Abstract
Grazing and topography have drastic effects on plant communities and soil properties. These effects are thought to influence arbuscular mycorrhizal (AM) fungi. However, the simultaneous impacts of grazing pressure (sheep ha−1) and topography on plant and soil factors and their relationship to the production of extra-radical AM hyphae are not well understood. Our 10-year study assessed relationships between grazing, plant species richness, aboveground plant productivity, soil nutrients, edaphic properties, and AM hyphal length density (HLD) in different topographic areas (flat or sloped). We found HLD linearly declined with increasing grazing pressure (1.5–9.0 sheep ha−1) in sloped areas, but HLD was greatest at moderate grazing pressure (4.5 sheep ha−1) in flat areas. Structural equation modeling indicates grazing reduces HLD by altering soil nutrient dynamics in sloped areas, but non-linearly influences HLD through plant community and edaphic changes in flat areas. Our findings highlight how topography influences key plant and soil factors, thus regulating the effects of grazing pressure on extra-radical hyphal production of AM fungi in grasslands. Understanding how grazing and topography influence AM fungi in semi-arid grasslands is vital, as globally, severe human population pressure and increasing demand for food aggravate the grazing intensity in grasslands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, grazing occurs on more than 25% of the terrestrial surface and is a substantial driver of ecosystem degradation and soil carbon loss (Asner et al. 2004; Conant et al. 2017). Grazing leads to significant alterations in plant communities as well as carbon (C) and nitrogen (N) cycling (Milchunas and Lauenroth 1993; Hoffmann et al. 2016; Zhou et al. 2017). Overgrazing threatens biodiversity and ecosystem multifunctionality with consequences to plant productivity, community stability, and soil C and N storage (Milchunas and Lauenroth 1993; Hoffmann et al. 2016; Zhou et al. 2017). Moderate grazing is known to increase plant diversity and community productivity (Milchunas et al. 1988; Cingolani et al. 2005; Li et al. 2017). The effects of grazing intensity on soil microbes, however, are less well understood (Murray et al. 2010; Chen et al. 2013). Understanding and predicting grazing effects in semi-arid grasslands are even more critical as grasslands are suffering from overgrazing at the global scale.
Arbuscular mycorrhizal (AM) fungi are soil microorganisms that colonize approximately 80% of terrestrial plants and are of significant importance in semi-arid grassland ecosystems (Smith and Read 2008; Brundrett 2009). Mycorrhizal fungi typically form mutualistic associations, promoting host-plant performance by increasing nutrient uptake and resistance to stress, in return for plant-produced carbon compounds (Smith and Read 2008; Jiang et al. 2017; Luginbuehl et al. 2017). These fungi also produce abundant extra-radical hyphae, radiating from plant roots into soil, contributing 20–30% of soil microbial biomass and ~ 15% of soil organic C (Leake et al. 2004). These extra-radical hyphae play an important role in plant-plant interactions, C and N cycling, formation of glomalin-related soil protein, and stabilization of soil aggregates (Wilson et al. 2009; Rillig et al. 2010; Horton 2015; Kohler et al. 2017; Lin et al. 2017).
Most studies of AMF in the context of grazing have focused on AM fungi colonization not extra-radical hyphae in the soil (Wearn and Gange 2007; Barto and Rillig 2010; Tawaraya et al. 2012). Mycorrhizal fungi obtain carbon compounds from host-plants, ranging between 10 and 20% of plant photosynthates, forming intra-radical hyphae in host plant roots and extra-radical hyphae in the soil (Simard et al. 2003; Smith and Read 2008; Wang et al. 2017). The carbon-limitation hypothesis predicts long-term grazing and/or clipping reduces C allocation to roots and root exudates, leading to decreased abundance of AM fungi and other soil organisms (Van der Heyde et al. 2017). A meta-analysis of 33 publications showed herbivory can reduce percent root colonization by AM fungi (Barto and Rillig 2010). Other studies suggest the percentage of roots colonized by AM fungi can be increased by herbivory (Eom et al. 2001; Wearn and Gange 2007; Nishida et al. 2009; Tawaraya et al. 2012). However, percent root colonization by AM fungi is a relative measure and may not reflect changes in carbon allocation by the plant. Extra-radical hyphal length density (HLD) may be the most accurate predictor of plant-produced carbon compounds flux to AM fungi, because extra-radical hyphae are the largest component of total AM fungi biomass (Smith and Read 2008; Grman 2012). Grazing has been shown to increase C allocation to roots in the short term but decrease allocation over long-term herbivory and/or clipping (Bardgett et al. 1998; Barto and Rillig 2010), indicating plant-produced carbon compound flux is related to herbivory intensity and duration. Therefore, we assessed the influence of grazing on HLD in a long-term field study, conducted in the semi-arid grasslands of Inner Mongolia.
Previous studies focused on the direct effect of grazing on soil microbes (Barto and Rillig 2010; Murray et al. 2010; Soliveres et al. 2016). Understanding of the mechanisms by which grazing directly and indirectly mediate AM fungi is essential for interpreting plant-soil interactions in grazed grasslands. Moreover, grazing not only directly affect plant growth and C allocation but also soil fertility (e.g., soil organic carbon, plant-available nitrogen, and phosphorus) and edaphic properties (e.g., soil moisture, bulk density, and soil pH) (Bardgett et al. 1998; Mikola et al. 2009; Sorensen et al. 2009; Liu et al. 2015). Grazing can increase nutrient availability as a result of dung and urine deposition (Antoninka et al. 2015; Liu et al. 2015). Plant-available phosphorus (P) additions can decrease HLD, while additional plant-available N can increase HLD (Liu et al. 2012; Johnson et al. 2015), indicating herbivore grazing could indirectly impact HLD through changes in soil nutrient availability. While Chen et al. (2013) found soil nematodes were influenced by grazing pressure via pathways of the plant community, soil nutrients, and edaphic properties, few studies have considered direct and indirect grazing influences on HLD of AM fungi.
Topography mediates grazing effects on plant community composition, structure, and soil properties through soil erosion and differential dung and urine accumulation (Murray et al. 2010; Kölbl et al. 2011; Collins and Calabrese 2012; Li et al. 2017; Liu et al. 2017). Previous research indicates plant community structure shifts and productivity reductions occur at sheep densities ≥ 3.75 sheep ha−1 in grassland areas with no slope (hereafter, flat areas) but occur at lower sheep densities (≥ 3.0 ha−1) in grassland areas with ~ 10° slope (hereafter, sloped areas) (Li et al. 2015, 2017). When plant communities are altered, extra-radical AM hyphae and soil C storage can be altered as well (Liu et al. 2012; Sochorova et al. 2016). In addition, plant communities and nutrient availability interacted with grazing to influence AM fungi composition across a topographical gradient (Murray et al. 2010; Liu et al. 2017). These findings indicate topography may interact with grazing to influence HLD via several pathways.
We investigated plant and soil responses to a gradient of grazing pressure (seven sheep densities) in flat and sloped grassland areas in a long-term (10 year) experiment. Previous studies conducted at the site found grazing pressure strongly influenced soil nutrients and edaphic properties, resulting in different plant community vegetation dynamics between sloped and flat areas (Kölbl et al. 2011; Hoffmann et al. 2016; Li et al. 2017). Our study tests the following hypotheses: (1) high grazing pressure will decrease HLD indirectly through alterations in aboveground plant productivity, plant richness, soil nutrients, and edaphic properties; (2) differences in soil nutrients and edaphic properties between sloped and flat grassland areas will cause grazing influences on HLD to differ.
Materials and methods
Experimental site
Our experiment was conducted at the Inner Mongolia Grassland Research Station (IMGERS, 43° 38′ N, 116° 42′ E, altitude 1200 m a.s.l.) of the Chinese Academy of Sciences (Bai et al. 2004). This semi-arid grassland is characterized by mean annual precipitation of 346.1 mm falling mainly in the growing season (May to September) and mean annual temperature of 0.3 °C (− 21.6 °C in January, 19.0 °C in July). The soil is classified as a Calcic Chernozem (IUSS Working Group WRB 2006). Leymus chinensis (Trin.) Tzvelev and Stipa grandis P.A.Smirn are two dominant C3 grasses in this area, together accounting for 75% of total aboveground biomass (Li et al. 2017). Our study was conducted in sloped and flat areas (topographic factor), with elevation ranging from 1200 to 1280 m a.s.l. and slopes of ~ 10° inclination (Hoffmann et al. 2008).
Grazing experimental design
A 160-ha grazing experiment located at the Sino-German grazing experiment site, was established in 2005 and maintained for 11 years. Our study is a landscape-scale experiment; therefore, to reduce inherent spatial heterogeneity in plant community composition, soil properties, and management expenses, replicated blocks for each grazing intensity were not utilized (Li et al. 2017). The plots were ~ 2 ha or ~ 4 ha (plot size enlarged to 4 ha when sheep density = 1.5 sheep ha−1 to ensure six sheep per plot). Plots were randomly assigned one of seven grazing pressures (GP) (GP = 0, 1.5, 3.0, 4.5, 6.0, 7.5, or 9.0 sheep ha−1) replicated twice per topographical factor (flat or sloped) for 28 total plots. From the beginning of June to mid-September, non-lactating female sheep with an average live weight of 35 kg were used for grazing. Experimental design was presented in detail in previous studies (Hoffmann et al. 2008; Kölbl et al. 2011; Hoffmann et al. 2016).
Plant and soil sampling
Three exclosure cages (2 × 3 m) were set up prior to grazing and moved monthly to estimate aboveground plant biomass inside (i) and outside (o) the cages in grazed plots. Each exclosure cage was sampled three times and averaged as one replicate. We calculated annual aboveground net primary productivity (ANPP) in grazed plots with the formula: ANPP = W1o + (W2i − W1o) + (W3i − W2o) + (W4i − W3o). Where Wi represents standing plant biomass at the start of each month (1 = June, 2 = July, 3 = August, and 4 = September). Plant species richness near each exclosure cage were assessed using nine 1 × 1 m quadrats. Peak standing biomass of plant communities was used to estimate ANPP in non-grazed plots. Soil nutrients and edaphic properties were measured in late August 2015, 10 years after initiation of the experiment. Three 50-m transects were randomly set in each plot, and we collected soil samples at three locations along each transect. At each location, five soil cores (diameter 3 cm, depth 10 cm) were taken within a 1-m2 area and homogenized. Nine soil samples were collected in each of the 28 grazing areas and sieved through 2 mm mesh to remove roots. Soil samples were separated into two parts: one part maintained fresh for plant-available N analysis; the other air-dried to assess extra-radical AM hyphae, plant-available P, soil organic C, and soil pH. In addition, nine soil samples (0–10 cm depth) were collected in each of the 28 grazing areas to assess soil moisture and bulk density using a soil bulk density auger (100 cm3 volume), then oven-dried at 105 °C for 48 h, and weighed to determine soil bulk density.
Soil measurement
Extra-radical AM hyphae were extracted using the membrane filter technique (Jakobsen et al. 1992). Briefly, 5 g of soil was blended in 250 ml deionized water. Hyphae in 5-ml suspension were collected on a 25-mm membrane filter (1.2 μm pore size) and stained with Trypan Blue. Aseptate hyphae with characteristic unilateral angular projections and thick yellowish cell walls are distinguishing features of AM extra-radical hyphae. Extra-radical HLD was measured under a microscope at ×200 magnification using the gridline intercept method. The HLD of each soil sample was determined for six replicate membranes and averaged.
Plant-available P was measured using the Olsen method (Carter and Gregorich 2008). Soil organic C was analyzed by the dry combustion method after inorganic C was removed with 1 M HCl prior to SOC determination (Multi N/C 2100, Analytik Jena, Germany). Plant-available N was also measured by Multi N/C 2100 after extraction with 50 ml of 2 M K2SO4 from 10 g fresh field soil. A 20-g subsample of field soil was oven-dried at 105 °C for 24 h to measure soil moisture content. A 10-g subsample of field soil was mixed with 25 ml of 1 M KCl solution to measure pH using a pH meter (PB-10, Sartorius, Germany). All results were presented on a dry weight basis.
Statistical analysis
To assess grazing effects on all related variables, linear and quadratic regression analyses with sheep densities (0, 1.5, 3.0, 4.5, 6.0, 7.5, or 9.0 sheep ha−1) in flat and sloped areas were performed and least squares methods with adjusted R 2 and lowest corrected Akaike information criterion (AIC) were used to perform curve fitting. By goodness-of-fit tests for different regression models, quadratic regressions with best adjusted R 2 were selected for addressing grazing-HLD relationships in the flat and sloped grasslands. The quadratic regression analyses of all related variables along with grazing intensities were all tested versus goodness of fit of linear models with higher adjusted R 2. The level of significance was P < 0.05. All data were log-transformed prior to analysis to ensure normality and homogeneity.
Structural equation modeling
Structural equation modeling (SEM) was used to analyze potential pathways that estimate grazing effects and the strength of direct and indirect relationships between plant community parameters, soil nutrients, and edaphic properties on extra-radical AM hyphae in flat or sloped areas (Eisenhauer et al. 2015). SEM allows testing of complex causality between multiple variables by predicting expected statistical relationships from hypothesized causality (Grace 2006; Veen et al. 2010). Prior to the SEM procedure, all bivariate relationships between plant community parameters, soil nutrients, edaphic properties, and extra-radical AM hyphae were analyzed with simple linear regressions and selected by statistically significance (p < 0.05) to ensure linear models were appropriate.
Plant community parameters (richness, ANPP), soil nutrients (organic C, available N, available P), and edaphic properties (soil moisture and soil bulk density) also were reduced separately through principal component analysis (PCA) (Grace 2006; Veen et al. 2010). The first principal component (PC1) in each group was used for the subsequent SEM analysis. PCA results showed, in sloped areas, PC1 explains 73, 65, and 91% of the total variance of plant community parameters, soil nutrients, and edaphic properties, respectively, while in flat areas, PC1 explains 81, 67, and 98% of the total variance of plant community parameters, soil nutrients, and edaphic properties, respectively (see Table S1). In the SEM analyses, grazing pressure and topography were assumed to affect extra-radical AM hyphae, both directly and indirectly by influencing plant community parameters, soil nutrients, and edaphic properties. The chi-square test and its associated p value were used to adjust the model (good fit when 0 ≤ χ 2 ≤ 2 and 0.05 < p ≤ 1.00). Root mean square error of approximation (RMSEA: good fit when 0 ≤ RMSEA ≤ 0.05 and 0.10 < p ≤ 1.00) and AIC (lower AIC indicating a better fit) were used to evaluate the fit of the model. SEM analyses and related statistical analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
HLD, plant, and soil responses to grazing pressure
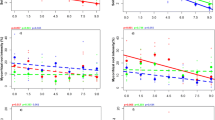
Mycorrhizal HLD was significantly negatively correlated with grazing pressure (1.5–9.0 sheep ha−1) in sloped areas (Fig. 1a). However, HLD peaked at moderate grazing pressure (4.5 sheep ha−1) in flat areas (Fig. 1b). Soil nutrient edaphic properties and plant community parameters significantly responded to grazing pressure, both in flat and sloped plots (Figs. 2 and 3). High grazing pressure reduced ANPP (Figs. 2a and 3a) and plant richness (Figs. 2b and 3b) in both flat and sloped plots.
Relationships between grazing pressure (sheep ha−1) and hyphal length density (HLD) in sloped (a) or flat (b) areas. Regressions were estimated using a linear or quadratic model with grazing pressure as a continuous predictor. Statistics (R 2 and p values) for the regressions are indicated; dotted lines represent the 95% confidence intervals
Relationships between grazing pressure (sheep ha−1) and aboveground net primary productivity (ANPP) (a), plant species richness (b), soil nutrients (c–e), and soil edaphic properties (f–h) in sloped areas. Regressions were estimated using a linear or quadratic model with grazing pressure as a continuous predictor. Statistics (R 2 and p values) for the regressions are indicated; dotted lines represent the 95% confidence intervals
Relationships between grazing pressure (sheep ha−1) and aboveground net primary productivity (ANPP) (a), plant species richness (b), soil nutrients (c–e), and edaphic properties (f–h) in flat areas. Regressions were estimated using a linear or quadratic model with grazing pressure as a continuous predictor. Statistics (R 2 and p values) for the regressions are indicated; dotted lines represent the 95% confidence intervals
High grazing pressure also directly altered soil nutrients by decreasing soil organic C (Figs. 2c and 3c) and plant-available N (Figs. 2d and 3d) in both areas, while increasing plant-available P in sloped areas but decreasing plant-available P in flat areas (Figs. 2e and 3e). The relationships between C, N, and P stoichiometry, and grazing pressure indicated N-limitation in sloped areas and P-limitation in flat areas (Fig. S1). High grazing pressure also affected edaphic properties by decreasing soil moisture (Figs. 2f and 3f) and increasing soil bulk density (Figs. 2g and 3g) in both flat and sloped areas. Soil pH was negatively related with grazing pressure in sloped areas (Fig. 2h) but was not related in flat areas (Fig. 3h).
The relationships between plant community parameters, soil properties, and HLD
In sloped areas, HLD showed no significant correlation with ANPP (Fig. 4a) but was positively related to species richness (Fig. 4b). HLD was positively correlated with soil organic C (Fig. 4c) and plant-available N (Fig. 4d) but negatively related to plant-available P (Fig. 4e) in sloped but not flat areas (Fig. 5c–e). The relationship between C, N, and P stoichiometry and HLD confirmed that a different nutrient limitation (P-limited or N-limited) drove HLD in flat compared with sloped areas (Fig. S2). Mycorrhizal HLD also was positively related to soil moisture (Fig. 4f) and soil pH (Fig. 4h) but negatively related to soil bulk density (Fig. 4g) in sloped areas. HLD had remarkably different relationships with plant and soil variables, however, in flat versus sloped areas. For example, HLD was positively related to ANPP (Fig. 5a) and showed no significant correlation with species richness in flat areas (Fig. 5b) and was negatively correlated with plant-available N (Fig. 5d) and soil moisture (Fig. 5f), with no significant correlations versus soil organic C, plant-available P, soil pH, or soil bulk density in flat areas (Fig. 5c, e, g, and h).
Relationships between hyphal length density (HLD) and aboveground net primary productivity (ANPP) (a), plant species richness (b), soil nutrients (c–e), and edaphic properties (f–h) in sloped areas. Regressions were estimated using a linear or quadratic model with grazing pressure as a continuous predictor. Statistics (R 2 and p values) for the regressions are indicated; dotted lines represent the 95% confidence intervals
Relationships between hyphal length density (HLD) and aboveground net primary productivity (ANPP) (a), plant species richness (b), soil nutrients (c–e), and soil edaphic properties (f–h) in flat areas. Regressions were estimated using a linear or quadratic model with grazing pressure as a continuous predictor. Statistics (R 2 and p values) for the regressions are indicated; dotted lines represent the 95% confidence intervals
Pathways determining HLD
Two SEMs reveal grazing altered HLD via different pathways in sloped versus flat areas, according to significant standardized path coefficients (Fig. 6a, b). In sloped areas, soil nutrients were the main pathway determining HLD. Grazing pressure was a direct negative pathway to HLD, suggesting other factors than soil nutrients alone might explain variation in sloped areas. In flat areas, however, edaphic properties and plant community parameters were the two crucial factors affecting HLD, and while soil nutrients interacted with edaphic properties, soil nutrients had no significant pathway to HLD.
Structural equation models (SEM) showing grazing pressure effects on hyphal length density (HLD) via pathways of plant community parameters, soil nutrients, and edaphic properties in sloped (a) (χ 2 = 1.76, p = 0.62, RMSEA = 0.00, AIC = 25.16) and flat (b) (χ 2 = 0.04, p = 0.84, RMSEA = 0.00, AIC = 28.04) plots. Square boxes denote variables included in the models. Plant community parameters include aboveground net primary productivity (ANPP) and plant species richness; soil nutrient variables include plant-available nitrogen (AN), plant-available phosphorus (AP), and soil organic carbon (SOC); edaphic variables include soil moisture (SM), soil bulk density (SBD), and soil pH. A significant increase or decrease of variables in relation to grazing pressure is shown by the “upward arrow” or “downward arrow,” respectively. Plant community parameters, soil nutrients, and edaphic properties are synthetic variables derived from the first axis of principal component analyses. The values of the mean change in response to grazing across different levels of pressure (i.e., 0, 1.5, 3, 4.5, 6, 7.5, or 9 sheep ha −1) are shown in each box. The width of arrows indicates the strength of the standardized path coefficient (**p < 0.01; *p < 0.05), solid lines indicate significant path coefficients, dashed lines indicate non-significant path coefficients, black lines indicate positive path coefficients, and gray lines indicate negative path coefficients; R 2values represent the proportion of variance explained for each endogenous variable
Discussion
A recent study suggested slope aspect influenced AM fungus communities (Liu et al. 2017). The present study shows topography mediated the effect of grazing pressure on the extra-radical hyphae of AM fungi. There was a negative linear relationship between grazing pressure and HLD in sloped grassland areas. An optimum of 4.5 sheep ha−1 was discovered in flat areas, however, both below and above which we measured fewer extra-radical AM hyphae. In grazed areas, changes in extra-radical hyphae were closely related to ANPP, plant species richness, soil nutrients, and edaphic properties. SEM analysis supports our prediction that grazing pressure effects on HLD differ in flat compared with sloped areas, and this is driven by differences in soil nutrients and edaphic properties of these grassland areas.
High grazing pressure should lead to reduced AM fungal abundance, according to the carbon-limitation hypothesis (Gehring and Whitham 2003). Hartnett and Wilson (2002) suggest that moderate levels of grazing may result in increased AM fungal abundance, however, which concurs with our findings that extra-radical hyphae increased when fewer than 4.5 sheep ha−1 grazed, but decreased with higher grazing pressure in flat areas (Fig. 1b). Barto and Rillig’s (2010) meta-analysis reported grazing can reduce intra-radical AM fungal abundance, but challenged the carbon-limitation hypothesis by concluding reductions were not biologically relevant in most systems. Our results suggest several factors may moderate the effects of grazing on AM fungal abundance.
Previously reported findings may differ because of differing ratios of intra-radical and extra-radical hyphae produced by different AM taxa (Miller et al. 1995; Hart and Reader 2002). A recent study suggested grazing decreased HLD, but increased spore density and did not alter root colonization, indicating that plant herbivory consequences for AM fungi can substantially differ as a result of fungal measurements (Van der Heyde et al. 2017). Mycorrhizal HLD relates to plant N and P uptake, plant growth, soil N and C cycling, and stabilization of soil aggregates (Wilson et al. 2009; Rillig et al. 2010; Horton 2015; Kohler et al. 2017; Lin et al. 2017). Overall, HLD is an important indicator of AM fungal biomass, AM fungal functioning, and potential host-plant benefits (Olsson 1999; Balser et al. 2005). Our results suggest grazing does decrease HLD in sloped areas, and thus, may lead to a decrease in AM fungi functioning.
Topography and grazing pressure have not always been simultaneously taken into consideration in previous studies. Long-term over-grazing can significantly decrease spore density and AM taxa richness (Su and Guo 2007), and even moderate grazing can decrease HLD (Van der Heyde et al. 2017). Mycorrhiza abundance also can be affected by plant community parameters, root/shoot ratio, and host-plant identity (Grman 2012; Liu et al. 2012), which are influenced by grazing. Our results suggest high grazing pressure negatively affected ANPP, with a concomitant decrease in HLD, especially in sloped areas. Chen et al. (2013) showed root biomass was negatively correlated with grazing pressure in semi-arid grasslands. Therefore, high-intensity grazing will reduce photosynthetic output and fixed carbon supplies for roots and associated AM fungi, corroborating the carbon-limitation hypothesis (Gehring and Whitham 2003).
We found a humped relationship between HLD and grazing pressure in flat areas, which might be explained by the intermediate-disturbance hypothesis (Collins et al. 1995). Milchunas et al. (1988) and Milchunas and Lauenroth (1993) developed a model predicting plant productivity was linearly related with grazing pressure in semi-arid grasslands but showed a humped relationship in sub-humid grasslands after long-term grazing. Previous research also confirms aboveground productivity, plant diversity, and belowground nematode diversity are best maintained at a moderate grazing (~ 4.5 sheep ha−1) (Chen et al. 2013). Our results did not find peak plant richness at a moderate grazing level but do suggest that the intermediate-disturbance hypothesis may be expanded to help explain grazing effects on soil microbial abundances.
Our findings indicate topography altered the pathway of grazing effects on HLD. The abundance of AM fungi is also influenced by soil nutrient availability (Liu et al. 2012; Hu et al. 2013; Johnson et al. 2015). Compared with flat areas, sloped areas are more likely to be nutrient and water limited, because of reduced infiltration and vulnerability to erosion (Kölbl et al. 2011). Liu et al. (2012) found HLD was negatively correlated with plant-available N and P in soil and positively related to soil pH. We found a positive relationship between N-to-P ratio and HLD in sloped areas, indicating N-limitation. In contrast, we found a negative relationship between N-to-P ratio and HLD in flat areas, indicating P-limitation. Johnson et al. (2015) showed host-plants were more reliant on AM fungi in P-limited than in N-limited systems, and this may explain why we found ANPP increased along with HLD.
SEM analysis of our results helps determine mechanisms and pathways of grazing effects on plant community parameters, soil nutrients and edaphic properties, ultimately explaining differences in extra-radical AM hyphae across semi-arid grassland areas. These changes depend not only on grazing pressure but also on topography. In our study, grazing pressure reduced extra-radical AM hyphae by altering soil nutrients in sloped areas, while promoting extra-radical AM hyphae (when < 4.5 sheep ha−1) in flat areas, by altering plant community parameters and edaphic properties. Slope aspect has been shown to affect the diversity of AM fungi by mediating available P, soil organic carbon, plant cover, and plant diversity (Liu et al. 2017). Our results, combined with previous results (Chen et al. 2013; Liu et al. 2017), strongly indicate grazing influences soil microorganisms via complex pathways. Grazing-induced changes in soil microorganisms may in turn modify grassland ecosystem functioning (Chen et al. 2013; Chen et al. 2017; Soliveres et al. 2016). Our findings provide a better understanding of grazing-plant-soil AM fungi dynamics and have important implications for grazing management.
References
Antoninka AJ, Ritchie ME, Johnson NC (2015) The hidden Serengeti-Mycorrhizal fungi respond to environmental gradients. Pedobiologia 58(5-6):165–176. https://doi.org/10.1016/j.pedobi.2015.08.001
Asner GP, Elmore AJ, Olander LP, Martin RE, Harris AT (2004) Grazing systems, ecosystem responses, and global change. Annu Rev Environ Resour 29(1):261–299. https://doi.org/10.1146/annurev.energy.29.062403.102142
Bai YF, Han XG, Wu JG, Chen ZZ, Li LH (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431(7005):181–184. https://doi.org/10.1038/nature02850
Balser TC, Treseder KK, Ekenler M (2005) Using lipid analysis and hyphal length to quantify AM and saprotrophic fungal abundance along a soil chronosequence. Soil Biol Biochem 37(3):601–604. https://doi.org/10.1016/j.soilbio.2004.08.019
Bardgett RD, Wardle DA, Yeates GW (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 30(14):1867–1878. https://doi.org/10.1016/S0038-0717(98)00069-8
Barto EK, Rillig MC (2010) Does herbivory really suppress mycorrhiza? A meta-analysis. J Ecol 98(4):745–753. https://doi.org/10.1111/j.1365-2745.2010.01658.x
Brundrett M (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320(1-2):37–77. https://doi.org/10.1007/s11104-008-9877-9
Carter MR, Gregorich EG (2008) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Chen DM, Zheng SX, Shan YM, Taube F, Bai YF (2013) Vertebrate herbivore-induced changes in plants and soils: linkages to ecosystem functioning in a semi-arid steppe. Funct Ecol 27(1):273–281. https://doi.org/10.1111/1365-2435.12027
Chen T, Christensen M, Nan Z, Hou F (2017) The effects of different intensities of long-term grazing on the direction and strength of plant–soil feedback in a semiarid grassland of Northwest China. Plant Soil 413(1-2):303–317. https://doi.org/10.1007/s11104-016-3103-y
Cingolani AM, Noy-Meir I, Diaz S (2005) Grazing effects on rangeland diversity: a synthesis of contemporary models. Ecol Appl 15(2):757–773. https://doi.org/10.1890/03-5272
Collins SL, Calabrese LB (2012) Effects of fire, grazing and topographic variation on vegetation structure in tallgrass prairie. J Veg Sci 23(3):563–575. https://doi.org/10.1111/j.1654-1103.2011.01369.x
Collins SL, Glenn SM, Gibson DJ (1995) Experimental-analysis of intermediate disturbance and initial floristic composition—decoupling cause and effect. Ecology 76(2):486–492. https://doi.org/10.2307/1941207
Conant RT, Cerri CEP, Osborne BB, Paustian K (2017) Grassland management impacts on soil carbon stocks: a new synthesis. Ecol Appl 27(2):662–668. https://doi.org/10.1002/eap.1473
Eisenhauer N, Bowker MA, Grace JB, Powell JR (2015) From patterns to causal understanding: structural equation modeling (SEM) in soil ecology. Pedobiologia 58(2–3):65–72. https://doi.org/10.1016/j.pedobi.2015.03.002
Eom AH, Wilson GWT, Hartnett DC (2001) Effects of ungulate grazers on arbuscular mycorrhizal symbiosis and fungal community structure in tallgrass prairie. Mycologia 93(2):233–242. https://doi.org/10.2307/3761643
Gehring CA, Whitham TG (2003) Mycorrhizae-herbivore interactions: population and community consequences. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 295–320. https://doi.org/10.1007/978-3-540-38364-2_12
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511617799
Grman E (2012) Plant species differ in their ability to reduce allocation to non-beneficial arbuscular mycorrhizal fungi. Ecology 93(4):711–718. https://doi.org/10.1890/11-1358.1
Hart MM, Reader RJ (2002) Does percent root length colonization and soil hyphal length reflect the extent of colonization for all AMF? Mycorrhiza 12(6):297–301. https://doi.org/10.1007/s00572-002-0186-5
Hartnett DC, Wilson GWT (2002) The role of mycorrhizas in plant community structure and dynamics: lessons from grasslands. Plant Soil 244(1/2):319–331. https://doi.org/10.1023/A:1020287726382
Hoffmann C, Funk R, Li Y, Sommer M (2008) Effect of grazing on wind driven carbon and nitrogen ratios in the grasslands of Inner Mongolia. Catena 75(2):182–190. https://doi.org/10.1016/j.catena.2008.06.003
Hoffmann C, Giese M, Dickhoefer U, Wan HW, Bai YF, Steffens M, Liu CY, Butterbach-Bahl K, Han XG (2016) Effects of grazing and climate variability on grassland ecosystem functions in Inner Mongolia: synthesis of a 6-year grazing experiment. J Arid Environ 135:50–63. https://doi.org/10.1016/j.jaridenv.2016.08.003
Horton TR (2015) Mycorrhizal networks. Springer, Dordrecht, Netherlands. https://doi.org/10.1007/978-94-017-7395-9_9
Hu YJ, Rillig MC, Xiang D, Hao ZP, Chen BD (2013) Changes of AM fungal abundance along environmental gradients in the arid and semi-arid grasslands of northern China. PLoS One 8(2):e57593. https://doi.org/10.1371/journal.pone.0057593
IUSS Working Group WRB (2006) World reference base for soil resources. World soil resources reports. FAO, Rome
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium Subterraneum L.1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120(3):371–380. https://doi.org/10.1111/j.1469-8137.1992.tb01077.x
Jiang YN, Wang WX, Xie Q, Liu N, Liu LX, Wang DP, Zhang XW, Yang C, Chen XY, Tang DZ, Wang ET (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356(6343):1172–1175. https://doi.org/10.1126/science.aam9970
Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the law of the minimum. New Phytol 205(4):1473–1484. https://doi.org/10.1111/nph.13172
Kölbl A, Steffens M, Wiesmeier M, Hoffmann C, Funk R, Krümmelbein J, Reszkowska A, Zhao Y, Peth S, Horn R, Giese M, Kögel-Knabner I (2011) Grazing changes topography-controlled topsoil properties and their interaction on different spatial scales in a semi-arid grassland of Inner Mongolia, P.R. China. Plant Soil 340(1-2):35–58. https://doi.org/10.1007/s11104-010-0473-4
Kohler J, Roldán A, Campoy M, Caravaca F (2017) Unraveling the role of hyphal networks from arbuscular mycorrhizal fungi in aggregate stabilization of semiarid soils with different textures and carbonate contents. Plant Soil 410(1-2):273–281. https://doi.org/10.1007/s11104-016-3001-3
Leake JR, Johnson D, Donnelly DP, Muckle GE, Boddy L, Read DJ (2004) Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can J Bot 82(8):1016–1045. https://doi.org/10.1139/b04-060
Li W, Xu F, Zheng S, Taube F, Bai Y (2017) Patterns and thresholds of grazing-induced changes in community structure and ecosystem functioning: species-level responses and the critical role of species traits. J Appl Ecol 54(3):963–975. https://doi.org/10.1111/1365-2664.12806
Li WH, Zhan SX, Lan ZC, Wu XB, Bai YF (2015) Scale-dependent patterns and mechanisms of grazing-induced biodiversity loss: evidence from a field manipulation experiment in semiarid steppe. Landsc Ecol 30(9):1751–1765. https://doi.org/10.1007/s10980-014-0146-4
Lin G, McCormack ML, Ma C, Guo D (2017) Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol 213(3):1440–1451. https://doi.org/10.1111/nph.14206
Liu N, Kan H, Yang G, Zhang Y (2015) Changes in plant, soil, and microbes in a typical steppe from simulated grazing: explaining potential change in soil C. Ecol Monogr 85(2):269–286. https://doi.org/10.1890/14-1368.1
Liu Y, Shi G, Mao L, Cheng G, Jiang S, Ma X, An L, Du G, Collins JN, Feng H (2012) Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol 194(2):523–535. https://doi.org/10.1111/j.1469-8137.2012.04050.x
Liu M, Zheng R, Bai SL, Bai YE, Wang JG (2017) Slope aspect influences arbuscular mycorrhizal fungus communities in arid ecosystems of the Daqingshan Mountains, Inner Mongolia, North China. Mycorrhiza 27(3):189–200. https://doi.org/10.1007/s00572-016-0739-7
Luginbuehl LH, Menard GN, Kurup S, Van Erp H, Radhakrishnan GV, Breakspear A, Oldroyd GED, Eastmond PJ (2017) Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356(6343):1175–1178. https://doi.org/10.1126/science.aan0081
Mikola J, Setala H, Virkajarvi P, Saarijarvi K, Ilmarinen K, Voigt W, Vestberg M (2009) Defoliation and patchy nutrient return drive grazing effects on plant and soil properties in a dairy cow pasture. Ecol Monogr 79(2):221–244. https://doi.org/10.1890/08-1846.1
Milchunas DG, Lauenroth WK (1993) Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol Monogr 63(4):327–366. https://doi.org/10.2307/2937150
Milchunas DG, Sala OE, Lauenroth WK (1988) A generalized-model of the effects of grazing by large herbivores on grassland community structure. Am Nat 132(1):87–106. https://doi.org/10.1086/284839
Miller RM, Reinhardt DR, Jastrow JD (1995) External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 103(1):17–23. https://doi.org/10.1007/BF00328420
Murray TR, Frank DA, Gehring CA (2010) Ungulate and topographic control of arbuscular mycorrhizal fungal spore community composition in a temperate grassland. Ecology 91(3):815–827. https://doi.org/10.1890/09-0209.1
Nishida T, Izumi T, Katayama N, Ohgushi T (2009) Short-term response of arbuscular mycorrhizal association to spider mite herbivory. Popul Ecol 51(2):329–334. https://doi.org/10.1007/s10144-008-0116-2
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29(4):303–310. https://doi.org/10.1016/S0168-6496(99)00021-5
Rillig MC, Mardatin NF, Leifheit EF, Antunes PM (2010) Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol Biochem 42(7):1189–1191. https://doi.org/10.1016/j.soilbio.2010.03.027
Simard SW, Jones MD, Durall DM (2003) Carbon and nutrient fluxes within and between mycorrhizal plants. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 33–74. https://doi.org/10.1007/978-3-540-38364-2_2
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 3rd edn. Elsevier, New York
Sochorova L, Jansa J, Verbruggen E, Hejcman M, Schellberg J, Kiers ET, Johnson NC (2016) Long-term agricultural management maximizing hay production can significantly reduce belowground C storage. Agric Ecosyst Environ 220:104–114. https://doi.org/10.1016/j.agee.2015.12.026
Soliveres S, van der Plas F, Manning P, Prati D, Gossner MM, Renner SC, Alt F, Arndt H, Baumgartner V, Binkenstein J, Birkhofer K, Blaser S, Blüthgen N, Boch S, Böhm S, Börschig C, Buscot F, Diekötter T, Heinze J, Hölzel N, Jung K, Klaus VH, Kleinebecker T, Klemmer S, Krauss J, Lange M, Morris EK, Müller J, Oelmann Y, Overmann J, Pašalić E, Rillig MC, Schaefer HM, Schloter M, Schmitt B, Schöning I, Schrumpf M, Sikorski J, Socher SA, Solly EF, Sonnemann I, Sorkau E, Steckel J, Steffan-Dewenter I, Stempfhuber B, Tschapka M, Türke M, Venter PC, Weiner CN, Weisser WW, Werner M, Westphal C, Wilcke W, Wolters V, Wubet T, Wurst S, Fischer M, Allan E (2016) Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536(7617):456–459. https://doi.org/10.1038/nature19092
Sorensen LI, Mikola J, Kytoviita MM, Olofsson J (2009) Trampling and spatial heterogeneity explain decomposer abundances in a sub-Arctic grassland subjected to simulated reindeer grazing. Ecosystems 12(5):830–842. https://doi.org/10.1007/s10021-009-9260-6
Su YY, Guo LD (2007) Arbuscular mycorrhizal fungi in non-grazed, restored and over-grazed grassland in the Inner Mongolia steppe. Mycorrhiza 17(8):689–693. https://doi.org/10.1007/s00572-007-0151-4
Tawaraya K, Shiozawa S, Ueda K, Murayama H, Nishizawa T, Toyomasu T, Murayama T, Sato S, Wagatsuma T, Yasuda H (2012) Leaf herbivory by Spodoptera litura increases arbuscular mycorrhizal colonization in roots of soybean. Soil Sci Plant Nutr 58(4):445–449. https://doi.org/10.1080/00380768.2012.704520
Van der Heyde M, Bennett JA, Pither J, Hart M (2017) Longterm effects of grazing on arbuscular mycorrhizal fungi. Agric Ecosyst Environ 243:27–33. https://doi.org/10.1016/j.agee.2017.04.003
Veen GF, Olff H, Duyts H, van der Putten WH (2010) Vertebrate herbivores influence soil nematodes by modifying plant communities. Ecology 91(3):828–835. https://doi.org/10.1890/09-0134.1
Wang WX, Shi JC, Xie QJ, Jiang YN, Yu N, Wang ET (2017) Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol Plant 10(9):1147–1158. https://doi.org/10.1016/j.molp.2017.07.012
Wearn JA, Gange AC (2007) Above-ground herbivory causes rapid and sustained changes in mycorrhizal colonization of grasses. Oecologia 153(4):959–971. https://doi.org/10.1007/s00442-007-0789-9
Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12(5):452–461. https://doi.org/10.1111/j.1461-0248.2009.01303.x
Zhou G, Zhou X, He Y, Shao J, Hu Z, Liu R, Zhou H, Hosseinibai S (2017) Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: a meta-analysis. Glob Chang Biol 23(3):1167–1179. https://doi.org/10.1111/gcb.13431
Acknowledgements
We are grateful to all the people who helped collect and process data over the years.
Funding
This project was supported by National Natural Science Foundation of China (31501996) and (31700389), Basic research program of Jiangsu province (Natural Science Foundation)-Youth Foundation (BK20160738) and (BK20150665). We acknowledge the Inner Mongolia Grassland Ecosystem Research Station (IMGERS) of the Chinese Academy of Sciences for providing field facilities and a long-term meteorological dataset.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 466 kb)
Rights and permissions
About this article
Cite this article
Ren, H., Gui, W., Bai, Y. et al. Long-term effects of grazing and topography on extra-radical hyphae of arbuscular mycorrhizal fungi in semi-arid grasslands. Mycorrhiza 28, 117–127 (2018). https://doi.org/10.1007/s00572-017-0812-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-017-0812-x