Abstract
Purpose
To characterize in vivo the transpedicular approach (TA) as an alternative route to study intervertebral disc (IVD) regeneration strategies in a sheep model.
Methods
48 IVD of 12 sheep were used. TA was performed under fluoroscopy, followed by nucleotomy (2-mm shaver resector). A polyurethane scaffold was used to repair the end-plate. X-ray and MRI images were acquired pre-, intra- and post-operatively (1, 3, 6 months). Complications were recorded.
Results
TA was feasible in all animals; nucleus pulposus (NP) from L1 to L5 was accessible. Nucleotomy followed by end-plate repair was achieved. Loss of NP signal intensity was shown in MRI images of the nucleotomy group.
Conclusions
TA is feasible in vivo, repeatable after only a short learning period and safely performed without significant morbidity. This animal model allows the study of IVD degeneration physiopathology and investigation of IVD regeneration techniques in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, strong efforts to develop an effective treatment for back pain (BP) have been made to prevent, slow down or reverse the degeneration of the intervertebral disc (IDD) that is known to be its leading cause. Recovering the ability of the disc to repair the extracellular matrix (ECM) and re-establishing the proteoglycan content may have a significant therapeutic effect, since they increase intervertebral disc (IVD) hydration improving its biomechanics. Recent studies have demonstrated that the use of growth factors, gene and stem cell therapies, and tissue engineering strategies might be feasible and effective approaches to prevent and treat IDD [1]. Indeed, very recently bone marrow mesenchymal stem cells have been used in a clinical phase I study in patients seeking BP with moderate IDD, showing safety and pain improvement [2]. However, several unsolved issues are still present in the translation of this new cell therapy to the clinic, such as the most reliable transplantation method, including the surgical route to approach the disc, and suitable animal models to test regenerative techniques. Indeed, novel cell/hydrogel-based treatments for regeneration of degenerated IVDs require to be tested in vivo animal models prior to translation into humans [3].
In particular, to study regenerative strategies of the nucleus pulposus (NP), it is paramount to maintain the annulus fibrosus (AF) intact. In fact, even a small AF lesion may biochemically and biomechanically alter the disc making it unable to support the physiological intradiscal pressure and avoid NP and/or cell/biomaterial leakage [4–6]. Therefore, to improve the efficacy of cells/hydrogel-based therapies, it is necessary to identify an alternative route to access the NP, which does not induce AF damage. We have recently described, in a cadaveric human and ovine spine in vitro study, that the transpedicular approach could be another way to enter the NP. In addition, the above study showed the feasibility and the relevance of such a technical approach to perform injections, nucleotomy and nucleoplasty without disrupting the AF [7].
Animal models are widely used to study IDD and to evaluate disc treatments because of the availability of the tissue, the decreased variability between subjects compared with humans and the feasibility to perform in vivo experiments. Several in vivo animal models have been described to study IDD and repair in different species [3]. However, there are several important aspects that have to be considered whenever animals are used to study IVD, such as the development and anatomy of the spine, loading and size differences, and the mechanical, biochemical and nutritional conditions. Considering all these aspects, sheep represents a suitable choice [8]. The sheep spine shares many anatomical and biomechanical similarities to the human spine, making it increasingly popular as a large animal model for preclinical spine surgery studies [3, 9]. They are readily available and show great homogeneity when selected for age, breed and sex [8]. Moreover, the ovine IVD has been used as model of IDD [10, 11] and as preclinical model to test biological therapies such as growth factor or stem cell treatments [12]. It has become more popular to test vertebral implants and fusion technologies [13] due to the similar size and volume of the vertebrae to humans, which allow the use of the same devices and instrumentation as under clinical conditions [8]. Furthermore, the biomechanical behavior of the ovine lumbar spine has been shown to be qualitatively similar to that of human in vitro [9]. Finally, ovine IVDs lack notochordal cells in adulthood, thus resembling the human condition [3].
The goal of this study was to characterize in vivo the transpedicular approach as an alternative route to study IDD and potential IVD regeneration strategies in a sheep model. The qualitative criteria used to evaluate our technique were feasibility, accuracy of the wire position in the NP and surgery technique-related adverse effects.
Materials and methods
All procedures involving live animals were approved by the Italian Ministry of Health. Twelve Biellese breed, approximately 3-year-old, skeletally mature, female sheep were used. In each sheep, four lumbar IVDs (L1–2, L2–3, L3–4, L4–5) were treated. All animal procedures and surgeries were performed at an accredited facility (Veterinary Teaching Hospital of the Department of Animal Medicine, Production and Health, University of Padua, Padua, Italy) where sheep were monitored at least once a day.
Anesthetic protocol
The animals were transferred to the operating suite and positioned in ventral recumbence on a warming blanket. The sheep received intramuscular (IM) injections of 0.2 mg/kg methadone and 5 μg/kg medetomidine prior to manipulation. Once the sheep were sedated, anesthesia was induced with intravenous (IV) injection of 2–4 mg/kg propofol and maintained with inhalation of 1–2 % isoflurane and IV injection via CRI of 60 μg/kg/h alfentanil. An endotracheal tube was attached to an anesthesia machine delivering oxygen, room air and isoflurane (1–3 %). Replacement fluids (lactated Ringer’s solution or 0.9 % NaCl) were administered via the IV catheter at an approximate rate of 10 mL/kg/h.
Surgical procedure
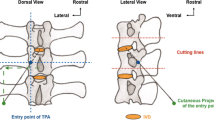
The central back portion in the lumbar region of the sheep was shaved. Then, the operative field was prepared in sterile fashion with betadine solution. A dorsal midline skin incision was extended from T10 to S1, identified by manual palpation. Fluoroscopy was used to identify the correct level in each animal. The dorso-lumbar fascia was exposed. The left side of the posterior vertebral arch was skeletonized to expose the articular and the transverse process (Fig. 1). Electrocautery was used throughout the exposure to minimize bleeding. Then, the transpedicular approach to the NP was performed.
The transpedicular approach
Throughout a posterior surgical access to the lumbar spine, a 2-mm tunnel was drilled using a Kirschner wire (k-wire) into the pedicle of the caudal vertebra to access the NP space of the spinal segment under fluoroscopy guidance, checking the wire direction (mean angle degree, ± standard deviation) in latero-lateral and anterior-posterior images (Fig. 2), as already described [7].
Nucleotomy
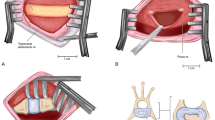
A 2-mm diameter shaver resector (Smith & Nephew Shaver Blades, 2.0-mm Full Radius, 7 cm length), powered by an arthroscopy shaver unit (Smith & Nephew, Dionics Inc. PS3500, Germany) and connected to a vacuum pump, was introduced through the 2-mm tunnel. NP tissue was removed maintaining the shaver blade in the disc space oscillating at 4,000 rpm for 5 min (Fig. 3a, b).
Surgical pictures in (a) and (b) showing the placement of the 2-mm shaver dissector through the transpedicular approach in the disc space, used to perform nucleotomy under aspiration. A porous polyurethane (PU) cylinder (arrow in c) was inserted using a 14-G cannula (Bonopty® Bone Biopsy System, AprioMed, Sweden) at the edge of the bonny tunnel to repair the end-plate and seal the disc space. (d) Representative fluoroscopy image control showing the cannula placed into the traspedicular tunnel during the scaffold positioning (arrow)
End-plate repair
The end-plate was repaired by sealing the edge of the tunnel using a press-fit porous polyurethane (PU) cylinder (2.2-mm diameter and 10-mm length) that was placed at the end-plate edge using a 14-G cannula (Bonopty® Bone Biopsy System, AprioMed, Sweden) (Fig. 3c) inserted through the tunnel as shown in Fig. 3d. The elastomeric PU cylinder was synthesized as previously described [14–16]. The scaffold was sterilized by cold ethylene oxide process and stored at room temperature until the time of surgery.
At the end of the surgical procedure, the deep fascia, the subcutaneous layers and the skin were closed in a simple continuous pattern.
Pain management and postoperative care
Following surgery, each animal that recovered from anesthesia was returned to pen housing. The animals recovered in a heated room. Buprenorphine IM 10 μg/kg daily for 2 days + ketoprofene IM 1–2 mg/kg twice daily for 5 days were administered. The animals were monitored for normal feeding behavior and cage activity twice daily to determine their general health. A wound inspection to check for possible infection or wound opening was performed on a daily basis until the surgical wound healed.
Methods for taking measurements
The effect of the nucleotomy on IVDs degeneration was evaluated through X-ray and MRI performed at 1, 3 and 6 months after surgery. Complications such as dural lesions, infections and fractures were recorded and prevalence rate evaluated.
Killing
At the time of killing, animals were administered an IM relaxant of romifidine IM 50 μg/kg + thiopentone IV 8–10 mg/kg followed by an IV injection of Tanax (embutramide, tetracaine hydrochloride and Mebezonium chloride).
Results
Surgical technique
The transpedicular approach was feasible in all animals entered in the study. The NPs of vertebrae from the L1 to L5 were accessible through the transpedicular approach. In vertebrae caudal to L5, problems arose because the pelvis and limbs of the animals interfered with lateral fluoroscopy. Intradiscal K-wire placement was achieved in all animals at the center of the disc space. The K-wire mean orientation (angle degree, ± standard deviation) assessed on the latero-lateral and anterior-posterior fluoroscopy images were 50.6° ± 3.1° on the frontal plane and 50.1° ± 6° on the sagittal plane.
Intraoperative complications
In two cases, cerebrospinal fluid leaked from the tunnel, showing a possible perforation of the dural sac (4.15 % complication rate). No animal showed adverse reactions during procedure, as confirmed by stable cardiovascular parameters including central venous pressure, pulmonary arterial pressure, cardiac output, heart rate, oxygen saturation and arterial blood gas parameters. All animals tolerated the procedure and were able to walk within 2 h. No muscular deficits of lower limb were observed. Blood loss was minimal and there were no anesthetic complications or postoperative pain management concerns with this technique. We have not experienced any clinically apparent neural or great vessel injury, and postoperative hernia or wound dehiscence did not occur.
Perioperative data
The average operation time was 92 min (range 74–110 min). The duration of the procedure to reach the NP, perform nucelotomy and place the scaffold became shorter over the course of the surgical procedures.
Postoperative complications
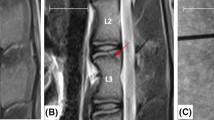
One sheep developed a fracture to the anterior aspect of the cranial vertebral body (Fig. 4a) due to the drilling of the cranial end-plate during the transpeducular approach at one level (2.1 % of complication rate). The fracture evolved into spondylarthritis after 3 months (Fig. 4b). One sheep developed spondylodiscitis (2.1 % of complication rate) at one single level (Fig. 4c, d). The sheep underwent antibiotic therapy and was killed at the first time point.
Nucleotomy
T2-weighted MRI images showed the loss of the NP signal intensity in the disc that underwent nucleotomy compered to controls. The transpedicular tunnel throughout the vertebral body and pedicle was detectable on MRI images (Fig. 5).
Discussion
The transpedicular technique represents a suitable way to approach the NP and an alternative route to deliver therapeutic agents to the IVD without disruption of the AF. Moreover, the approach can be used to perform nucleotomy to trigger a degenerative cascade studying IDD or to allow successful delivery of cell/biomaterial constructs into the disc to study their effects [7]. Indeed, normal discs have a high swelling pressure in sheep and resist the injection of any volume of substance [6]. Therefore, the injection of an adequate amount of cells and hydrogel into the IVD requires nucleotomy, which is often performed through the AF with impairment of structural integrity [5].
This pilot study on 12 sheep describes the technique in detail with the aim of investigating its in vivo feasibility and relevance in the preclinical field. Indeed, injections, nucleotomy and nucleoplasty are feasible through this approach as already described [7]. Even if some practice is required, the learning curve of the technique gets better over time. We observed a low rate of intraoperative and postoperative complications such as dural tears, anterior vertebral body fracture and spondylodiscitis (Fig. 4), comparable to those of human procedures [17].
The transpedicular approach can be performed from the right or left side without limitations, but it must be noticed, when passing instruments, that in sheep the spinal cord continues into the lumbar and sacral region. However, as already described in other studies, the posterior longitudinal ligament is often partially calcified or even ossified serving as a protective barrier [18]. For these same reasons, to study implants and regenerative disc strategies in vivo, the ovine lumbar IVDs have traditionally been accessed via an anterior or anterolateral approach [5, 19]. This retroperitoneal or transperitoneal approach carries risks that include bowel and great vessel injury, neural injury and hernia formation. The procedure also requires a large abdominal incision with greater retraction of abdominal viscera, which can be harmful to the animal. In contrast, the transpedicular approach avoids all these problems being an easy and straightforward procedure to perform.
This technique is also appropriate for testing new surgical devices. Indeed, the sheep lumbar spine model is widely used because it is comparable with the human spine [3, 8, 9]. It allows researchers to perform surgical procedures in a manner simulating the clinical situation. Moreover, bone formation rates in sheep are similar to those in humans and compares favorably with small laboratory animals [20].
An experimental model of IDD based on end-plate fractures or nucleus herniation through the end-plate has been described by Holm et al. Through a retroperitoneal approach in pigs, the cranial end-plate of the lumbar vertebra was perforated using a 3.5-mm drill bit inserted from the lateral cortex at mid-height, angulated at 45° so as to reach the central part of the end-plate. This model has shown to trigger similar degenerative changes as those observed in degenerated human discs, such as alteration of water, proteoglycan and cellular contents [21]. However, this IDD model is based on a large damage of the end-plate, without any sealing strategy, allowing Schmorl’s nodes herniation. In the present study, to limit the degradation cascade, we sealed the end-plate tunnel using conductive scaffolds (Fig. 5d); [14, 16, 22]. The evaluation of the level of the tunnel’s repair with an accurate histological analysis will be the goal of future studies. We are aware that one potential limitation of this approach is the damage caused to the end-plate tissue, which is a paramount structure in controlling nutrient supply to the center of the IVD.
Nowadays, the international scientific community is strongly interested in developing new and effective tissue engineering and regenerative strategies to solve degenerative and traumatic diseases. The expenses arising from degenerative disorder of the musculoskeletal system are enormous for the National Health Systems. This approach will deliver to the scientific community interested in the field of IVD regeneration a new powerful model to rigorously test growth factors, gene therapy, stem cells and tissue engineering-based regenerative strategies. Indeed, tissue regeneration has made rapid progresses in recent years, making possible the synthesis of constructs that closely mimic the structure and properties of native tissues. Many new promising biomaterials have been recently described [23–25] and need to be tested in valuable animal models. Various agencies, such as Medicines and Healthcare products Regulatory Agency (MHRA), National Institute for Health and Clinical Excellence (NICE), Central Office for Research Ethics Committees (COREC) and US Food and Drug Administration (FDA), will require evidences of successful animal works, to sanitize any permissions given to proceed to human work [3].
This novel preclinical ovine model would enable biological and biomechanical study of the new NP regenerative therapies [1]. This will be a significant contribution and the previous step to the clinical translation of new regenerative strategies for biological restoration of early and mild degenerative changes in IVD, which is crucial to improve present clinical treatments and life quality of several patients.
Conclusion
The reported in vivo transpedicular approach is feasible, repeatable after only a short learning period and can be safely performed without significant morbidity to the animals. This animal model allows to study IDD physiopathology and to investigate in vivo IVD regeneration techniques such as new cell therapies and tissue engineering-based strategies.
References
Vadala G, Russo F, Di Martino A, Denaro V (2013) Intervertebral disc regeneration: from the degenerative cascade to molecular therapy and tissue engineering. J Tissue Eng Regen Med. doi:10.1002/term.1719
Orozco L, Soler R, Morera C, Alberca M, Sanchez A, Garcia-Sancho J (2011) Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92:822–828. doi:10.1097/TP.0b013e3182298a15
Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ (2008) Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17:2–19. doi:10.1007/s00586-007-0414-y
Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R (2009) ISSLS prize winner: does discography cause accelerated progression of degeneration dpchanges in the lumbar disc: a ten-year matched cohort study. Spine (Phila Pa 1976) 34:2338–2345. doi:10.1097/BRS.0b013e3181ab5432
Melrose J, Shu C, Young C, Ho R, Smith MM, Young AA, Smith SS, Gooden B, Dart A, Podadera J, Appleyard RC, Little CB (2012) Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine (Phila Pa1976) 37:18–25. doi:10.1097/BRS.0b013e31820cd8d5
Vadala G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD (2012) Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 6:348–355. doi:10.1002/term.433
Vadala G, Russo F, Pattappa G, Schiuma D, Peroglio M, Benneker LM, Grad S, Alini M, Denaro V (2013) The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine (Phila Pa1976) 38:E319–E324. doi:10.1097/BRS.0b013e318285bc4a
Wilke HJ, Kettler A, Wenger KH, Claes LE (1997) Anatomy of the sheep spine and its comparison to the human spine. Anat Rec 247:542–555
Wilke HJ, Kettler A, Claes LE (1997) Are sheep spines a valid biomechanical model for human spines? Spine (Phila Pa 1976) 22:2365–2374
Osti OL, Vernon-Roberts B, Fraser RD (1990) 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976) 15:762–767
Sasaki M, Takahashi T, Miyahara K, Hirose aT (2001) Effects of chondroitinase ABC on intradiscal pressure in sheep: an in vivo study. Spine(Phila Pa 1976) 26:463–468
Goldschlager T, Rosenfeld JV, Ghosh P, Itescu S, Blecher C, McLean C, Jenkin G (2011) Cervical interbody fusion is enhanced by allogeneic mesenchymal precursor cells in an ovine model. Spine (Phila Pa 1976) 36:615–623. doi:10.1097/BRS.0b013e3181dfcec9
Benneker LM, Gisep A, Krebs J, Boger A, Heini PF, Boner V (2012) Development of an in vivo experimental model for percutaneous vertebroplasty in sheep. Vet Comp Orthop Traumatol 25:173–177. doi:10.3415/VCOT-11-02-0026
Eglin D, Griffon S, Alini M (2010) Thiol-containing degradable poly(thiourethane-urethane)s for tissue engineering. J Biomater Sci Polym Ed 21:477–491. doi:10.1163/156856209X424404
Laschke MW, Strohe A, Menger MD, Alini M, Eglin D (2010) In vitro and in vivo evaluation of a novel nanosize hydroxyapatite particles/poly(ester-urethane) composite scaffold for bone tissue engineering. Acta Biomater 6:2020–2027. doi:10.1016/j.actbio.2009.12.004
Laschke MW, Strohe A, Scheuer C, Eglin D, Verrier S, Alini M, Pohlemann T, Menger MD (2009) In vivo biocompatibility and vascularization of biodegradable porous polyurethane scaffolds for tissue engineering. Acta Biomater 5:1991–2001. doi:10.1016/j.actbio.2009.02.006
Di Martino A, Papapietro N, Lanotte A, Russo F, Vadala G, Denaro V (2012) Spondylodiscitis: standards of current treatment. Curr Med Res Opin 28:689–699. doi:10.1185/03007995.2012.678939
Oehme D, Goldschlager T, Rosenfeld J, Danks A, Ghosh P, Gibbon A, Jenkin G (2012) Lateral surgical approach to lumbar intervertebral discs in an ovine model. TheScientificWorldJournal 2012:873726. doi:10.1100/2012/873726
Baramki HG, Papin P, Steffen T (2000) A surgical approach to the ventral aspect of the lumbar vertebrae in the sheep model. SRA 22:25–27
Shi L, Wang L, Zhang Y, Guo Z, Wu ZX, Liu D, Gao MX, Chen H, Fu SC, Lei W (2012) Improving fixation strength of pedicle screw by microarc oxidation treatment: an experimental study of osteoporotic spine in sheep. J Orthop Res 30:1296–1303. doi:10.1002/jor.22072
Holm S, Holm AK, Ekstrom L, Karladani A, Hansson T (2004) Experimental disc degeneration due to endplate injury. J Spinal Disord Tech 17:64–71. doi:00024720-200402000-00012
Piazzolla A, De Giorgi G, Solarino G (2011) Vertebral body recollapse without trauma after kyphoplasty with calcium phosphate cement. Musculoskeletal surgery 95:141–145. doi:10.1007/s12306-011-0130-y
Mortisen D, Peroglio M, Alini M, Eglin D (2010) Tailoring thermoreversible hyaluronan hydrogels by “click” chemistry and RAFT polymerization for cell and drug therapy. Biomacromolecules 11:1261–1272. doi:10.1021/bm100046n
Vadala G, Mozetic P, Rainer A, Centola M, Loppini M, Trombetta M, Denaro V (2012) Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur Spine J 21(Suppl 1):S20–S26. doi:10.1007/s00586-012-2235-x
Di Martino A, Liverani L, Rainer A, Salvatore G, Trombetta M, Denaro V (2011) Electrospun scaffolds for bone tissue engineering. Musculoskelet surg 95:69–80. doi:10.1007/s12306-011-0097-8
Acknowledgments
The authors would like to acknowledge Giulia De Benedictis for the anesthesiology support, Fabrizio Russo for the surgical support and Mauro Alini and David Eglin, AO Research Institute Davos, for providing the scaffold for tunnel repair. The support of the Italian Ministry of Instruction, University and Research Grant (PRIN-200938NT8Z), the Young Investigator Research Grant of the Italian Ministry of Health (GR-2010-2318448), the Research Grant for Scientific and Technological Cooperation of the Italian Ministry of Foreign Affairs (MAE, Direzione generale per la promozione del sistema paese - PGR00009-USA), the BIOSPINA Award of the Italian Society of Spine Surgery (SICV&GIS) and the NASS Research Travelling Grant are gratefully acknowledged.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vadalà, G., De Strobel, F., Bernardini, M. et al. The transpedicular approach for the study of intervertebral disc regeneration strategies: in vivo characterization. Eur Spine J 22 (Suppl 6), 972–978 (2013). https://doi.org/10.1007/s00586-013-3007-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-013-3007-y