Abstract
Purpose
Cognitive dysfunction is more frequent after cardiac surgery. However, the preoperative cognitive state is seldom assessed when the effects of cardiac surgery on cognition are investigated. Postoperative cognitive dysfunction may be associated with the preoperative cognitive state and the existence of cerebral ischemic lesions in patients who undergo cardiac surgery.
Methods
Data were collected prospectively on 362 consecutive patients scheduled to undergo elective cardiac surgery. The brains of all patients were imaged by magnetic resonance imaging (MRI) to assess prior cerebral infarctions, carotid artery stenosis and intracranial arterial stenosis, and diffusion-weighted imaging (DWI) was used to assess acute cerebral ischemia. Patients were classified with impaired cognitive function prior to surgery if their score on the Hasegawa dementia rating scale was <24. Postoperative cognitive dysfunction from baseline was determined using four neuropsychological tests.
Results
Prior to surgery 40 patients (11%) were assessed with impaired cognition. Relative to the other patients, these patients were older and less well educated, and they had significantly higher rates of peripheral vascular disease, white matter lesions, cerebral infarction on MRI, carotid artery stenosis and postoperative cognitive dysfunction. Of these 40 cognitively impaired patients, seven (18%) had cerebral ischemia, based on DWI images before surgery; in comparison, nine of the 322 patients (3%) without preoperative cognitive impairment were found to have abnormalities on the DWI images (P < 0.001). An analysis by stepwise logistic regression demonstrated that the significant risks for preoperative cognitive impairment were advanced age, lower attained level of education, peripheral artery disease, prior cerebral infarctions, and abnormalities on DWI images.
Conclusions
These findings suggest that preoperative cognitive impairment associated with cerebral ischemic lesions and an increased risk of postoperative cognitive dysfunction existed in our patient cohort undergoing cardiac surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cognitive decline is being increasingly recognized as a postoperative complication of cardiac surgery [1–3]. The etiologies for postoperative cognitive dysfunction have been linked to cardiopulmonary bypass (CPB), microembolic and macroembolic events, hypoperfusion, and systemic inflammatory response. Although most studies have attempted to identify specific aspects of the surgical procedures that lead to dysfunction, there has as yet been no assessment of the preoperative cognitive state of patients experiencing dysfunction and its relation to postoperative cognitive decline.
Atherosclerosis and many of its risk factors, such as hypertension [4], diabetes mellitus [5], hyperlipidemia [6], and peripheral arterial disease [7], are known to disturb cognitive function. In a recent study carried out by our group, we reported that cerebral ischemic disorders, such as small infarctions, can be seen on diffusion-weighted imaging (DWI) scans of patients before they underwent cardiac surgery [8]. DWI identifies cerebral ischemia lesions with a high sensitivity and specificity and can distinguish between acute and chronic infarction. Vermeer et al. reported that although most of the patients with small infarctions in their patient cohort were asymptomatic and manifested no clinical signs, these asymptomatic small infarctions were an important factor related to the development of cognitive impairment and dementia [9].
Psychometric tests, such as the Mini-Mental State Examination (MMSE), are commonly administered screening assessments of cognitive function. Jensen et al. [10] found that 20% of their candidates for coronary bypass graft (CABG) obtained an MMSE score in the impaired range (<24) prior to surgery. Nevertheless, there is relatively little currently information available on the risk factors or on existing cerebral ischemic lesions in patients with cognitive impairment. Identifying underlying ischemic cerebral disease prior to surgery may help to assess the likelihood and etiology of postoperative neurologic complications. In the study reported here, we performed cerebral magnetic resonance imaging (MRI) on consecutive patients scheduled to undergo cardiac surgery with the aim of evaluating the relationship between existing acute and chronic cerebral ischemia and preoperative cognitive impairment. We also examined whether preoperative cognitive impairment was associated with systemic atherosclerosis and postoperative cognitive dysfunction in patients undergoing cardiac surgery.
Materials and methods
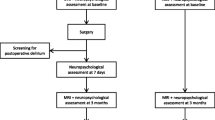
A total of 429 patients were prospectively and consecutively enrolled in the study between September 2004 and August 2008. Institutional Research Ethics Committee approval was granted, and written informed consent was obtained from all patients. The present data set includes data from a cohort of patients previously reported on for prevalence of cerebral abnormalities identified by DWI before cardiac surgery [8]. All patients were scheduled to receive CABG with CPB (on-pump) or without (off-pump), mitral valve repair or replacement, or aortic valve replacement. In all, 67 patients were excluded from the study, including those with contraindications based on MRI results (43 patients), severe visual disorders (11), and current severe psychiatric disease (2), and 11 patients who had prolonged tracheal intubation for postoperative pulmonary complications and could not participate in the postoperative testing period. All of the remaining 362 patients (84%) agreed to participate.
Neurologic evaluation
Cognitive status was assessed by four tests administered preoperatively and 1 week postsurgery to all patients when they were free from the effects of sedatives. The examination included several cognitive domains:
-
1.
Orientation and global cognition was assessed using the Hasegawa-dementia score (HDS), which is a modification of the MMSE, with a score ranging from 0 to 30, in which higher scores represent a better cognitive state [11]. In this study, patients were classified as having existing impaired cognition function if their HDS score was <24.
-
2.
Verbal memory was assessed using a digit span subtest of the Wechsler Memory Scale-Revised: the tasks require short-term memory (digit span forward) and working memory (digit span backward) for verbal material.
-
3.
Attentional performance was assessed with the digit symbol substitution test of the Wechsler Adult Intelligence Scale, in which participants transcribed number–symbol pairs under timed conditions.
-
4.
The Kana pick-out test measured executive function. Patients read a children’s fable and pick out all vowels, such as a, I, u, e, and o, while also remembering the meaning of the sentences.
These tests were performed prior to surgery by one of three trained investigators who were assigned randomly to the patients. All tests were repeated postoperatively by a single investigator who was blinded to the surgical procedure performed. Postoperative cognitive dysfunction was defined as a decrease of at least 20% from baseline in an individual’s performance in more than two tests [1].
MRI scans
An MRI scan and magnetic resonance angiograph (MRA) were obtained for each patient 1–14 days before surgery and after each (if any) cardiac catheterization. The MRI examinations were performed using a 1.5 T system (Gyroscan Intera Achieva Nova Dual; Philips Medical Systems, Eindhoven, The Netherlands). The imaging protocol included a diffusion weighted, single-shot, spin-echo echoplanar sequence [diffusion gradient b values of 0 and 1000 s/mm2, repetition time (T R) 3000 ms, echo time (T E) 51 ms, slice thickness 5 mm, with a 1-mm intersection gap, matrix 112 × 256 pixels, 230 mm field of view], turbo fluid-attenuated inversion recovery, and T2-weighted turbo spin-echo sequences. In DWI, the diffusion gradients for total acquisition were successively and separately applied in three orthogonal directions for a total acquisition time of 30 s. Trace images were then generated and maps of apparent diffusion coefficient (ADC) calculated with a dedicated software tool. The MRA included intracranial and neck vessels and was performed using a three-dimensional time-of-flight technique.
MRI findings (fluid-attenuated inversion recovery and T2) were classified as: no infarcts; single infarct with a diameter ≥3 mm; multiple infarcts at the same location (cortical, subcortical, or cerebellar); multiple infarcts in the multiple locations. The degree of stenosis of the intracranial arteries was graded bilaterally from MRA as: almost normal; moderate narrowing >50%; occluded [12]. The degree of stenosis in the carotid arteries was graded based on MRA as: normal or mild narrowing <50%; moderate narrowing of 50–75%; severe narrowing (>75%) or obstructed [13, 14]. A Fazekas [15] rating scale was used to grade the lesion load of MRI hyperintensities in the white matter of the brain. White matter lesions were defined as punctuate foci of MRI hyperintensities, beginning confluence of foci, and large confluent areas. DWI scan results were considered to be abnormal if, relative scans of normal brains, an area of hyperintensity was present on the DWI scan and an area of hypointensity was present on the apparent diffusion coefficient (ADC) maps, signifying cerebral ischemia. The apparent lesions on the MRI, MRA and DWI scans were evaluated by two experienced neuroradiologists blinded to the clinical and neuropsychological data.
Postoperative brain MRI or computed tomography (CT) was performed only on patients with neurological deficits that lasted more than 24 h. Stroke was defined as a new postoperative neurological deficit that could be confirmed by postoperative MRI or CT of the brain and further verified by neurologists.
Patient management and evaluation of arteriosclerosis in the aorta
Diazepam and fentanyl were used to induce and maintain anesthesia, supplemented with isoflurane or sevoflurane during surgery. Surgery with CPB was performed using a membrane oxygenator and roller pump under alpha-stat pH management and moderate hypothermia (28–34°C), as described previously [16]. Blood from cardiotomy suction catheters was separated from the pump circuit and washed with a cell-saving device. Patients with a history of prior cerebral infarction or severe carotid stenosis were maintained at a mean arterial pressure >70 mmHg during CPB and rewarmed to maintain no more than a 3°C difference between rectal and CPB perfusion temperatures. The mean arterial pressure in other patients was maintained between 50 and 70 mmHg, and the difference between rectal and CPB perfusate temperature was restricted to 5–6°C during rewarming. Off-pump CABG surgery was performed with a Medtronic Octopus IV (Medtronic, Minneapolis, MN). A deep pericardial traction suture was applied for cardiac displacement.
All patients underwent epiaortic ultrasound scans at the time of surgery to screen for atherosclerosis of the ascending aorta. We divided the ascending aorta from the aortic valve to the innominate artery into three segments and assessed intimal thickening off-line on videotape records, as described previously [17]. The degree of atherosclerosis in the ascending aorta was graded as: almost normal or mild atherosclerosis (<3 mm); moderate (atheroma ≥3 mm in one segment of the ascending aorta); severe (atheroma ≥3 mm in two or all three segments, or mobile atheroma). All patients were examined by one echocardiograph technician. Decisions to modify cannulation, clamping, proximal graft anastomotic sites, and/or cardioplegia cannulation sites were determined by the cardiac surgeons based on findings from real-time imaging.
Statistical analysis
Differences in continuous variables between groups with and without preoperative cognitive impairment were tested for significance using one-way analysis of variance (ANOVA) and Student’s t test. Significance among categorical variables was determined using Fisher’s exact test. A P value <0.05 was considered to be significant. Stepwise logistic regression analysis was performed to separately assess for predictors of preoperative cognitive impairment. Variables included in the multivariate model included all those found to be associated with this condition at <0.20 on univariate analysis and potential risk factors for preoperative cognitive impairment. Odds ratios (OR) were calculated for each variable. Goodness of fit was assessed by the Hosmer–Lemeshow statistic. Tests were performed using the SAS Institute statistics package (ver. 9.1; SAS, Cary, NC).
Results
The 362 patients enrolled in this study had average age of 69.5 ± 9.7 years. Their mean HDS score was 26.7 ± 3.0 (range 12–30). Prior to cardiac surgery 40 (11%) patients had an existing cognitive impairment. Preoperative characteristics for patients with and without preoperative cognitive impairment are summarized in Table 1. Relative to patients without cognitive impairment, those who had impaired cognition prior to surgery were older, had a lower level of education, and had a significantly higher rate of peripheral vascular disease. The neuropsychological test results for patients with and without preoperative cognitive impairment are listed in Table 1. Differences were noted in all test results between patients with and without preoperative cognitive impairment. There appeared to be a higher incidence of a history of cerebrovascular disease in patients with preoperative cognitive impairment (23%) than in unimpaired patients (12%), but this difference was not significant (P = 0.079).
Preoperative MRI findings and postoperative neurologic complications are shown in Table 2. The group with impaired cognition before surgery had significantly higher rates of white matter lesions, multiple cerebral infarctions, carotid artery stenosis, and postoperative cognitive dysfunction. There was no significant difference between any of the types of surgical procedure and the prevalence of postoperative cognitive dysfunction (Table 3).
Of the 40 patients with preoperative cognitive impairment, seven (18%) had DWI abnormalities on the preoperative scan, of whom four (57%) had their elective surgery delayed by 2–5 weeks (24 ± 6 days) and follow-up imaging studies. None of these four patients showed relevant ischemic lesion on preoperative follow-up studies. However, one (25%) patient showed a decline in cognitive state in the postoperative testing period, and two of the three (67%) DWI patients without follow-up imaging studies had cognitive deficits on postoperative testing. Of these, one patient had a small area with a diffusion abnormality that was larger on the postoperative MRI sequence; he developed dysarthria and right hemiparesis. In contrast, of the 322 patients without preoperative cognitive impairment, nine patients (3%) had DWI abnormalities on the preoperative scan; however, these patients did not show a decline in cognitive state in the postoperative testing period.
Stepwise logistic regression analysis identified age (OR per 10 years 1.848, P = 0.025), lower level of education (OR per grade 1.696, P = 0.044), peripheral arterial disease (OR 2.747, P = 0.033), cerebral infarctions (OR per grade 1.526, P = 0.027), and abnormalities in the brain detected by DWI (OR 3.423, P = 0.036) as being associated with preoperative cognitive impairment (Table 4). The final study model had an area under the receiver operating characteristic curve (“c” index) of 0.773 with an adjusted Hosmer–Lemeshow test statistic of 0.556.
Discussion
The results of this study demonstrate that, among our patient cohort, patients with a low cognitive performance score on psychometric tests before surgery (baseline) had higher rates of multiple cerebral infarctions and evidence of preoperative acute cerebral ischemia on the DWI image. Our findings also suggest that preoperative cognitive impairment led to an increased risk for postoperative cognitive dysfunction in patients who underwent cardiac surgery. Multivariate analysis revealed that advanced age, lower educational level, peripheral arterial disease, cerebral infarctions, and abnormalities in the brain detected by DWI were independent predictors of preoperative cognitive impairment.
Previous investigations have excluded subjects with low baseline cognitive performance scores; however, longitudinal and cross-sectional studies have indicated that higher degrees of cardiovascular disease can impair cognitive performance. Selnes et al. [18] found that patients with coronary artery disease had lower cognitive performance scores and a more rapid decline over 72 months compared with those having no vascular risk factors. Millar et al. [19] reported that 16% of patients performed poorly on the Stroop test before CABG surgery, an indication of impaired cognition, and Evered et al. [20] found that 35% of CABG patients were cognitively impaired before their procedure. In our study, impaired cognition was present in 11% of patients before surgery; a lower proportion than that found in earlier studies. The difference was influenced by the type of surgery, including valve surgery, and the methods used to assess cognitive function. However, because of the systemic nature of atherosclerotic disease, the cardiovascular surgical population may be at particular risk for cognitive impairment.
Performance on psychometric tests is influenced by demographic characteristics: scores decrease with advancing age and a lower level of education. Similar to our observations, Hogue et al. [21] found that age and level of education were associated with cognitive impairment. Although the association between education and cognitive function is not fully known, one possible explanation is the concept of cognitive reserve [22, 23]; for example, more education may simply reflect a tendency to make better health or other behavioral choices that, in turn, lead to the maintenance of cognitive function.
White matter lesions and multiple infarctions detected by MRI were more frequent in patients with cognitive impairment, but a history of cerebrovascular disease was not independently associated with cognitive impairment. White matter lesions appear to be related to chronic microvascular disease and hypoperfusion [24] and are known to increase with age [25]. However, most patients with white matter lesions or multiple infarctions do not present a history of clinical stroke, although many may be found with cognitive decline [9]. Previous studies have found a higher incidence of cognitive impairment and dementia in the presence of multiple infarctions compared to single or no infarctions [9, 26]. Therefore, low scores on psychometric tests, such as the HDS or MMSE, are considered to reflect an early stage of cognitive impairment and may be useful for identifying asymptomatic cerebral infarctions in elderly patients undergoing cardiac surgery.
Preoperative cerebral ischemia detected by DWI was one of the strongest predictors of preoperative cognitive impairment in this series. However, the relation between abnormalities uncovered by DWI and cognitive state is unclear. Restrepo et al. [27] studied 13 patients and found that four patients with postoperative defects visible on DWI scans had a greater degree of neurocognitive decline than patients with normal DWI. Barber et al. [28] reported that 43% of patients with postoperative DWI had new ischemic lesions and that cognitive decline was evident in all patients with postoperative ischemic lesions. In contrast, no association was found between DWI lesions and cognitive decline in a number of other studies of patients after cardiac surgery [29–31]. However, CPB may aggravate existing neurological ischemia and potentiate cerebral edema in areas where the blood brain barrier is disrupted. Patients with preoperative cerebral injury may be more vulnerable to the adverse effects of cardiac surgery. Therefore, we suggest that cardiac surgery may be safer if it can be delayed for 2–3 weeks. DWI may provide useful clinical information to determine the optimal timing of strategies in cardiac surgery. Further studies are needed to determine the relationship between DWI lesions and cognitive dysfunction.
There were several limitations to the present analysis. First, our patient data originated from a single institution. Thus, we cannot exclude the possibility that our results were biased by institutional standards and patient population. Second, we used only the HDS as a tool to assess preoperative cognitive function. However, the HDS and MMSE psychometric tests are commonly used instruments for psychometric screening of cognitive function, and we studied a relatively large sample of 362 subjects to lessen the impact of bias. Third, postoperative MRI or DWI findings were not assessed in our patients, so the correlation between the presence of preoperative cognitive impairment and postoperative new cerebral ischemic lesions was uncertain.
In conclusion, the results of this study demonstrate that cognitive impairment before surgery was associated with existing cerebral ischemic lesions. Our findings also suggest that preoperative cognitive impairment was derived from systemic atherosclerosis (i.e., prior cerebral infarction or peripheral arterial disease) and led to an increased risk of postoperative neurological dysfunction in patients who underwent coronary surgery. Routine screening for preoperative cognitive evaluation should allow underlying ischemic cerebral disease to be identified and supported by strategies aimed at improving neurologic outcome.
References
Shaw PJ, Bates D, Cartlidge NE, French JM, Heaviside D, Julian DG, Shaw DA. Neurologic and neuropsychological morbidity following major surgery: comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987;18:700–7.
Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, Aggarwal A, Marschall K, Graham SH, Ley C. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335:1857–63.
Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA, Neurological Outcome Research Group and the Cardiothoracic Anesthesiology Research Endeavors Investigators. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402.
Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–40.
Elias PK, Elias MF, D’Agostino RB, Cupples LA, Wilson PW, Silbershatz H, Wolf PA. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20:1388–95.
Kivipelto M, Helkala EL, Hänninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–9.
Phillips NA, Mate-Kole CC. Cognitive deficits in peripheral vascular disease. A comparison of mild stroke patients and normal control subjects. Stroke. 1997;28:777–84.
Maekawa K, Goto T, Baba T, Yoshitake A, Morishita S, Koshiji T. Abnormalities in the brain before elective cardiac surgery detected by diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2008;86:1563–9.
Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–22.
Jensen BO, Hughes P, Rasmussen LS, Pedersen PU, Steinbrüchel DA. Cognitive outcomes in elderly high-risk patients after off-pump versus conventional coronary artery bypass grafting: a randomized trial. Circulation. 2006;113:2790–5.
Kato S, Shimogami H, Onodera A, Okita K, Ikeda K, Kosaka A, Imai Y, Hasegawa K. Development of the revised version of Hasegawa’s dementia scale. Rounen Seisin Igaku Zashi (Jpn J Geriatr Psychiatry). 1991;2:1339–47 (in Japanese).
Heiserman JE, Drayer BP, Keller PJ, Fram EK. Intracranial vascular stenosis and occlusion: evaluation with three-dimensional time-of-flight MR angiography. Radiology. 1992;185:667–73.
Atlas SW. MR angiography in neurologic disease. Radiology. 1994;193:1–16.
Masaryk TJ, Lewin JS, Laub G. Magnetic resonance angiography. In: Stark DD, Bradley WG, editors. Magnetic resonance imaging. 2nd ed. St. Louis: Mosby Year Book; 1992. p. 299–334.
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–6.
Goto T, Baba T, Honma K, Shibata Y, Arai Y, Uozumi H, Okuda T. Magnetic resonance imaging findings and postoperative neurologic dysfunction in elderly patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2001;72:137–42.
Goto T, Baba T, Matsuyama K, Honma K, Ura M, Koshiji T. Aortic atherosclerosis and postoperative neurological dysfunction in elderly coronary surgical patients. Ann Thorac Surg. 2003;75:1912–8.
Selnes OA, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, McKhann GM. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg. 2009;88:445–54.
Millar K, Asbury AJ, Murray GD. Pre-existing cognitive impairment as a factor influencing outcome after cardiac surgery. Br J Anaesth. 2001;86:63–7.
Evered LA, Silbert BS, Scott DA, Maruff P, Laughton KM, Volitakis I, Cowie T, Cherny RA, Masters CL, Li QX. Plasma amyloid beta42 and amyloid beta40 levels are associated with early cognitive dysfunction after cardiac surgery. Ann Thorac Surg. 2009;88:1426–32.
Hogue CW Jr, Hershey T, Dixon D, Fucetola R, Nassief A, Freedland KE, Thomas B, Schechtman K. Preexisting cognitive impairment in women before cardiac surgery and its relationship with C-reactive protein concentrations. Anesth Analg. 2006;102:1602–8.
Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20.
Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–10.
Tomimoto H, Ihara M, Wakita H, Ohtani R, Lin JX, Akiguchi I, Kinoshita M, Shibasaki H. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003;106:527–34.
Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–32.
Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–8.
Restrepo L, Wityk RJ, Grega MA, Borowicz L Jr, Barker PB, Jacobs MA, Beauchamp NJ, Hillis AE, McKhann GM. Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke. 2002;33:2909–15.
Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–33.
Knipp SC, Matatko N, Wilhelm H, Schlamann M, Thielmann M, Lösch C, Diener HC, Jakob H. Cognitive outcomes three years after coronary artery bypass surgery: relation to diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2008;85:872–9.
Stolz E, Gerriets T, Kluge A, Klovekorn WP, Kaps M, Bachmann G. Diffusion-weighted magnetic resonance imaging and neurobiochemical markers after aortic valve replacement: implications for future neuroprotective trials? Stroke. 2004;35:888–92.
Cook DJ, Huston J 3rd, Trenerry MR, Brown RD Jr, Zehr KJ, Sundt TM 3rd. Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83:1389–95.
Acknowledgments
The authors thank Dr. Jon Moon for his editorial assistance and Dr. Akira Kitagawa for his statistical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Maekawa, K., Goto, T., Baba, T. et al. Impaired cognition preceding cardiac surgery is related to cerebral ischemic lesions. J Anesth 25, 330–336 (2011). https://doi.org/10.1007/s00540-011-1108-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-011-1108-5