration in humans. Usually, animal models have a better IVD nutrition status, compared to the degenerating human IVD, and because the process of IVD degeneration is not uniform, it is almost impossible to establish an animal model for naturally occurring human disc degeneration. For these reasons, the researcher is challenged to match a model to the objectives of the study [19, 32].
The second concern is the method of cell delivery. Animal studies present several ways to transplant cells to the IVD. Injection by an open procedure or fluoroscopy-guided discography is ideal. However, there may be other methods, such as the use of various scaffolding or drug delivery systems. These should also be tested to find the most efficient method to specifically match cell type and procedure.
The third concern is deciding which patients are appropriate for the application of IVD cell therapy, and this is a major concern. Biologically less active IVDs, particularly Thompson grade 4–5 discs, are not realistic candidates because of their inactive IVD microenvironment. IVDs with Thompson grades 2–3 with mild to moderate degeneration may be better recipients, or more realistically, we may only target iatrogenic IVD degeneration induced post discectomy or by degeneration of the adjacent discs [34]. The answers to these questions need to be clarified by thorough investigation before experimental studies are translated to the clinic.
Fourth, although most of the research reported is in NP cell supplementation, it must be acknowledged that the AF and EP should also be looked at as therapeutic targets. As described above, the interaction with AF cells increases the biological activity of NP cells, and the EP is the site that provides nutrition. Facilitation of AF and EP regeneration is clearly needed to synergistically improve the efficacy of cell-based therapy.
The final concern is the issue of ensuring safety in the performance of these newly developed techniques in a clinical setting. It should always be kept in mind that degeneration of recipient tissue may be a balance to maintain homeostasis. Introducing cells from an outside environment may not always be beneficial, and could cause unexpected reactions, such as tumourigenesis, transformation, or karyotypic abnormalities of the cells. In vitro techniques and reagents used in cell culture may induce adverse changes in cells. These issues need to be assessed thoroughly before any clinical applications are considered.
Future of cell utilisation for disc disease
Despite the growing number of research data on cell-based experimental therapy for IVD disease, it is clear that we do not know much about native disc cells and their microenvironment. This lack of information is a major obstacle in building a strategy for the treatment of IVD disease. Investigating the specific differentiation status of native IVD cells and their homeostasis will surely provide more ideas and clues for efficient therapeutic approaches. Although cell-based therapy for IVD disease is still in its infancy, the stage of testing a variety of cells for injection should be toned. To progress to the next step, we should be investigating what exactly IVD cells are, and how they control their homeostasis, in parallel with various studies optimising parameters, such as cell dosage and culture period and the severity of IVD degeneration in the recipient.
Conclusions
Studies on cell-based therapy using various cell sources in animal models are emerging. The results of animal models are promising in proving efficacy under limited conditions. Future studies are required to optimise efficacy, methodology, and safety. To take research on cell-based therapy for IVD disease a step forward, a better understanding of the phenotype and differentiation of IVD cells and of their microenvironment is essential.
References
An HS, Thonar EJ, Masuda K (2003) Biological repair of intervertebral disc. Spine 28:S86–S92
Anderson DG, Albert TJ, Fraser JK, Risbud M, Wuisman P, Meisel HJ, Tannoury C, Shapiro I, Vaccaro AR (2005) Cellular therapy for disc degeneration. Spine 30:S14–S19
Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M (1996) The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98:996–1003
Alini M, Roughley PJ, Antoniou J, Stoll T, Aebi M (2002) A biological approach to treating disc degeneration: not for today, but may be for tomorrow. Eur Spine J 11:S215–S220
Buckwalter JA (1995) Aging and degeneration of the human intervertebral disc. Spine 20:1307–1314
Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, Berven S (2004) Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 32:430–434
Frymoyer JW, Cats-Baril WL (1991) An overview of the incidence and costs of low back pain. Orthop Clin North Am 22:263–271
Fujita N, Miyamoto T, Imai J, Hosogane N, Suzuki T, Yagi M, Morita K, Ninomiya K, Miyamoto K, Takaishi H, Matsumoto M, Morioka H, Yabe H, Chiba K, Watanabe S, Toyama Y, Suda T (2005) CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun 338:1890–1896
Ganey TM, Meisel HJ (2002) A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J 11(Suppl 2):S206–S214
Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC (2003) Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine 28:2609–2620
Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN Jr (2002) Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine 27:1626–1633
Gorensek M, Jaksimović C, Kregar-Velikonja N, Gorensek M, Knezevic M, Jeras M, Pavlovcic V, Cör A (2004) Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett 9:363–373
Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D (2008) Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res 26:589–600
Hoogendoorn RJ, Lu ZF, Kroeze RJ, Bank RA, Wuisman PI, Helder MN (2008) Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future. J Cell Mol Med [Epub ahead of print]
Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S (2007) A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J 16:2174–2185
Leung VY, Chan D, Cheung KM (2006) Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J 15:S406–S413
Lu ZF, Zandieh Doulabi B, Wuisman PI, Bank RA, Helder MN (2007) Differentiation of adipose stem cells by nucleus pulposus cells: configuration effect. Biochem Biophys Res Commun 359:991–996
Maniadakis N (2000) Gray A (2000) The economic burden of back pain in the UK. Pain 84:95–103
Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS (2005) A novel rabbit model of mild, reproducible disc degeneration by an annulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14
Meisel HJ, Ganey T, Hutton WC, Libera J, Minkus Y, Alasevic O (2006) Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J 15:S397–S405
Mwale F, Roughley P, Antoniou J (2004) Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater 8:58–63
Nishimura K, Mochida J (1998) Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine 23:1531–1538
Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K (2001) Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop 389:94–101
Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K (2000) Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res 3:988–997
Park JB, Chang H, Kim KW (2001) Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine 26:618–621
Phillips FM, Reuben J, Wetzel FT (2002) Intervertebral disc degeneration adjacent to a lumbar fusion. An experimental rabbit model. J Bone Joint Surg Br 84:289–294
Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM (2006) Nucleus pulposus cells express HIF–1alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem 98:152–159
Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, Nakai T, Ando K, Hotta T (2003) Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials 24:3531–3541
Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T (2005) Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration7. Spine 30:2379–2387
Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, Nakai T, Ando K, Hotta T (2006) Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 27:335–345
Semba K, Araki K, Li Z, Matsumoto K, Suzuki M, Nakagata N, Takagi K, Takeya M, Yoshinobu K, Araki M, Imai K, Abe K, Yamamura K (2006) A novel murine gene, Sickle tail, linked to the Danforth’s short tail locus, is required for normal development of the intervertebral disc. Genetics 172:445–456
Singh K, Masuda K, An HS (2005) Animal models for human disc degeneration. Spine J 5:267S–279S
Takada T, Nishida K, Doita M, Kurosaka M (2002) Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine 27:1526–1530
Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB (1990) Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine 15:411–415
Yamamoto Y, Mochida J, Sakai D, Nakai T, Nishimura K, Kawada H, Hotta T (2004) Upregulation of the viability of nucleus pulposus cells by bone-marrow-derived stromal cells: significance of direct cell-to-cell contact in co-culture system. Spine 29:1508–1514
Zhang YG, Guo X, Xu P, Kang LL, Li J (2005) Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res 430:219–226
Acknowledgments
This work is supported by a grant from the Academic Frontier Project of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and a grant from AO Spine International.
Conflict of interest statement
None of the authors has any potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakai, D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J 17 (Suppl 4), 452–458 (2008). https://doi.org/10.1007/s00586-008-0743-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-008-0743-5