Abstract
Persons with recurrent low back pain (LBP) have been observed to have altered proprioceptive postural control. These patients seem to adopt a body and trunk stiffening strategy and rely more on ankle proprioception to control their posture during quiet upright standing. The aim of this study is to determine the effect of changing postural condition (stable and unstable support surface) on postural stability and proprioceptive postural control strategy in persons with recurrent LBP. Postural sway characteristics of 21 persons with recurrent LBP and 24 healthy individuals were evaluated in upright posture with or without standing on “foam” for the conditions as follows: (1) control (no vibration); (2) vibration of the triceps surae muscles; (3) paraspinal muscle vibration; (4) vibration of the tibialis anterior muscles. Vision was occluded in all conditions except for one control trial. All trials lasted 60 s. Vibration (60 Hz, 0.5 mm), as a potent stimulus for muscle spindles, was initiated 15 s after the start of the trial for a duration of 15 s. Persons with recurrent LBP showed significantly different postural control strategies favoring ankle muscle proprioceptive control (ratio closer to 1) instead of paraspinal muscle proprioceptive control (ratio closer to 0) for both standing without foam (ratio ankle muscle/paraspinal muscle control = 0.83) (P < 0.0001) and on foam (ratio ankle muscle/paraspinal muscle control = 0.87; P < 0.0001) compared to healthy individuals (0.67 and 0.46, respectively). It is concluded that young persons with recurrent LBP seem to use the same proprioceptive postural control strategy even in conditions when this ankle strategy is not the most appropriate such as standing on an unstable support surface. The adopted proprioceptive postural control strategy might be effective in simple conditions, however, when used in all postural conditions this could be a mechanism to undue spinal loading, pain and recurrences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimal postural control is an essential requirement to perform daily activities. The central nervous system (CNS) must identify and selectively focus on the sensory inputs (visual, vestibular, proprioceptive) that are providing the functionally most reliable information [9]. This ability to select and reweigh (multi-) sensory signals adaptively in conflicting and demanding situations is one of the most critical factors for postural control. In addition, reweighting of sensory signals based on location rather than on modality (i.e., within the proprioceptive system) have been demonstrated in healthy persons and in persons with low back pain (LBP) [6].
Healthy persons should have control of sufficient variability in motor learning and control, i.e., variability in the postural task constituents, which enables adaptation to altered postural demands without running the risk to jeopardize the performance (e.g., postural stability) [16, 27]. The capacity of the central integrative mechanisms that reorganize the hierarchy among sensory inputs seem to be different between healthy young people and elite sportsmen (e.g., gymnasts). Elite gymnasts have the ability to extract and weigh relevant sensory cues faster compared to healthy youngsters [35]. However, little is known about the capacity of persons with LBP to reweigh somatosensory signals adaptively to changing postural conditions.
Persons with LBP have been observed to have altered lumbosacral proprioceptive acuity [6, 33], dysfunction in trunk muscle control [20, 21] and altered postural balance [19, 30]. The underlying mechanisms of trunk muscle dysfunction and altered postural control in patients with LBP, however, is still obscure [10]. Pain might be a confounding factor for variability in postural task constituents and could induce a loss of normal variability of the postural strategy. Moseley and Hodges [31, 32] showed a decrease in transversus abdominis control variability when pain is induced and even in some subjects when pain stopped. This decrease in variability of the postural strategy might increase further back problems.
The aim of this study was to address two questions based on our previous observations that persons with recurrent LBP have altered their postural control during quiet standing [6]. Persons with recurrent LBP showed an increased gain of ankle proprioceptive signals for postural control compared to healthy persons. The first question investigated was: do persons with recurrent LBP use the same postural strategy despite changing postural conditions? This will support the hypothesis that persons with recurrent LBP have decreased posturomotor variability. The second question addressed was: does the adopted proprioceptive postural control strategy lead to postural instability? If so, this could be a mechanism to undue loading of the lumbar spine and the high recurrence rate. Therefore two postural conditions were created: the subjects had to stand on a stable and unstable support surface (“foam”). The “foam” condition should force the subjects to decrease reliance on ankle proprioceptive afference [25]. Muscle vibration was used as an experimental probe to quantify the weighting of proprioceptive afference from the lumbar multifidus muscle and the ankle muscles. Muscle vibration is known as a powerful stimulus of muscle spindles and can evoke illusory sensations of joint displacement, which most of the time correspond with a perceived lengthening of the vibrated muscle [12, 17, 34]. When postural muscles are vibrated and when the CNS uses these signals for postural control, the kinaesthetic illusions will cause excessive corrective displacement of the center of mass to avoid falling. For example, during standing vibration of triceps surae muscles can give the illusion of forward leaning and therefore the subject will compensate with a backwards shift of the center of mass, even to the point of falling [6, 14]. A brief report of this study was previously published as an abstract [7].
Materials and methods
Subjects
Forty-five young individuals voluntarily participated in this study. A medical screening by a physician was performed to include and exclude subjects in this study. Individuals with a history of vestibular disorder, neurological or respiratory disease, previous spinal surgery, acute radicular pathology, a recent (<6 months) musculoskeletal problem of the lower limb or serious neck problems were excluded. The persons with recurrent LBP did not have a more specific medical diagnosis than non-specific mechanical LBP. Subjects were included in the group with LBP when they had experienced non-specific mechanical LBP for more than 6 months and had at least three self-reported recurrent episodes of LBP (most liberal definition of LBP recurrence) [28]. Subjects were tested when they were not in a recurrence of their LBP and their pain ratings were lower than 3/10 at the moment of testing [29]. The group with recurrent LBP (n = 21) included 14 women and 7 men. The healthy group (n = 24) consisted of 13 women and 11 men. After giving their written informed consent, two questionnaires were administered: a physical activity questionnaire [2] and the oswestry disability index (ODI-2) [15]. In addition, the subjects were asked to score the pain at that moment on a visual analogue scale (VAS pain).
Subjects wore a short and T-shirt during the measurements. Height and weight were recorded. Characteristics of the subjects are presented in Table 1.
All procedures were approved by the institutional Medical Research Ethics Committee and were applied with respect to the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects).
Movement analysis
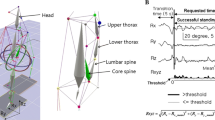
Postural sway characteristics were measured using a six-channel force plate (Bertec corporation, OH, USA). Force plate data were sampled at 500 Hz using a Micro1401 data-acquisition system and Spike2 software (Cambridge Electronic Design, UK) and low pass filtered with a cutoff frequency of 5 Hz. To evaluate trunk position in space, two piezo-resistive accelerometers (ICSensors, UK), also connected with the data-acquisition system, were placed on the spinous processes of T1 and S2 vertebra in upright posture.
Muscle vibration
In six trials mechanical vibration was used to stimulate muscle spindles specifically. Therefore two muscle vibrators (Maxon motors, Switzerland) were used. Vibration was applied bilaterally to triceps surae muscles, tibialis anterior muscles, or lumbar multifidus muscles, respectively. These muscles were selected, based on our previous experiments (unpublished data), to represent the muscles used in ankle strategy or a multi-segmental postural strategy, respectively. Bilateral vibration of vastus medialis muscle or gluteal muscles give similar results on postural control as paraspinal muscle vibration, hence, for pragmatic reason only ankle and back muscles were vibrated to give information on proprioceptive control. Muscle vibration was initiated 15 s after the start of the trial for the duration of 15 s. Activation and deactivation of the vibrators were manually controlled. The frequency of vibration was set at 60 Hz and the amplitude was approximately 0.5 mm. These characteristics of vibration were chosen to induce maximal illusory joint movement and were demonstrated to induce a significant muscle-lengthening illusion in healthy individuals [12, 34]. Moreover, when triceps surae muscles are vibrated in a healthy subject, a postural sway in backward direction is expected. When lumbar multifidus and tibialis anterior muscles are vibrated, a healthy subject is expected to show a postural sway in forward direction [6].
Test procedure
To appraise postural stability and proprioceptive postural control, two test conditions were used: (1) an upright standing condition on stable support surface and (2) an upright standing condition on an unstable support surface (“foam”), respectively (see Table 2). Both conditions consisted of three control trials and three muscle vibration trials. All trials lasted 1 min.
In Condition 1 (see Fig. 1a), each subject had to stand barefoot on the force plate and the arms loosely hanging along the body. The heels were 10 cm apart with the forefeet in a free splayed out position. Only in the first trial the subject was instructed to look straightforward to a white wall. In the other trials vision was occluded by means of 3D goggles (3Scope, VRLogic, Germany). When wearing the goggles, the subjects were asked to keep the eyes open (looking to a white display) and to keep the gaze in a straight-ahead direction [24].
For each trial the subjects were instructed to remain as immobile, but relaxed as possible in the upright standing posture.
Condition 2 (see Fig. 1b) consisted of six trials on “foam”. This condition was chosen to create a postural condition whereby ankle proprioceptive signals are less reliable and therefore the CNS should rely on other proprioceptive signals to control posture [25].
Data reduction and statistical analysis
Postural sway characteristics from the force plate readings were collected and calculated using Spike2 and Microsoft Excel software, for the 12 experimental conditions and for the two groups. Displacements of the center of pressure (COP) in anterior–posterior direction were estimated from the raw force plate data using the equation:
Further data reduction was performed by calculating the root mean square (RMS) values of the COP displacements for the stability trials (first three trials of each condition) and the mean values for the muscle vibration trials in order to appraise the directional effect of muscle vibration on COP displacement. The COP displacements in the muscle vibration trials were analyzed over two periods: the 15 s preceding and the 15 s during muscle vibration. Positive values correspond to forward COP displacement, negative values with backward COP displacement. Furthermore, proprioceptive control strategy or relative proprioceptive weighting (RW) was appraised by using the equation:
where abs TS is the absolute value of the mean COP displacement during triceps surae muscle vibration and abs LM is the absolute value of the mean COP displacement during lumbar multifidus muscle vibration. A score equal to 1 corresponds to 100% reliance on triceps surae muscle afference. A score equal to 0 corresponds to 100% reliance on lumbar multifidus muscle afference.
Differences in RMS and mean values of COP displacement between the conditions, between the trials, and between the LBP and healthy group were compared, based on repeated measures analysis of variance (ANOVA/MANOVA). Where a significant main and interaction effect was found post hoc tests (Tukey’s unequal N HSD) were performed to further analyze the detailed effects. All data are presented as means ± standard deviations (SD). The level of statistical significance was set at P < 0.05. The statistical analysis was performed with Statistica 7.1 (Statsoft, OK, USA).
Results
Postural stability measures
A significant interaction between groups and the postural stability trials (Trials 1–3, 7–9) was found [F(5,43) = 11.26, P < 0.000]. Further post hoc analyses revealed no significant differences between both groups during quiet standing on a firm support surface for the three trials without muscle vibration (P > 0.05). However, when standing on an unstable support surface (“foam”) the persons with recurrent LBP showed significantly larger sways compared to the healthy subjects during respectively the vision occlusion (P < 0.05) and ballistic arm movement trials (P < 0.0001). Table 3 displays the mean RMS values of COP displacements for the three postural stability trials for both groups and for both conditions.
Proprioceptive postural control
A significant interaction between groups and the muscle vibration trials (Trials 4–6, 10–12) was found [F(5,43) = 3.53, P < 0.005]. Further post hoc analyses showed significantly larger backward sways in the group with recurrent LBP compared to the healthy group during triceps surae muscle vibration (Trial 4) in standing on a stable support surface, with mean values of COP displacement −10.0 ± 3.2 cm and −8.1 ± 3.0 cm, respectively (P < 0.05). For tibialis anterior muscle vibration (Trial 6), no significant differences exist between the two groups (P > 0.05). In contrast, during the lumbar multifidus muscle vibration (Trial 5), the healthy subjects showed significantly larger forward sways in comparison with the group with recurrent LBP, with mean values of COP displacement 3.7 ± 1.2 cm and 1.3 ± 1.7 cm, respectively (P < 0.0001). Accordingly, the proprioceptive postural control strategy or relative weighting was significantly different between the two groups (P < 0.0001), showing higher RW ratio’s for the subjects with recurrent LBP, RW TS/LM = 0.83 ± 0.06, compared to healthy subjects, RW TS/LM = 0.67 ± 0.13 (see Fig. 2).
Means and standard deviations of the relative proprioceptive weighting triceps surae muscle/lumbar multifidus muscle of healthy persons and persons with recurrent low back pain when standing on a stable support surface (white) and on an unstable support surface (striped pattern). Higher numbers mean more use of proprioceptive input from the ankle muscles
On “foam”, healthy subjects showed significantly decreased effect of ankle muscle vibration (−5.8 ± 2.3 cm) and increased effect of lumbar multifidus muscle vibration (7.5 ± 4.5 cm) compared to the persons with recurrent LBP (−8.2 ± 2.7 cm and 1.0 ± 1.1 cm, respectively) (P < 0.005; P < 0.0001, respectively). Accordingly, the proprioceptive postural control strategy was significantly different between the two groups [F(3,43) = 16.86, P < 0.000), showing significantly higher RW ratio’s for the subjects with recurrent LBP (RW TS/LM = 0.87 ± 0.07) compared to Condition 1 (P < 0.0001), while the healthy subjects significantly decreased their RW ratio’s (RW TS/LM = 0.46 ± 0.14) compared to the stable support surface condition (P < 0.0001) (see Fig. 2).
Based on the RW ratios a sub-classification of subjects has been made. The cutoff value of 0.77 is derived from the mean RW TS/LM of the patient groups subtracted with one standard deviation. Note that the healthy persons who fall in the subclass RW TS/LM > 0.77 in Condition 1 all changed strategy (i.e., more back muscle afference reliance) during Condition 2 compared to the persons with recurrent LBP who did not (see Fig. 3a, b).
a Means and standard deviations of the relative proprioceptive weighting triceps surae muscle/lumbar multifidus of sub-classified persons with low back pain when standing on a stable support surface (white) and on foam (striped pattern). b Means and standard deviations of the relative proprioceptive weighting triceps surae muscle/lumbar multifidus of sub-classified healthy persons when standing on a stable support surface (white) and on foam (striped pattern)
Discussion
The main result of this study is that young patients with recurrent LBP showed a stronger ankle steered proprioceptive postural control for both normal stance and during standing on “foam” conditions compared to healthy persons. This decreased variability in postural strategy induced postural instability when postural demands (on “foam”) increased.
Postural stability
No significant differences in COP displacement were found between the persons with and without LBP for the stable support surface condition. A trunk stiffening strategy or ankle strategy (i.e., inverted pendulum control) could be sufficient for controlling posture in simple postural conditions, leading to tighter control of center of mass (and COP) and as a result smaller postural sways. Recently, in 140 young persons, negative correlations between the postural sway on a stable support surface and the increase in postural sway observed during standing on “foam” were found [23]. These results support the hypothesis that small postural sways during quiet upright standing do not guarantee postural stability in more complex postural conditions.
Only when the persons with LBP were standing on “foam” without vision (Trials 8 and 9) significant differences in postural sway were demonstrated compared to the healthy persons. These results are in agreement with previous studies where no significant differences in postural sways could be found between persons with and without LBP during quiet standing conditions [13, 30]. These authors observed that when the complexity of the task increased, the postural stability decreased in persons with LBP compared to healthy controls. Moreover, preparatory postural responses are necessary to limit the impact of limb movements (e.g., Trial 9) on other body parts [1]. Accordingly, patients with LBP have been observed to have altered anticipatory control during arm movements [22].
Proprioceptive postural control
In bipedal barefoot standing on a stable support surface (Condition 1), the persons with LBP showed significantly higher sensitivity to both ankle muscle vibrations compared to the healthy persons, expressed by larger COP displacements. For lumbar paraspinal muscle vibrations also significant differences in sensitivity could be found between the persons with and without LBP, showing higher sensitivity in the healthy persons.
During standing on “foam” the CNS of the healthy persons significantly upweighted the proprioceptive signals from the paraspinal muscles and downweighted those from the ankle muscles to control postural balance. In contrast, during ankle muscle vibration, the CNS of persons with recurrent LBP still used the ankle proprioceptive signals to control postural stability, while back muscle afference seemed to be ignored. The ability to gate sensory input in accordance with the internal representation of the current posture by the CNS, so as to avoid undesirable responses triggered by internal or external perturbations, is known to be an important property of the postural control system [18]. Reweighting sensory information adaptively is considered critical for flexible postural control [9, 35]. Inherent to standing on “foam” is less reliable proprioceptive input from the ankle joints [25]. Therefore, the CNS should rely more on the proprioceptive input from other joints, such as the lumbosacral region to keep the postural balance. These findings suggest strongly that the persons with recurrent LBP have altered postural control. Moreover, the CNS of the persons with LBP seemed to select the same postural control strategy (i.e., proprioceptive control at the ankles) as in normal bipedal standing on stable support surface, showing a decrease in postural control variability. This postural strategy could lead to less stable postures when postural demands increase. This is in agreement with recent studies showing in some subjects a decrease in variability in abdominal muscle control when pain was induced and even when pain stopped [31]. These authors concluded that these subjects, due to pain-related cognitions (e.g., perception of threat to the back), exerted tighter evaluative control over variability of the postural strategy [32]. In addition to pain other factors might influence the normal variability of postural control, e.g., fear of falling [8] and diminished lumbosacral proprioceptive acuity [5, 36]. Future studies should address the relative contribution of each factor to the reduction in postural variability and its relation to causing or sustaining LBP.
Clinical implications
The importance of classifying patients with (chronic) LBP based on variables relevant to physical rehabilitation into homogeneous sub-groups has been emphasized [3, 26]. Insight into the mechanisms of proprioceptive postural control in relation to LBP might help to develop these classification systems.
Specific evaluation of alterations in postural variability due to pain, fear (of falling) or diminished proprioception might be important to direct the treatment of patients with recurrent LBP and to prevent further progression of spinal problems. Assessment in several postural conditions with different degrees of complexity will be necessary as simple postural conditions would not be sufficient to detect differences [13, 23, 30]. Hereby, muscle vibration could play an important role as experimental probe.
Based on proprioceptive RW ratios a sub-classification of subjects was performed in this study (i.e., RW TS/LM = 0.77 as cutoff point). The healthy young subjects with high RW ratios during stable conditions had still the capacity to switch postural strategy during the “foam” condition compared to the persons with recurrent LBP. Few studies (only one to the author’s knowledge) exist on whether posturomotor control impairments are the cause or simply the result of LBP [11]. It could prove fruitful to perform a prospective study to investigate if high proprioceptive RW TS/LM ratios could be a preexisting risk factor for developing LBP.
Even if posturomotor control impairments and decreased postural variability are not the cause of LBP, addressing these impairments could be effective in preventing further recurrences. Specific training could improve the efficiency of the integration process leading to the optimal reweighting of sensory information [35].
Limitations
Several methodological issues warrant discussion. First, despite the accuracy and reliability of piezo-resistive accelerometers in measuring trunk position in space [4], 3D-motion analysis systems could provide more detailed information on position and movement of all body segments and therefore an estimation of center of mass could be made. Second, electromyographic recordings of the trunk muscles and lower leg muscles could give additional insight in the postural control mechanisms. Third, we acknowledge that the results of this study cannot be generalized to a more typical LBP patient population, i.e., older age and more disabled. However, the findings of this study might shed additional light on the mechanism of the high recurrence rate observed in persons with LBP. Therefore, future research comparing young persons to patients with LBP of older age and with higher disability would further test the proposed hypothesis.
Conclusions
The results of this study show that young persons with recurrent LBP have an altered postural control strategy favoring ankle proprioceptive control. Furthermore, these persons present a decrease in postural control variability, which could lead to disability, pain and recurrences. Further study of older and more disabled patients with LBP could prove fruitful.
References
Aruin AS, Latash ML (1995) Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res 103:323–332
Baecke JAH, Burema J, Frijters JER (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36:936–942
Borkan JM, Cherkin DC (1996) An agenda for primary care research on low back pain. Spine 21:2880–2884
Brumagne S, Lysens R, Spaepen A (1999) Lumbosacral repositioning accuracy in standing posture: a combined electrogoniometric and videographic evaluation. Clin Biomech 14:361–363
Brumagne S, Cordo P, Lysens R et al (2000) The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine 25:989–994
Brumagne S, Cordo P, Verschueren S (2004) Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neurosci Lett 366:63–66
Brumagne S, Paulus I, Van Deun S et al (2006) Persons with recurrent low back pain exhibit a rigid postural control strategy. Eur Spine J 15(Suppl. 4):S490
Carpenter MG, Frank JS, Silcher CP et al (2001) The influence of postural threat on the control of upright stance. Exp Brain Res 138:210–218
Carver S, Kiemel T, Jeka JJ (2006) Modeling the dynamics of sensory reweighting. Biol Cybern 95:123–134
Cholewicki J, van Dieën JH, Arsenault AB (2003) Muscle function and dysfunction in the spine. J Electromyogr Kinesiol 13:303–304
Cholewicki J, Silfies SP, Shah RA et al (2005) Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine 30:2614–2620
Cordo PJ, Gurfinkel VS, Brumagne S et al (2005) Effect of slow, small movement on the vibration-evoked kinesthetic illusion. Exp Brain Res 167:324–334
della Volpe R, Popa T, Ginanneschi F et al (2006) Changes in coordination of postural control during dynamic stance in chronic low back pain patients. Gait Posture 24:349–355
Eklund G (1972) General features of vibration-induced effect on balance. Ups J Med Sci 77:112–124
Fairbank JCT, Pynsent PB (2000) The oswestry disability index. Spine 25:2940–2953
Fomin SV, Gurfinkel VS, Feldman AG et al (1976) Moments in human leg joints during walking. Biofizika 21:556–561
Goodwin GM, McCloskey DI, Matthews PBC (1972) The contribution of muscle afferents to kinesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95:705–748
Gurfinkel VS, Ivanenko Y, Levik Y et al (1995) Kinesthetic reference for human orthograde posture. Neuroscience 68:229–243
Henry SM, Hitt JR, Jones SL et al (2006) Decreased limits of stability in response to postural perturbations in subjects with low back pain. Clin Biomech 21:881–892
Hides JA, Richardson CA, Jull GA (1996) Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine 21:2763–2769
Hodges PW, Richardson CA (1996) Inefficient muscular stabilization of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine 21:2640–2650
Hodges PW, Richardson CA (1999) Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil 80:1005–1012
Isableu B, Vuillerme N (2006) Differential integration of kinaesthetic signals to postural control. Exp Brain Res 174:763–768
Ivanenko YP, Grasso R, Lacquaniti F (1999) Effect of gaze on postural responses to neck proprioceptive and vestibular stimulation in humans. J Physiol 519.1:301–314
Ivanenko YP, Solopova IA, Levik YS (2000) The direction of postural instability affects postural reactions to ankle muscle vibration in humans. Neurosci Lett 292:103–106
Kent P, Keating JL (2005) Classification in nonspecific low back pain: what methods do primary care clinicians currently use? Spine 30:1433–1440
Latash ML (2000) There is no motor redundancy in human movements. There is motor abundance. Motor Control 4:259–261
Marras WS, Ferguson SA, Burr D et al (2007) Low back pain recurrence in occupational environments. Spine 32:2387–2397
McGorry RW, Webster BS, Snook SH, Hsiang SM (2000) The relation between pain intensity, disability, and the episodic nature of chronic and recurrent low back pain. Spine 25:834–841
Mientjes MI, Frank JS (1999) Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin Biomech 14:710–716
Moseley GL, Hodges PW (2005) Are the changes in postural control associated with low back pain caused by pain interference? Clin J Pain 21:323–329
Moseley GL, Hodges PW (2006) Reduced variability of postural strategy prevents normalization of motor changes induced by back pain: a risk factor for chronic trouble? Behav Neurosci 120:474–476
Newcomer K, Laskowski ER, Yu B et al (2000) Differences in repositioning error among patients with low back pain compared with control subjects. Spine 25:2488–2493
Roll JP, Vedel JP (1982) Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47:177–190
Vuillerme N, Teasdale N, Nougier V (2001) The effect of expertise in gymnastics on proprioceptive sensory integration in human subjects. Neurosci Lett 311:73–76
Wijnberg N (2001) Posture in Parkinson patients: a proprioceptive problem? In: Duysens J, Smits-Engelsman BCM, Kingma H (eds) Control of posture and gait. International Society for Postural and Gait, Maastricht, pp 758–761
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brumagne, S., Janssens, L., Knapen, S. et al. Persons with recurrent low back pain exhibit a rigid postural control strategy. Eur Spine J 17, 1177–1184 (2008). https://doi.org/10.1007/s00586-008-0709-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-008-0709-7